Abstract
Corticotrophs in the fetal sheep become increasingly responsive to arginine vasopressin (AVP) in late gestation. We previously reported that this may be due in part to corresponding increases in signal transduction (inositol 1,4,5-trisphosphate, IP3). These ontogenic changes are prevented by hypothalamo-pituitary disconnection (HPD), which also prevents fetal plasma cortisol concentrations from increasing in late gestation. This led us to hypothesize that cortisol is involved in mediating the changes in pituitary responsiveness. HPD was performed on fetal sheep at 120 days gestational age (dGA). Half of the HPD fetuses were infused with cortisol for 3 days beginning at 135–137 dGA (HPD+C). The remaining HPD fetuses and a group of sham-operated control fetuses were infused with saline. Pituitary cells were isolated and cultured. After 48 h, a subset of cells was stimulated with 100 nM AVP for 2 h, and the medium was collected for ACTH analysis. Another subset of cells was stimulated with 100 nM AVP for 30 min, and the formation of IP3 was determined. Plasma cortisol concentrations increased rapidly within the first 6 h after infusion (5.2 ± 1.9 to 29.7 ± 4.9 ng/ml) but did not increase thereafter. Cells from HPD+C and sham-operated fetuses secreted significantly more ACTH than those from HPD fetuses (% increase from control: 33.0 ± 8.8%, 47.9 ± 10.6%, and 11.9 ± 2.4%, respectively). IP3 formation was significantly increased in cells from HPD+C and sham-operated compared with HPD fetuses (% increase from control: 17.7 ± 4.4%, 18.9 ± 4.3%, and 4.6 ± 1.5%, respectively). These findings support the idea that cortisol plays a role in mediating the increase in pituitary responsiveness to AVP in the late-gestation fetal sheep.
Keywords: inositol 1,4,5-trisphosphate; ovine fetus; cortisol replacement; adrenocorticotropic hormone
fetal plasma cortisol concentrations increase markedly close to parturition. This is well illustrated in fetal sheep, in which plasma cortisol concentrations are negligible at 120 days gestational age (dGA) but increase to ∼90 ng/ml after 140 dGA (7). Gestation is ∼145–147 days in this species. The late-gestation cortisol “surge” is a critical mediator of lung and other organ development. Several changes in the hypothalamic-pituitary-adrenal (HPA) axis have been proposed to underlie the surge. These include increased plasma bioactive ACTH concentrations (13, 15) and heightened adrenal responsiveness (10, 12, 19, 24). Another important change is increased pituitary responsiveness to arginine vasopressin (AVP). It has been demonstrated that corticotroph maturation in fetal sheep is both morphological and functional in nature. Corticotrophs isolated from 100-dGA fetal sheep pituitaries are “fetal” in appearance and are more responsive to stimulation by corticotropin-releasing factor than AVP. Conversely, corticotrophs from 140-dGA fetuses are morphologically similar to those found in adult pituitaries and are more responsive to AVP (2, 9, 16, 17).
Until recently, the reason for this increased pituitary responsiveness to AVP remained unclear. AVP-induced ACTH release is propagated through a series of steps, beginning with the hormone binding to its G protein-coupled V1b receptor (14, 23). This binding induces a conformational change in the receptor structure, leading to G protein dissociation and activation of phospholipase C (14, 23). Subsequent phospholipase C-mediated cleavage of phosphatidylinositol 4,5-bisphosphate leads to the formation of inositol 1,4,5-trisphosphate (IP3) (3). IP3 then diffuses into the cytosol, where it triggers the release of calcium from endoplasmic reticulum (22). The calcium in turn stimulates release of ACTH from storage granules (21). In a previous study (6), we hypothesized that the increased pituitary responsiveness to AVP in the late-gestation sheep fetus was related to increased second messenger formation. Indeed, this appears to be the case. We reported that the formation of IPs (including IP3) after AVP stimulation increased with advancing gestational age in pituitary cells obtained from fetal sheep. Interestingly, in the same study (6), we also found that hypothalamo-pituitary disconnection (HPD) performed at 120 dGA significantly impaired this ontogenic development. This procedure also prevented the developmental increase in cortisol from occurring (6). This finding led us to further hypothesize that cortisol may play a role in mediating the increased second messenger response apparent with advancing gestation. We tested this hypothesis in the present study by infusing late-gestation sheep fetuses with cortisol after HPD and subsequently assessing pituitary cell responsiveness (IP formation and ACTH secretion) to AVP.
MATERIALS AND METHODS
Animals.
Time-dated, mixed-breed pregnant sheep were obtained from local suppliers and maintained in straw-lined pens with ad libitum access to food and water. Animals were housed for a minimum of 4–5 days before surgery. All procedures were approved by the Wake Forest University Animal Care and Use Committee.
Hypothalamo-pituitary disconnection.
HPD of fetal sheep was performed at ∼120 dGA with a modified (25), previously described technique (1). Control fetuses were sham operated, where the only difference in treatment was that the pituitary stalk was not severed. Fetal jugular and carotid catheters were inserted for later cortisol infusion and blood sampling, respectively. Maternal femoral venous catheters were also inserted, and antibiotics (gentamicin, Abbott Laboratories, North Chicago, IL; ampicillin, American Pharmaceutical Partners, Schaumburg, IL) and an anti-inflammatory (ketoprofen) were administered for 2 days after surgery. Ewes were returned to pens immediately after surgery. All catheters were flushed with saline and refilled with heparin every second day.
Cortisol infusion.
At ∼133 dGA, ewes carrying HPD fetuses were transferred to metabolic carts, where they remained for the duration of the experiment. Intravenous cortisol (hydrocortisone, Sigma, St. Louis, MO) infusion was initiated in half of the HPD fetuses (HPD+C, n = 6) at 135 dGA (the other half were infused with isotonic saline, n = 6). Sham-operated fetuses were also infused with isotonic saline (n = 5). The cortisol dose of 1.2 μg·kg−1·min−1 (administered at a rate of 0.046 ml/min) was given to raise fetal plasma cortisol concentrations to levels comparable with those normally evident close to term. The dose was calculated by assuming that the average 135-dGA fetus weighs 3.5 kg. The cortisol stock solution was prepared as 1 mg/ml in 50% ethanol. This solution was diluted in isotonic saline to obtain the appropriate concentration for infusion. The infusion was continuous for 72 h (fresh solution was prepared daily). Ethanol was hence infused at a rate of 0.03 mg·kg−1·min−1 (our previous unpublished studies indicate that the late-gestation fetus is able to completely metabolize such a low dose of ethanol before plasma accumulation). Fetal blood samples were obtained at 0, 6 (except from sham-operated fetuses), 24, 48, and 72 h to measure plasma cortisol concentrations and assess fetal health (as indicated by blood gases and pH). Animals were killed by pentobarbital sodium overdose at the completion of infusion.
Pituitary cell culture.
Fetal anterior pituitaries were immediately dissected and placed in ice-cold HEPES dissociation buffer (HDB). Completeness of HPD was visually confirmed at this time. Pituitaries were then cut into small pieces with sterile scalpel blades, washed several times with HDB, and placed in 0.04% collagenase II (Worthington Biochemical, Lakewood, NJ). Tissue was dissociated over ∼2 h by gentle rocking at 37°C. DNase (150 U, Sigma) was added after 1 h. Five milliliters of DMEM-F-12 medium containing 10% fetal calf serum (complete medium) was then added to the solution. This was followed by centrifugation. Cells were washed with complete medium several times and then plated on 48-well plates at densities of 0.6 × 105 or 2.0 × 105 cells/well (volume was 500 μl).
After 24 h of culture, 0.5 μl of [3H]-myo-inositol (Perkin Elmer, Boston MA) was added to each well in which cells were plated at a density of 0.6 × 105 cells/well. At 46 h, complete medium was removed and replaced with DMEM-F-12 containing 0.1% Polypep (Sigma) and 10 mM LiCl (incomplete medium). LiCl inhibits the recycling of IP to inositol. At 48 h, half of the cells were stimulated for 30 min with incomplete medium containing 100 nM AVP (Sigma). Medium in the remaining wells (containing control cells) was replaced with incomplete medium alone. Medium was then removed, and the stimulation was halted by addition of 0.5 ml of ice-cold stop solution (1 M KOH, 18 mM Na borate, 3.8 mM EDTA, 7.6 mM NaOH) and neutralized with 0.5 ml of 7.5% HCl. The resultant solution, containing lysed cells, was stored at −80°C for later IP analysis.
Cells plated at 2.0 × 105 cells/well were used to assess AVP-induced ACTH secretion. These cells were cultured for 46 h in complete medium and then incubated for 2 h with DMEM-F-12 containing 0.1% Polypep (incomplete). Subsequently, cells were treated for 2 h with incomplete medium alone (control) or incomplete medium containing 100 nM AVP. Medium was then immediately stored at −80°C for later ACTH analysis.
Anion exchange chromatography and ACTH and cortisol measurement.
IP3 and total IPs were determined as previously described (4, 6).
Cell medium ACTH and fetal plasma cortisol concentrations were measured with commercial RIA kits (DSL, Webster, TX), in which minimum detection limits were 3.5 pg/ml (ACTH) and 0.6 ng/ml (cortisol). Coefficients of variation were 6.0% intra-assay and 7.0% interassay for ACTH and 4.2% intra-assay and 7.0% interassay for cortisol.
Statistical analysis.
All data were compared by one- or two-way analysis of variance and (when appropriate) Dunnett's T3 or Dunn's post hoc test. Dunnett's test is used for comparing groups with unequal variances. All analyses were performed with SPSS statistical software (Chicago, IL). Data are presented as means ± SE, and differences were considered to be significant when P < 0.05.
RESULTS
Fetal blood gases and pH and body weight.
There were no between-group differences with regard to blood gases or pH, nor did any of these parameters change with cortisol infusion. Values fell within the following ranges: pH 7.28–7.41, Po2 17–26 mmHg, Pco2 41–57 mmHg.
There were no between-group differences in fetal body weight as determined at study completion. Mean weights were 4.2 ± 0.4, 4.0 ± 0.4, and 4.1 ± 0.4 kg for the sham-operated, HPD, and HPD+C groups, respectively. The mean cortisol dose (taking into account postmortem fetal body weight) was 1.1 ± 0.1 μg·kg−1·min−1.
Plasma cortisol concentrations.
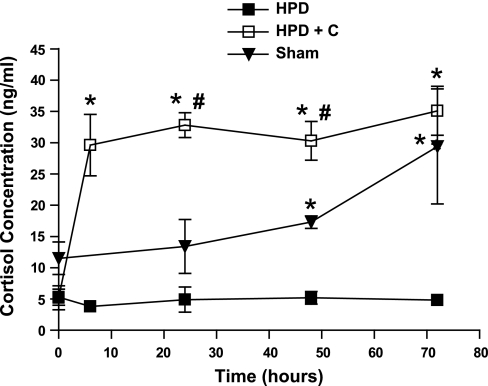
Plasma cortisol concentrations increased sharply within the first 6 h of infusion in HPD+C fetuses (Fig. 1, P <0.05). Cortisol concentrations in these fetuses did not change significantly over the ensuing 66 h. In sham-operated fetuses, cortisol concentrations increased significantly between 0 h and 72 h (P <0.05). As expected, plasma cortisol concentrations did not change in saline-infused HPD fetuses (Fig. 1). Cortisol concentrations were significantly higher in HPD+C fetuses at 6, 24, 48, and 72 h and at 24 and 48 h than in HPD and sham-operated fetuses, respectively (P <0.05 for all). Cortisol concentrations were significantly higher in sham-operated compared with HPD fetuses at 48 and 72 h (P <0.05 for both).
Fig. 1.
Plasma cortisol concentrations in sham-operated and hypothalamo-pituitary disconnected (HPD) fetuses infused with saline (n = 5 and 6, respectively) and HPD fetuses infused with cortisol (HPD+C, n = 6) for 72 h. *Significant difference vs. HPD group for a given time (P < 0.05); #significant difference between HPD+C and sham-operated groups for a given time (P < 0.05). Plasma cortisol concentrations increased significantly between 0 and 6 h in HPD+C fetuses (P < 0.05). Plasma cortisol concentrations were significantly higher in sham-operated fetuses at 72 h compared with 0 h (P > 0.05).
Inositol phosphate formation.
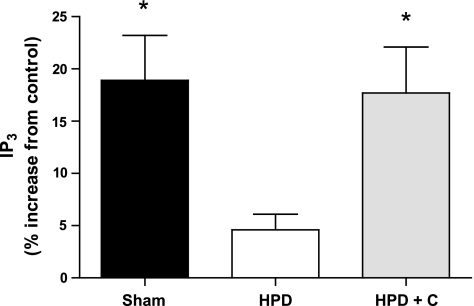
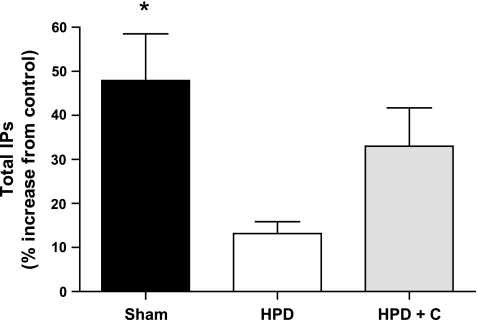
The results pertaining to IP3 and total IP formation following AVP stimulation for each group are presented in Figs. 2 and 3. IP3 and total IP levels were significantly higher in cells from sham-operated compared with HPD fetuses (P = 0.01 and 0.007, respectively). Similarly, IP3 levels were significantly higher in cells from HPD+C compared with HPD pituitaries (P = 0.023). There was a trend for total IP formation to be higher cells in from HPD+C vs. HPD fetuses (P = 0.08). There were no differences between the sham-operated and HPD+C groups for either of these variables.
Fig. 2.
Vasopressin-induced inositol 1,4,5-trisphosphate (IP3) formation in pituitary cells isolated from sham-operated (n = 5), HPD (n = 6), and HPD+C (n = 6) fetuses. *Significant difference vs. HPD group (P < 0.05).
Fig. 3.
Vasopressin-induced total IP formation in pituitary cells isolated from sham-operated (n = 5), HPD (n = 6), and HPD+C (n = 6) fetuses. *Significant difference vs. HPD group (P < 0.05).
ACTH secretion.
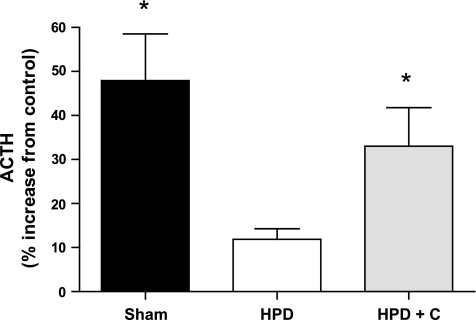
In keeping with the IP findings, ACTH secretion from pituitary cells following AVP stimulation was significantly higher in both the sham-operated and HPD+C groups compared with the HPD group (P = 0.037 and 0.047, respectively; Fig. 4). Again, there was no difference between the sham-operated and HPD+C groups.
Fig. 4.
Vasopressin-induced ACTH secretion from pituitary cells isolated from sham-operated (n = 5), HPD (n = 6), and HPD+C (n = 6) fetuses. *Significant difference vs. HPD group (P < 0.05).
DISCUSSION
In a previous study of fetal sheep (6), we demonstrated that heightened pituitary responsiveness to AVP in late gestation appears to be due at least in part to an increased capacity for signal transduction (IP3 formation). In the present study we demonstrate that cortisol is likely a key mediator of this change.
The late-gestation increase in fetal plasma cortisol levels is a pivotal developmental event. The mechanisms underlying this surge are incompletely understood; however, changes are apparent at several levels of the HPA axis. Concentrations of bioactive ACTH in the plasma increase in concert with cortisol levels (7). In the adrenal, ACTH receptor expression is increased and steroidogenic capacity heightened. As previously noted, there is also compelling evidence suggesting that the fetal sheep pituitary becomes increasingly responsive to AVP with advancing gestation (9, 16), while responsiveness to corticotropin-releasing hormone concurrently declines. This occurs despite the fact that pituitary AVP receptor expression actually decreases in late gestation (25). We suggest that the change in AVP sensitivity is essential for continued ACTH secretion. Further to this, we previously found (6) that the amount of second message formed on AVP binding to its receptor increases toward parturition. This led us to conclude that the heightened capacity for signal transduction would appear to be a key factor underlying the increased pituitary responsiveness to AVP.
In this study we found that pituitary cells isolated from HPD fetuses that were infused with cortisol in late gestation exhibited responsiveness to AVP in terms of IP3 formation and ACTH secretion that was equivalent to that evident in cells from sham-operated control fetuses. Conversely, cells obtained from HPD fetuses that were not infused with cortisol exhibited markedly decreased responsiveness. Hence the cortisol infusion restored the capacity of pituitary cells to fully respond to AVP stimulation. These findings indicate that cortisol is sufficient to drive the increased AVP signal transduction capacity, which in turn underlies the heightened pituitary responsiveness to AVP in the late-gestation sheep fetus.
We performed HPD of fetuses at ∼120 dGA in this study. At this stage in development, HPA axis activity is relatively low compared with later in gestation. Cortisol infusion was initiated at ∼135 dGA and was continued for 72 h. At this time, bioactive ACTH and cortisol concentrations are increasing in non-HPD fetuses. The aim of this infusion was to elevate cortisol concentrations in HPD fetuses to levels comparable to those within the normal physiological range for this period of gestation. We were successful in achieving this, as indicated by the fact that the concentrations attained after infusion (which were stable at ∼30 ng/ml after the first 6 h) were comparable to concentrations in sham-operated fetuses. These levels are also in accordance with previous reports concerning similarly aged non-HPD fetuses (6, 25). We acknowledge that the rapid increase in cortisol concentrations after the initiation of infusion is not overly physiological in terms of mimicking the cortisol surge. However, given that fetal plasma cortisol concentrations were stable for 66 h thereafter, we are confident that any effect of the initial rapid increase on pituitary function would have been negligible by the time the fetuses were posted.
Several previously published studies support our finding that cortisol plays a role in mediating pituitary cell responsiveness to AVP. For instance, Rabadan-Diehl and Aguilera (18) reported that AVP-induced IP formation was increased in whole pituitaries isolated from dexamethasone-treated rats. In the same study, these researchers also found that IP formation was increased in pituitary cells cultured in medium containing dexamethasone or corticosterone after AVP stimulation (18). Other studies performed in fetal sheep in which the HPA axis has been manipulated also highlight the purported link between plasma cortisol levels and pituitary AVP responsiveness. Specifically, pituitary cells obtained from late-gestation bilateral adrenalectomized sheep fetuses, in which the cortisol surge does not occur, secrete less ACTH after AVP stimulation than cells from normal fetuses in which the cortisol surge is unimpaired (9). It should be noted that Bilezikjian et al. (5) found that glucocorticoid exposure did not affect AVP-induced IP3 formation in adult rat pituitary cells. The relatively short (compared with our and Rabadan-Diehl and Aguilera's studies) dexamethasone exposure time may explain this disparity.
We did not investigate precisely how cortisol might be influencing fetal pituitary AVP signal transduction in the present study. However, findings from a number of reports suggest that increased G protein expression may play a role to this end. As noted above, the AVP signal is initiated when the ligand binds to its G protein-coupled receptor. This leads to activation of phospholipase C, followed by IP3 formation, subsequent intracellular calcium release, and finally ACTH secretion. Hence there are several steps in this cascade that could potentially be regulated by glucocorticoids. In a study using adult rat pituitary cells, Rabadan-Diehl and Aguilera (18) examined each step in the AVP signal transduction cascade and found that dexamethasone pretreatment resulted in upregulated G protein expression (type Gq). Neither phospholipase C nor calcium appeared to be involved (18). Other researchers have also variously reported that glucocorticoids can modulate G protein expression (8, 11, 20). It remains to be determined whether cortisol exerts a stimulatory effect on AVP receptor-coupled G protein expression in the late-gestation sheep fetus; however, the aforementioned findings suggest this is likely. We hope to examine this possibility in future studies.
In the adult sheep, cortisol exerts an inhibitory effect on ACTH secretion. In the late-gestation sheep fetus, however, concentrations of both bioactive ACTH and cortisol increase in concert (7, 13, 14). These are classic negative and positive feedback loops, respectively. It is unclear why there is such a distinct difference between adult and fetus in this regard. We can only conclude that there are other unique factors at play in the fetus, specific to this particular period of gestation, that influence HPA axis function. Such factors could be hormonal and could be of maternal, fetal, or placental origin. Presumably parturition breaks this positive feedback phenomenon apparent in the fetus, and “normal” negative feedback ensues.
In summary, we have demonstrated in this study that pituitary cells isolated from HPD sheep fetuses infused with cortisol exhibit increased responsiveness to AVP in terms of IP3 formation and ACTH secretion compared with cells isolated from control and noninfused HPD fetuses. These findings support the hypothesis that cortisol is an important modulator of pituitary responsiveness to AVP in the late-gestation sheep fetus.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grant HD-11210. L. C. Carey was supported by an RJR-Leon Golberg fellowship in Pharmacology and Toxicology.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Antolovich GC, Clarke IJ, McMillen IC, Perry RA, Robinson PM, Silver M, Young R. Hypothalamo-pituitary disconnection in the fetal sheep. Neuroendocrinology 51: 1–9, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Antolovich GC, Perry RA, Trahair JF, Silver M, Robinson PM. The development of corticotrophs in the fetal sheep pars distalis: the effect of adrenalectomy or cortisol infusion. Endocrinology 124: 1333–1339, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ Inositol trisphosphate and calcium signaling. Ann NY Acad Sci 766: 31–43, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J 206: 587–595, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilezikjian LM, Blount AL, Vale WW. The cellular actions of vasopressin on corticotrophs of the anterior pituitary: resistance to glucocorticoid action. Mol Endocrinol 1: 451–458, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Carey LC, Tatter SB, Rose JC. Ontogeny and effects of hypothalamic pituitary disconnection on formation of inositol trisphosphate in fetal sheep pituitary cells. Endocrinology 148: 1440–1444, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev 10: 182–204, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Chang FH, Bourne HR. Dexamethasone increases adenylyl cyclase activity and expression of the alpha-subunit of Gs in GH3 cells. Endocrinology 121: 1711–1715, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Fora MA, Butler TG, Rose JC, Schwartz J. Adrenocorticotropin secretion by fetal sheep anterior and intermediate lobe pituitary cells in vitro: effects of gestation and adrenalectomy. Endocrinology 137: 3394–3400, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Glickman JA, Challis JR. The changing response pattern of sheep fetal adrenal cells throughout the course of gestation. Endocrinology 106: 1371–1376, 1980. [DOI] [PubMed] [Google Scholar]
- 11.Haigh RM, Jones CT, Milligan G. Glucocorticoids regulate the amount of G proteins in rat aorta. J Mol Endocrinol 5: 185–188, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Jones CT, Boddy K, Robinson JS, Ratcliffe JG. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol 72: 279–292, 1977. [DOI] [PubMed] [Google Scholar]
- 13.Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res 29: 111–159, 1973. [DOI] [PubMed] [Google Scholar]
- 14.Lolait SJ, O'Carroll AM, Mahan LC, Felder CC, Button DC, Young WS 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA 92: 6783–6787, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology 107: 155–159, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Perez FM, Schwartz J, Rose JC. Developmental changes in ovine corticotrophs in vitro. Endocrinology 138: 916–921, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Perry RA, Mulvogue HM, McMillen IC, Robinson PM. Immunohistochemical localization of ACTH in the adult and fetal sheep pituitary. J Dev Physiol 7: 397–404, 1985. [PubMed] [Google Scholar]
- 18.Rabadan-Diehl C, Aguilera G. Glucocorticoids increase vasopressin V1b receptor coupling to phospholipase C. Endocrinology 139: 3220–3226, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Rose JC, Meis PJ, Urban RR, Greiss FC Jr. In vivo evidence for increased adrenal sensitivity to adrenocorticotropin-(1–24) in the lamb fetus late in gestation. Endocrinology 111: 80–85, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Saito N, Guitart X, Hayward M, Tallman JF, Duman RS, Nestler EJ. Corticosterone differentially regulates the expression of Gsalpha and Gialpha messenger RNA and protein in rat cerebral cortex. Proc Natl Acad Sci USA 86: 3906–3910, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shears SB Metabolism of the inositol phosphates produced upon receptor activation. Biochem J 260: 313–324, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem 269: 27088–27092, 1994. [PubMed] [Google Scholar]
- 24.Wintour EM, Brown EH, Denton DA, Hardy KJ, McDougall JG, Oddie CJ, Whipp GT. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinol (Copenh) 79: 301–316, 1975. [DOI] [PubMed] [Google Scholar]
- 25.Young SF, Tatter SB, Valego NK, Figueroa JP, Thompson J, Rose JC. The role of hypothalamic input on corticotroph maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 284: R1621–R1630, 2003. [DOI] [PubMed] [Google Scholar]