Abstract
Protein kinase C-θ (PKCθ) is critical for TCR initiated signaling in mature T cells, but initial reports found no requirement for PKCθ in thymocyte development. Thymocytes and peripheral T cells utilize many of the same signaling components, and given the significant role of PKCθ in peripheral T cells, it was surprising that it was not involved at all in TCR signaling in thymocytes. We decided to reevaluate the role of PKCθ in thymocyte development using the well-characterized class-II restricted n3.L2 TCR transgenic TCR model. Analysis of n3.L2 PKCθ-/- mice revealed a defect in thymocyte positive selection, resulting in a 50% reduction in the generation of n3.L2 CD4 single positive thymocytes and n3.L2 CD4 mature T cells. Competition between n3.L2 WT and n3.L2 PKCθ-/- thymocytes in bone marrow chimeras revealed a more dramatic defect, with a >80% reduction in generation of n3.L2 CD4 single positive thymocytes derived from PKCθ-/- mice. Inefficient positive selection of n3.L2 PKCθ-/- CD4 single positive cells resulted from “weaker” signaling through the TCR and correlated with diminished ERK activation. The defect in positive selection was not complete in the PKCθ-/- mice, most likely accounted for by compensation by other PKC isoforms, not evident in peripheral cells. Similar decreased positive selection of both CD4 and CD8 single positive thymocytes was also seen in non-transgenic PKCθ-/- mice. These findings now place PKCθ as a key signaling molecule in the positive selection of thymocytes as well as in the activation of mature T cells.
Keywords: thymocyte, thymocyte development, protein kinases/phosphatases, signal transduction
Introduction
PKCθ is a serine/threonine kinase important for TCR signaling and mature T cell function (1-4). PKCθ is localized to the immunological synapse in mature T cells (5, 6) and has been linked to multiple pathways downstream of TCR signaling, including but not limited to calcium activation and activation of ERK and I-κ kinase. (7-13). The downstream effects of these multiple signaling roles for PKCθ are revealed by the multiple activation defects of PKCθ-/- T cells. Mature T cells from PKCθ-/- mice do not produce IL-2 and do not proliferate in response to antigen stimulation in vitro and are deficient in switching to the Th2 phenotype in vivo (7, 9, 14, 15). Furthermore, development of NK1.1 and CD25+FoxP3+ regulatory T (Treg) cells was diminished in PKCθ-/- mice (16).
Thymic development of α/β-TCR expressing T cells in PKCθ-/- mice has not yet been tested using a class-II restricted transgenic TCR. Given the central role for PKCθ in mature T cell signaling, and given that PKCθ is upregulated during transition from the double negative to the double positive stage (17), it was not unreasonable to expect that PKCθ would participate in thymocyte development. However, initial examination of non-transgenic PKCθ-/- mice found no obvious differences in α/β T cell development (7, 9), and positive and negative selection appeared unaffected using the class I-restricted transgenic TCR H-Y in female and male PKCθ-/- mice, respectively (7). However, in non-transgenic mice, TCR repertoire shifts can obscure moderate differences in TCR signaling strength. We hypothesized that by using a class-II restricted transgenic TCR to fix the TCR repertoire, a role for PKCθ in thymocyte TCR signaling would be revealed.
The CD4-restricted n3.L2 transgenic TCR has been previously described and is specific for Hb(64-76)/I-Ek (18-20). n3.L2+ thymocytes are positively selected in H-2k mice (18, 19). Useful reagents for this TCR have been established, including a clonotypic antibody (Cab) and purified, refolded I-Ek with antigenic peptide (pI-Ek), both of which can be used for in vitro stimulation. In addition, a panel of altered peptide ligands (APLs) has been established for the n3.L2 TCR, which enable an anaylsis of TCR signaling strength. Thus, these reagents make the n3.L2 system ideal for examining thymic development of CD4+ T cells. To address whether CD4+ T cell development was altered in PKCθ-/- mice, n3.L2 TCR transgenic, PKCθ-/-, H-2k mice were generated.
Here we report that generation of CD4 single positive (CD4SP) n3.L2 thymocytes was significantly reduced in n3.L2 PKCθ-/- mice as compared to n3.L2 WT mice. This developmental defect of CD4SP thymocytes in n3.L2 PKCθ-/- mice resulted from diminished positive selection and correlated with reduced TCR-stimulated phosphorylation of ERK kinase. Once we had established the defect in positive selection in the n3.L2 transgenic system, we evaluated non-transgenic PKCθ-/- mice and confirmed reduced positive selection of both CD4SP and CD8SP thymocytes. We hypothesized that the reduction in generation of CD4SP thymocytes might explain the previously reported deficit of CD25+, FoxP3+ Treg cells in PKCθ-/- mice (16). However, the failure of CD25+FoxP3+ cell development was apparent even when controlling for the reduced development of CD4SP thymocytes. Thus, here we provide evidence for a previously unrecognized role for PKCθ in TCR signaling in thymocytes.
Materials and Methods
Mice
PKCθ-/- mice back-crossed onto a B6 background for at least 15 generations were the kind gift of Dan Littman (Skirball Institute of Biomolecular Medicine, New York University School of Medicine, New York, NY) (14). n3.L2 transgenic mice have been previously described and are on the background B6.AKR-H-2k (B6.K) (19, 20). Breeding the PKCθ-/- mice to the n3.L2 B6.K mice generated n3.L2 PKCθ-/- mice on a B6 background that is H-2k and would thus positively select the n3.L2 TCR. Non-TCR transgenic mice were generated by breeding the PKCθ-/- mice to non-transgenic B6.K mice. Generation of a pure population of n3.L2+ CD4/CD8 double positive (DP) thymocytes was accomplished by crossing the n3.L2 transgenic mouse to the non-selecting H-2b, RAG-1-deficient strain (C57Bl/6J-RAG1tm1 Mom; The Jackson Laboratory, Bar Harbor, ME) and has been previously described (19). For biochemistry experiments n3.L2 PKCθ-/- RAG1-/- H-2b mice were generated by breeding n3.L2 PKCθ-/- B6.K mice to n3.L2 RAG1-/- H-2b mice. The phenotypes of mice were determined either by PCR for PKCθ, RAG1, and n3.L2 or by flow cytometry for H-2k or H-2b. All mice were housed in specific pathogen free environments at the Washington University and maintained according to approved practices. All experiments were performed according to protocols approved by the Washington University Animal Studies Committee. Mice were used between the ages of 6 and 12 weeks.
Flow cytometry
Single cell suspensions of thymocytes, splenocytes, and lymph node (axillary and inguinal) cells were incubated on ice with the indicated antibodies in FACS buffer (0.5% BSA and 0.1% sodium azide in PBS). The clonotypic antibody Cab recognizes the n3.L2 TCR and has been previously described (18). Fluorescently conjugated antibodies purchased from commercial sources were FITC-anti-CD4, allophycocyanin (APC)-anti-CD4, APC/Cy7-anti-CD4, PE-anti-CD8, PE-anti-CD69, PE-anti-CD24, FITC-anti-CD25, PE-anti-CD25, PE-anti-CD44, PE-anti-γ/δ TCR, PE-anti-H-2b (Biolegend, San Diego, CA); PE-anti-CD4, PerCP-anti-CD8, PE-anti-Bcl-2, PE-anti-NK1.1, FITC-anti-H-2k, PE/Cy5-strepavidin (BD Biosciences, San Jose, CA), FITC-anti-CD8, APC-anti-CD8, PE-anti-FoxP3, PE-anti-IL-7Rα, APC/AlexaFluor750-anti-CD8, and PE/Cy7-anti-CD45.1 (eBiosciences, San Diego, CA). Intracellular staining for FoxP3 and Bcl-2 was performed according to the manufacturer's instructions. Stained cells were analyzed on a FACScan, FACSCalibur, or FACSCanto and data analyzed using either CellQuest (BD Biosciences) or FlowJo (Tree Star Inc., Ashland, OR) software. Statistical significance was determined using unpaired, two-tailed Student's t test with p < 0.05 being significant (GraphPad Prism 4; GraphPad Software, Inc., San Diego, CA). As data in Figure 6 were collected in separate experiments, each using a single pair of age- and sex-matched mice, paired, two-tailed Student's t test was used for analysis.
Figure 6.
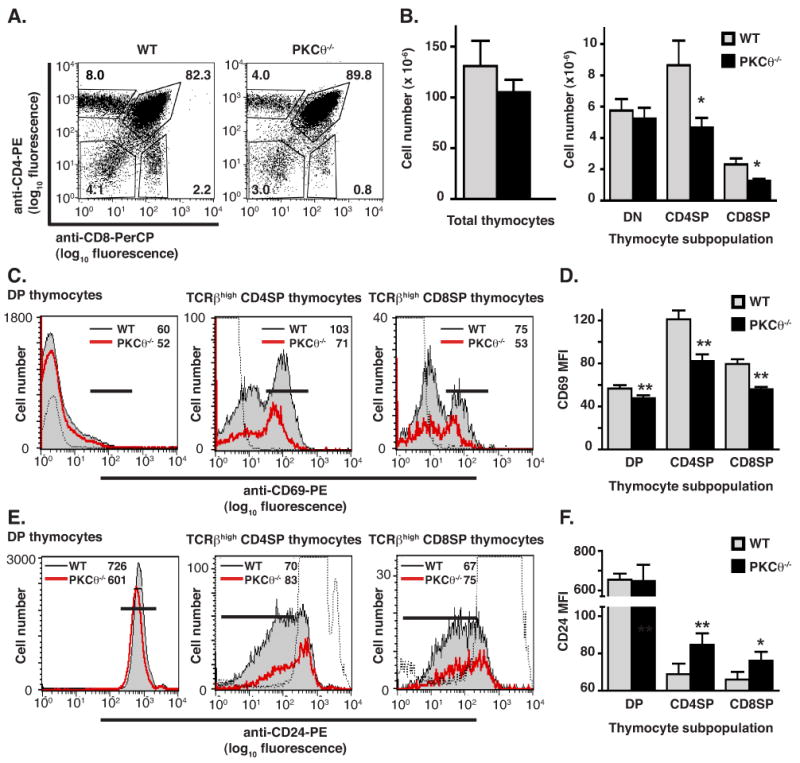
Positive selection is reduced in non-transgenic PKCθ-/- mice. (A) Thymocytes isolated from either PKCθ-/- or B6.K WT mice were stained with anti-CD4-PE and anti-CD8-PerCP and analyzed by flow cytometry. Percentages of DN, DP, CD4SP and CD8SP populations are given. Data are representative of 9 pairs of age-matched mice. (B) Total number of thymocytes isolated from non-transgenic WT (grey bars) and PKCθ-/- (filled bars) mice and numbers of cells that are DN, CD4SP or CD8SP are shown. Data shown are mean ± SEM (n = 10 for WT and n = 9 for PKCθ-/- mice). Reduction of number of cells that are CD4SP or CD8SP was statistically significant (*p < 0.05). (C) Reduced CD69 expression on CD4SP and CD8SP thymocytes isolated from non-transgenic PKCθ-/- mice. Thymocytes isolated from either WT (filled grey histogram) or PKCθ-/- (red line) mice were stained for TCRβ, CD69, CD4 and CD8. CD69 expression of cells gated on all live thymocytes and as indicated is shown. Isotype control is depicted as dotted line. The MFI of CD69 expression for WT and PKCθ-/- mice is given in the upper right hand corner of each histogram. (D) The average MFI of CD69 expression on DP, CD4SP and CD8SP thymocytes isolated from PKCθ-/- mice is reduced compared to the average MFI of CD69 expression on DP, CD4SP and CD8SP thymocytes isolated from WT mice. Data is presented as mean ± SEM (n = 9 pairs of mice for DP and CD4SP cells; n = 4 pairs of mice for CD8SP cells). Reduction of MFI expression is statistically significant with **p < 0.01, using paired Student's t test. (E) Increased CD24 expression on CD4SP and CD8SP thymocytes isolated from non-transgenic PKCθ-/- mice. Thymocytes isolated from either WT (filled grey histogram) or PKCθ-/- (red line) mice were stained for TCRβ, CD24, CD8 and CD4. CD24 expression of cells gated on all live thymocytes and as indicated is shown. Isotype control is depicted as dotted line. The MFI of CD24 expression for WT and PKCθ-/- mice is given in the upper right hand corner of each histogram. (F) The average MFI of CD24 expression on CD4SP and CD8SP thymocytes isolated from PKCθ-/- mice is increased compared to the average MFI of CD24 expression on CD4SP and CD8SP thymocytes isolated from WT mice. Data is presented as mean ± SEM (n = 4 pairs of mice). Increased MFI expression is statistically significant with *p < 0.05 and **p < 0.01, using paired Student's t test.
In vitro stimulation assays
CD69 upregulation
Plates (Corning Costar, Corning, NY) for stimulation were prepared by coating with avidin (10 μg/ml) in PBS, followed by incubation with biotinylated refolded I-Ek with antigenic peptide (pI-Ek; 14 μg/ml) or biotinylated Cab. Biotinylated pI-Ek was refolded as described (21). Unconjugated anti-CD3 (145-2C11; BD Biosciences) and Cab (10 μg/ml) was used in some experiments; no difference between using biotinylated Cab and unconjugated Cab was seen (data not shown).
Plates for stimulation with peptide-pulsed APCs were prepared by plating 5 × 105 DCEK Hi7 cells per well of a 24-well plate and incubating overnight with the indicated concentrations of stimulatory peptides. The fibroblast cell line DCEK Hi7 has been previously described (22). DCEK Hi7 cells were maintained in selection medium containing RPMI, 10% bovine growth serum, 2 mM glutamine (GlutaMAX, Invitrogen Life Technologies, Carlsbad, CA), 50 μg/ml gentamicin, 50 μM 2-mercaptoethanol, 0.5 mg/ml hygromycin and 0.5 mg/ml G418. Peptides were synthesized, purified and analyzed as previously described (18). The peptide sequence of the antigenic peptide, Hb(64-76), is as follows: GKKVITAFNEGLK. A weak agonist peptide was generated by substituting a threonine for the asparagine at position 72, such that its sequence is: GKKVITAFTEGLK. An antagonist peptide was generated by substituting an isoleucine for the asparagine at position 72, such that its sequence is: GKKVITAFIEGLK.
Plates were washed three times prior to addition of thymocytes. Thymocytes were incubated in I10 (IMDM, 10% fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM glutamine, 50 μg/ml gentamycin, 100 μM NEAA, 1 mM sodium pyruvate, 10 mM Hepes) overnight in plates. Thymocytes were washed and stained as indicated. Thymocytes were gated for n3.L2+ DP thymocytes and percent of cells positive for CD69 determined using unstimulated cells as a negative control.
Cell death assay
Thymocytes were incubated overnight in I10 on plates coated with the indicated concentrations of anti-CD3 (145-2C11) and anti-CD28 (37.51; BD Biosciences Pharmingen). Cells were harvested and stained with PerCP-anti-CD4, PE-anti-CD8, and Annexin V, Alexa Fluor 488 conjugate (Invitrogen-Molecular Probes). Binding buffer for Annexin V was 10 mM Hepes, 140 mM sodium chloride and 2.5 mM calcium chloride, as recommended by the manufacturer. Data were acquired by flow cytometry and analyzed.
Proliferation and IL-2 production
Cells isolated from spleens and lymph nodes were purified using CD4+-MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturers' instructions, except that the column buffer used was 10% bovine growth serum in PBS. Cell purity was consistently >90% as assessed by cytometry (data not shown). Cells were stained for CD4 and for expression of the n3.L2 TCR and populations normalized such that an equivalent number of CD4+, n3.L2+ T cells were added to each well. APCs used for proliferation and IL-2 production were irradiated splenocytes (2000 rads) isolated from B6.K mice.
For proliferation assays, 3-5 × 104 CD4+, n3.L2+ T cells were added to each well of a 96-well plate along with 5 × 105 APCs pulsed with the indicated concentrations of antigenic Hb(64-76) peptide. Cells were incubated in I10 for a total of 72 hours, to which 0.4 μCi/well 3H-thymidine was added for the last 24 hours.
For IL-2 production, 5 × 105 CD4+ T cells were added to each well of a 24-well plate along with 2.5 × 106 APCs pulsed with the indicated concentration of antigenic Hb(64-76) peptide. Cells were incubated in I10 for approximately 36 hours, after which time cells were removed from plates, restimulated with PMA and ionomycin in the presence of brefeldin, and stained for CD4 and n3.L2 on ice and fixed with 2% paraformaldehyde. Cells were then permeabilized with 0.5% saponin and stained for IL-2 using PE-anti-IL-2 (Biolegend). Data was acquired by flow cytometry and analyzed (23). Cells were gated on CD4+, n3.L2+ T cells and percent of cells positive for IL-2 determined using stained, unstimulated cells as a negative control.
Generation of bone marrow chimeras
B6.K or B6.K/CD45.1 mice were lethally irradiated (1000 rad) and retro-orbitally injected with 100 μL HBSS containing unfractionated bone marrow cells from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 RAG1-/- H-2k mice mixed in a 1:1 ratio. The ratio of H-2Kb:H-2Kk bone marrow cells was confirmed by flow cytometric analysis. Thymus and lymph nodes were harvested from recipient mice 5-6 wks after transfer and analyzed by flow cytometry for expression of CD4, CD8, n3.L2 TCR, H-2Kk and H-2Kb. Statistical significance was determined using a paired, two-tailed, non-parametric test (Wilcoxon signed rank test), as data were not normally distributed.
Biochemical assays
Immunoblots
Western blot assays were performed essentially as described (24). Thymocytes were stimulated on plates coated with 10 μg/ml anti-CD3 (145-2C11) and 10 μg/ml anti-CD28 (37.51) for the indicated periods of time. Stimulation was stopped by lysing cells on plates with lysis buffer (1% NP-40, 150 mM NaCl, 25 mM Hepes, 1 mM EDTA pH 7.4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM PMSF, and 1 mM sodium orthovanadate). Samples were incubated on ice for 20 minutes, and particulate matter removed by centrifugation at 21,000g for 10 min at 4°C. Supernatants were mixed with 2X SDS Laemmli sample buffer (Sigma). All samples were then boiled and separated by SDS-PAGE (12% acrylamide; Protogel, National Diagnostics, Atlanta, GA). Proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), blocked with 1:1 PBS:blocking buffer (LI-COR, Lincoln, NE), and then probed with the indicated primary antibodies. Antibodies used for immunoblots included monoclonal anti-ERK (BD Biosciences Pharmingen) and rabbit polyclonal anti-phosphorylated ERK (Cell Signaling Technology, Danvers, MA). The anti-ERK and anti-phosphoERK antibodies recognize both the p42 and p44 MAP kinases. Secondary antibodies for immunoblots included IRDye 800 conjugated goat anti-mouse IgG (Rockland Immunochemcals, Gilbertsville, PA) and AlexaFluor680 conjugated goat anti-rabbit IgG (Invitrogen, Molecular Probes). Bound antibodies were detected with the Odyssey infrared imaging system (LI-COR) according to the manufacturer's instructions. Integrated intensity of each band was determined using Odyssey software (LI-COR).
Calcium imaging
Calcium imaging assays were performed as previously described (23). In brief, thymocytes were loaded with 1 μM fura 2-AM (Invitrogen, Molecular Probes) in Ringers imaging solution (150 mM NaCl, 10 mM glucose, 5 mM HEPES, 5 mM KCl, 1 mM MgCl2, and 2 mM CaCl2) and washed immediately prior to use. Fura-2 loaded thymocytes (2-3 × 106/sample) were added to 8-chambered coverglass slides (Lab-Tek, Nalge Nunc International, Rochester, NY) that had been coated with peptide-pulsed DCEK Hi7 cells. Calcium imaging was performed on a Zeiss axiovert 200M microscope equipped with a xenon arc lamp. Fura-2 loaded thymocytes were excited using 340 and 380 excitation filters (71000a set; Chroma Technology, Rockingham, VT) and a polychroic mirror (73100bs; Chroma Technology). Fluorescence was passed through a 510 ± 40 wide band emission filter (Chroma Technology) and captured with a Cascade 512B camera (Roper Scientific, Tucson, AZ). Ratio measurements of fluorescence emission (340/380) were recorded at 3-second intervals. Data are presented as the 340/380 ratio, which is proportional to the intracellular calcium concentration. Peak calcium level, mean calcium level, and degree of oscillatory behavior were calculated as previously described (23). Degree of oscillatory behavior was determined by calculating the standard deviation upon linear regression analysis of calcium levels assessed after the initial calcium spike. Standard deviations were calculated by determining the sy.x value, which is the standard deviation of the vertical distances of the data points from the regression line (GraphPad Prism) (23).
EMSA for NF-κB activation
EMSA to assess activation of NF-κB was performed as previously described (25). Thymocytes were stimulated in serum-free IMDM by incubating 30-40 × 106 cells with soluble, biotinylated anti-CD3 (145-2C11) and anti-CD4 (RM4-5; 10 μg/ml each) and cross-linking with avidin (10 μg/ml). Samples were incubated at 37°C for the indicated time periods. Stimulation with TNF (a kind gift of R. Schreiber) at 20 ng/ml was used as a positive control. Stimulation was stopped by the addition of an excess volume ice-cold IMDM. Twenty micrograms of nuclear extract was incubated with IRDye700-labeled DNA probe for NF-κB (Ig κ promoter; LI-COR) and IRDye800-labeled Y-box (Eα promoter; LI-COR) DNA probe for the constitutively active NF-Y complex. Protein-DNA complexes were resolved on a 4-15% gradient gel (Bio-Rad) and imaged with the Odyssey infrared imaging system (LI-COR). Integrated intensity of each band was determined using Odyssey software. The intensity of the NF-κB band was normalized to the intensity of the NF-Y band to control for loading of the gel.
Results
Reduction of mature CD4SP thymocytes in n3.L2 PKCθ-/- mice
To determine whether PKCθ was required for maturation of n3.L2+ T cells, thymocyte subpopulations were analyzed by flow cytometry (Fig. 1). The percentage and absolute number of CD4SP thymocytes was significantly decreased in n3.L2 PKCθ-/- mice, though the total numbers of thymocytes isolated were equivalent (Fig. 1A and 1B). The decrease in CD4SP thymocytes appeared to be offset by a slight, though not statistically significant, increase in CD4/CD8 double positive (DP) thymocytes (Fig. 1A and 1B). The percentage of TCR-high cells was reduced in n3.L2 PKCθ-/- mice (Fig. 1C), and the percentage of TCR-high cells that were CD4SP was diminished in n3.L2 PKCθ-/- mice (Fig. 1D). Finally, the absolute number of n3.L2+ CD4SP cells was reduced by ∼50% (Fig. 1E). The decrease in TCR-high cells appeared to be offset by an accumulation of TCR-intermediate cells in n3.L2 PKCθ-/- thymocytes, consistent with the slight increase in DP thymocytes (Fig. 1B, 1C, 1D).
Figure 1.
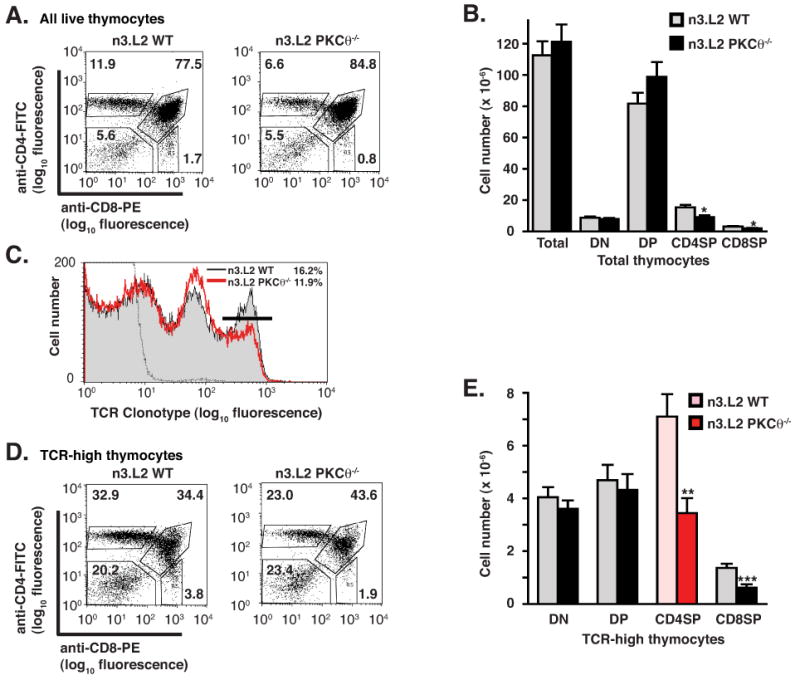
Reduction of mature n3.L2+ CD4SP thymocytes in n3.L2 PKCθ-/- mice. (A) Representative flow cytometric analysis of n3.L2 WT and n3.L2 PKCθ-/- thymocytes. Thymocytes from indicated mice were stained with anti-CD4-FITC, anti-CD8-PE and biotinylated-Cab/strepavidin-PerCP. Percentages of DN, DP, CD4SP and CD8SP thymocytes, as gated on all live thymocytes, are given. Representative of 15 pairs of mice. (B) Absolute thymocyte numbers in n3.L2 WT (grey bars) and n3.L2 PKCθ-/- mice (filled bars). All values given are mean ± SEM with n=15 for n3.L2 WT and n=16 for n3.L2 PKCθ-/- mice. The decrease in number of CD4SP and CD8SP thymocytes in n3.L2 PKCθ-/- mice as compared to n3.L2 WT mice is statistically significant with *p < 0.05. (C) n3.L2 TCR staining with the clonotypic mAb Cab of all live thymocytes in n3.L2 WT (shaded histogram) and n3.L2 PKCθ-/- (red line) thymocytes. Negative staining control shown as dotted line. Percentages of cells with high levels of TCR expression are indicated by the marker and given in the upper right corner. (D) Representative flow cytometric analysis of n3.L2 WT and n3.L2 PKCθ-/- thymocytes, performed as in (A) but gated on n3.L2 TCR-high thymocytes. (E) Absolute cell numbers of TCR-high thymocytes in n3.L2 WT and n3.L2 PKCθ-/- mice. All values given are mean ± SEM with n=15 for n3.L2 WT and n=16 for n3.L2 PKCθ-/- mice. There was a significant decrease in n3.L2+ CD4SP thymocytes in n3.L2 PKCθ-/- mice (red bar) as compared to n3.L2 WT mice (pink bar) when gating on 3L2+ thymocytes (**p < 0.01). There was also a statistically significant decrease in CD8 SP thymocytes (***p < 0.001).
The bulk CD4SP thymocyte population contains a few cells other than naive α/β TCR CD4SP thymocytes, such as natural killer T (NKT) cells, recirculating memory cells, Treg cells and γ/δ-TCR T cells. It was possible that the decrease in n3.L2 CD4SP thymocytes in n3.L2 PKCθ-/- mice reflected a decrease in another of these lineages. Excluding these non-naive CD4+ cells from analysis (26) revealed that 92% of n3.L2 CD4SP thymocytes were naive α/β T cells in n3.L2 WT mice and 94% of n3.L2 CD4SP thymocytes were naive α/β T cells in n3.L2 PKCθ-/- mice (data not shown). Though there was a slight decrease in the number of NKT, γ/δ, Treg and memory T cells in the n3.L2 CD4SP population in n3.L2 PKCθ-/- mice, this difference was not large enough to explain the 50% decrease in the number of n3.L2 CD4SP cells recovered.
The reduction in numbers of CD4SP thymocytes was reflected in a decreased percentage and number of peripheral n3.L2+ mature T cells (Fig. 2). The total number of T cells isolated from lymph nodes was reduced in n3.L2 PKCθ-/- mice compared to n3.L2 WT though total splenocyte numbers were equivalent (Fig. 2B, 2D). The number of n3.L2+ CD4+ mature T cells present in spleen and lymph nodes of n3.L2 PKCθ-/- mice was reduced approximately 50% as compared to n3.L2 WT mice, similar to the 50% reduction of n3.L2+ CD4SP thymocytes (Fig. 2B, 2D). Exclusion of NKT, γ/δ, Treg and memory T cells from the n3.L2 CD4+ T cell population when determining cell numbers did not alter the observed decrease in n3.L2 CD4+ T cells in n3.L2 PKCθ-/- mice (data not shown). Thus, production of n3.L2 CD4SP thymocytes and n3.L2 CD4+ mature T cells was reduced by half in the absence of PKCθ, demonstrating a significant role for PKCθ in the development of CD4+ T cells.
Figure 2.
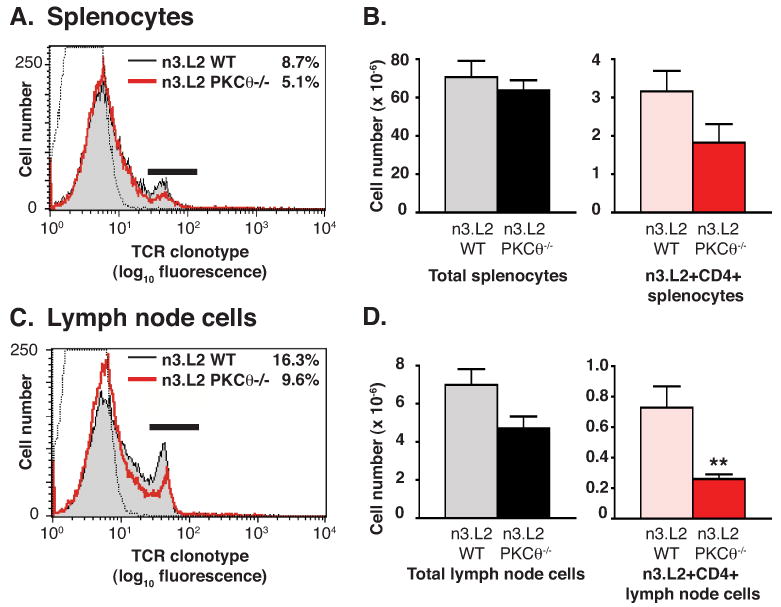
Reduced thymic maturation of n3.L2+ CD4SP cells resulted in reduced numbers of n3.L2+ CD4+ mature T cells in spleens (A-B) and lymph nodes (C-D) of n3.L2 PKCθ-/- mice as compared to n3.L2 WT mice. Splenocytes and lymph node cells from n3.L2 WT and n3.L2 PKCθ-/- mice were stained with Cab-FITC, anti-CD8-PerCP and anti-CD4-APC. (A) Reduced percentage of n3.L2+ splenocytes in n3.L2 PKCθ-/- (red line) vs n3.L2 WT (shaded histogram) mice. Negative staining control shown as dotted line. Percentage of n3.L2+ cells is given in upper right corner. (B) Total splenocyte cell numbers and number of n3.L2+ CD4+ splenocytes in n3.L2 WT (grey or pink) and n3.L2 PKCθ-/- (filled or red) mice. Values given are mean ± SEM with n=6. Difference between numbers of WT n3.L2+ CD4+ splenocytes and PKCθ-/- n3.L2+ CD4+ splenocytes is not statistically significant. (C) Reduced percentage of n3.L2+ lymph node cells in n3.L2 PKCθ-/- (red line) vs n3.L2 WT (shaded histogram) mice. Negative staining control shown as dotted line. Percentage of n3.L2+ cells is given in upper right corner. (D) Total cell number isolated from four lymph nodes and absolute number of n3.L2+ CD4+ lymph node cells in n3.L2 WT (grey or pink) and n3.L2 PKCθ-/- (red or filled) mice. Values given are mean ± SEM with n=6. Difference between n3.L2 WT Cab+ CD4+ and n3.L2 PKCθ-/- Cab+ CD4+ lymph node cells is statistically significant with **p<0.01.
Mature n3.L2 PKCθ-/- T cells deficient in proliferative response and IL-2 production
The developmental defect of thymocytes observed in n3.L2 PKCθ-/- mice varied from prior reports (7, 9). To determine whether mature T cells from n3.L2 PKCθ-/- mice exhibited activation defects similar to those previously demonstrated (7, 9), we assessed IL-2 production and proliferation following TCR stimulation. n3.L2 PKCθ-/- mature T cells did not produce IL-2 (Fig. 3A) or proliferate (Fig. 3B) in response to antigen stimulation as robustly as n3.L2 WT T cells. Thus, n3.L2 PKCθ-/- mature T cells that did develop exhibited the same activation defects that have been previously reported (7, 9).
Figure 3.
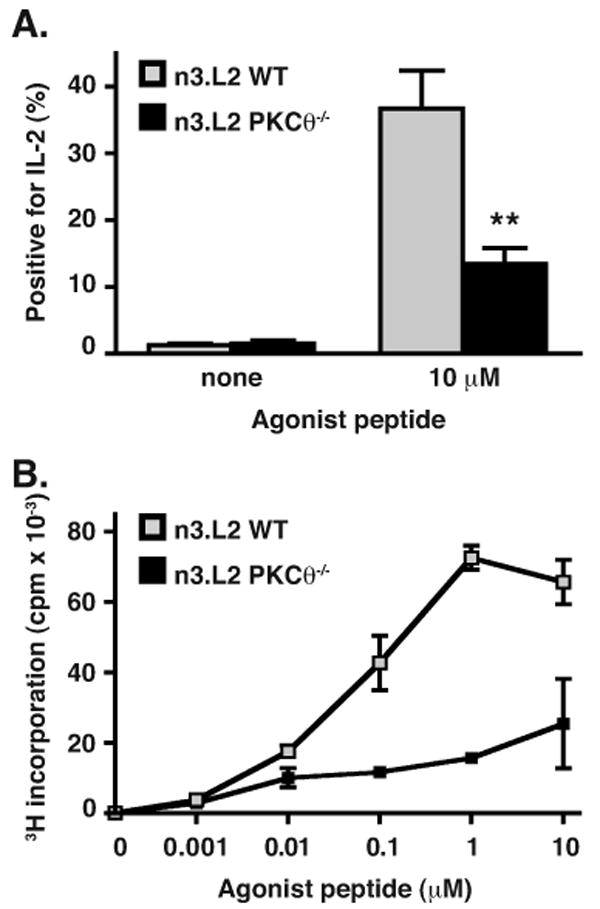
Proliferation and IL-2 production of n3.L2+ mature T cells is inhibited in n3.L2 PKCθ-/- cells as compared to n3.L2 WT cells. (A) CD4+ lymphocytes from n3.L2 WT (grey bars) or n3.L2 PKCθ-/- (filled bars) were incubated for 36 hours with irradiated B6.K splenocytes pulsed with 10 μM agonist peptide. IL-2 production was determined by intracellular cytokine staining and percentage of cells positive for IL-2 is shown. Difference was statistically significant with **p < 0.01, n = 4 independent experiments. (B) CD4+ lymphocytes from n3.L2 WT (grey symbols) or n3.L2 PKCθ-/- (filled symbols) mice, normalized for number of n3.L2+ CD4+ cells, were incubated for 72 hours with irradiated B6.K splenocytes pulsed with the indicated concentration of agonist peptide and proliferation determined by 3H-thymidine incorporation. Data given as the mean ± SD of triplicate samples within the same experiment. Data representative of three independent experiments.
Decrease in n3.L2 PKCθ-/- CD4SP thymocytes due to diminished positive selection
Given the slight increase in DP thymocytes and a significant decrease in CD4SP thymocytes in n3.L2 PKCθ-/- mice (Fig. 1), we hypothesized that DP thymocytes were not efficiently transitioning from the DP to the CD4SP stage due to inefficient positive selection. Positive selection is associated with changes in the expression of surface molecules, including an increase of CD69 and a decrease of CD24 (27, 28). CD69 expression was lower and CD24 expression was higher on both n3.L2+ PKCθ-/- DP and n3.L2+ PKCθ-/- CD4SP thymocytes, compared to n3.L2+ WT DP and n3.L2+ WT CD4SP thymocytes (Fig. 4). These differences in CD69 and CD24 expression indicate that positive selection is reduced in n3.L2 PKCθ-/- mice.
Figure 4.
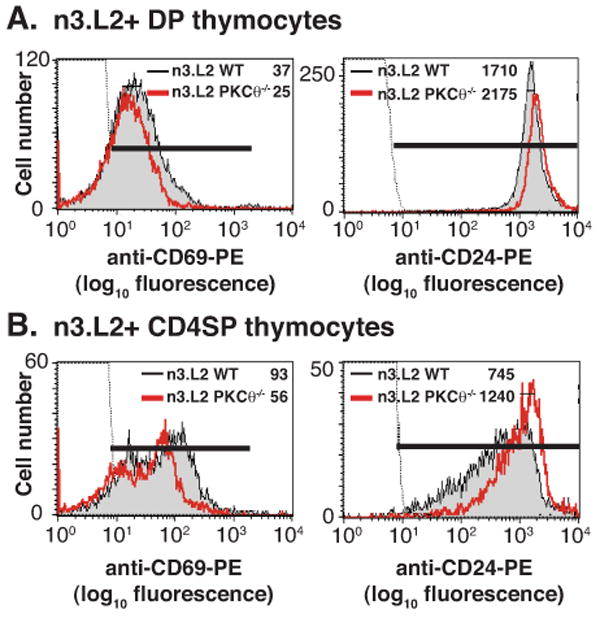
Flow cytometric analysis of markers of positive selection in n3.L2 PKCθ-/- and n3.L2 WT (A) DP and (B) CD4SP thymocytes. Thymocytes were stained with Cab-FITC, anti-CD8-PerCP, anti-CD4-APC and either anti-CD69-PE or anti-CD24-PE. Thymocytes were gated on Cab-FITC positive population and then gated on CD4-APC and CD8-PerCP populations to determine DP and CD4SP populations. CD69-PE and CD24-PE staining of the indicated populations is presented. The MFI of CD69 and CD24 expression is given in each histogram. Negative staining control depicted as dotted line. Data representative of at least 3 experiments.
Anti-CD3 mediated upregulation of CD69 reduced in n3.L2 PKCθ-/- thymocytes
If inefficient positive selection of n3.L2 PKCθ-/- CD4SP thymocytes was due to diminished TCR signaling, then in vitro TCR-mediated stimulation of CD69 upregulation should have been decreased in n3.L2 PKCθ-/- thymocytes. To test this prediction, thymocytes from n3.L2 WT and n3.L2 PKCθ-/- mice were incubated overnight on plate-bound anti-CD3, anti-TCR, or recombinant I-Ek with peptide (pI-Ek; Fig. 5A). Regardless of stimulus, upregulation of CD69 was diminished on n3.L2 PKCθ-/- DP thymocytes, as assessed by both the percentage and the mean fluorescence intensity (MFI) of cells positive for CD69 (Fig. 5A and data not shown). Upregulation of CD69 after stimulation with PMA and ionomycin, used as a positive control, was equivalent in PKCθ-/- and WT DP thymocytes (82% and 80%, respectively). The reduction of CD69 upregulation following anti-CD3 or anti-TCR stimulation is consistent with the hypothesis that TCR signaling is diminished in n3.L2 PKCθ-/- thymocytes.
Figure 5.
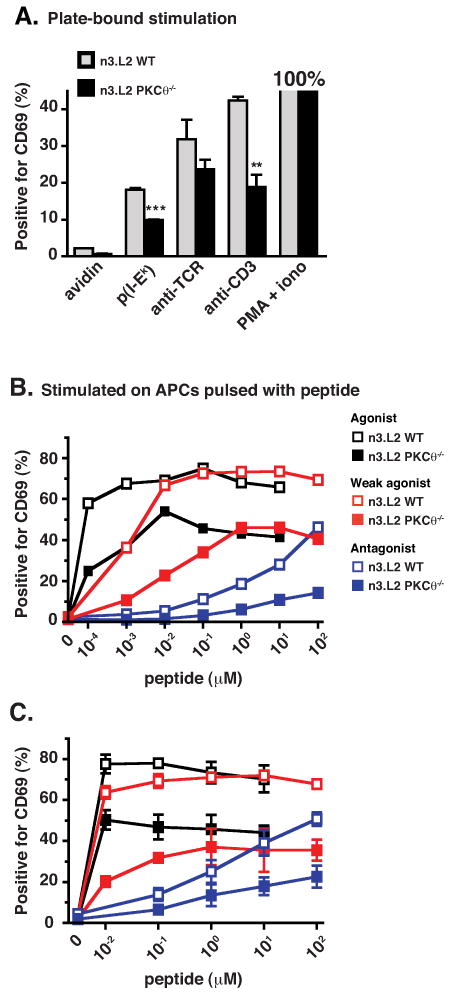
In vitro stimulation of CD69 upregulation is impaired in n3.L2 PKCθ-/- thymocytes (filled bars or symbols) as compared to n3.L2 WT thymocytes (grey bars or open symbols). (A) Thymocytes were incubated overnight on plate-bound anti-CD3 (10 μg/ml), plate-bound anti-n3.L2 (Cab, 10 μg/ml), pI-Ek, avidin, or PMA and ionomycin. Cells were stained with anti-CD8-FITC, anti-CD69-PE, Cab-biot/SA-PerCP, and anti-CD4-APC. Cells were gated to determine the percentage of n3.L2+ DP thymocytes that were also CD69 positive. This percentage was normalized to the percent of CD69+ cells stimulated with PMA and ionomycin. Mean ± SEM of triplicate samples is given. Difference is statistically significant as indicated (** p < 0.01; *** p < 0.001). Data representative of two independent experiments. (B, C) Thymocytes were incubated overnight on DCEK Hi7 APCs pulsed with agonist, weak agonist or antagonist peptide at the indicated concentrations. Thymocytes were stained with anti-TCR-FITC, anti-CD69-PE, anti-CD8-PerCP and anti-CD4-APC. Percentage of n3.L2+ DP thymocytes positive for CD69 staining is shown. (B) Panel represents one experiment, demonstrating that a dose response to the agonist peptide is apparent at very low concentrations. (C) Panel presents values for mean ± SEM from two (weak agonist) or three (agonist and antagonist peptide) independent experiments.
To further test the hypothesis that the strength of TCR signaling was altered in n3.L2 PKCθ-/- thymocytes, we used APLs previously identified for the n3.L2 TCR (29). If TCR signaling in n3.L2 PKCθ-/- thymocytes was weaker, then upregulation of CD69 upon stimulation with APLs should be reduced in n3.L2 PKCθ-/- thymocytes, compared to n3.L2 WT thymocytes. However, if TCR signaling was stronger in n3.L2 PKCθ-/- thymocytes, then APL-stimulated upregulation of CD69 of n3.L2 PKCθ-/- should be increased, compared to n3.L2 WT thymocytes. To differentiate between these possibilities, thymocytes were stimulated with an agonist, a weak agonist or an antagonist peptide. Stimulation with all APLs revealed diminished upregulation of CD69 in n3.L2 PKCθ-/- DP thymocytes, compared to n3.L2 WT DP thymocytes, as assessed by both percentage and MFI of cells positive for CD69 (Fig. 5B, 5C and data not shown). The dose response of the agonist peptide was more apparent when assessing the percentage of cells positive for CD69 at low concentrations of agonist peptide (Fig. 5B). These findings support the hypothesis of weaker TCR signaling in n3.L2 PKCθ-/- thymocytes.
Diminished positive selection of CD4SP and CD8SP thymocytes in non-transgenic PKCθ-/- mice
To examine whether development of CD8SP thymocytes was also dependent on PKCθ, thymocyte populations from non-transgenic PKCθ-/- and B6.K mice were compared (Fig. 6). A decrease in the percentage of cells that were CD4SP or CD8SP and a slight increase in the DP population were again seen (Fig. 6A). The numbers of CD4SP and CD8SP cells were reduced in PKCθ-/- mice as compared to WT mice by about 50% (Fig. 6B). Exclusion of NK1.1+, γ/δ-TCR+, CD25+ and CD44+ cells from the analysis revealed that 85% of CD4SP thymocytes in WT mice were naive T cells while 92% of CD4SP thymocytes in PKCθ-/- mice were naive T cells (data not shown). This slight reduction of non-naive T cell numbers was insufficient to explain the diminished numbers of CD4SP thymocytes in PKCθ-/- mice. These data revealed that the diminished positive selection of CD4SP thymocytes in n3.L2 PKCθ-/- mice was not limited to the n3.L2 transgenic system, and that the disturbance of thymocyte maturation in PKCθ-/- mice was not restricted to the CD4 lineage. Furthermore, CD69 expression was decreased and CD24 expression was increased on PKCθ-/- CD4SP and CD8SP cells as compared to WT CD4SP and CD8SP thymocytes (Fig. 6C, 6D, 6E, 6F), as was seen in n3.L2 PKCθ-/- CD4SP thymocytes. Diminished thymic production of CD4SP and CD8SP cells was reflected in a decrease in the total number of CD4+ and CD8+ cells isolated from the lymph nodes of PKCθ-/- mice, although no difference in splenocyte numbers was apparent (data not shown). Thus, a dependence for thymocyte development on PKCθ for both CD4SP and CD8SP cells was seen in the non-transgenic PKCθ-/- mice.
Reduction of PKCθ-/- CD4SP thymocytes not due to decreased survival or to change in DN thymocyte population
The decrease in CD4SP thymocytes in PKCθ-/- mice could also be due to decreased survival of PKCθ-/- thymocytes. PKCθ-/- thymocytes could theoretically be more likely to undergo death by neglect, or be more sensitive to activation-induced cell death (AICD). To address these possibilities, PKCθ-/- and WT thymocytes were incubated overnight with or without plate-bound anti-CD3 and anti-CD28 stimulation. Cell death was assessed by surface staining with Annexin V. The percentage of Annexin V-positive PKCθ-/- thymocytes was not increased compared to WT thymocytes, indicating that PKCθ-/- thymocytes were not more likely to undergo apoptosis either from neglect or from AICD (Fig. 7A). There was also no difference in survival between WT and PKCθ-/- peripheral cells when cells were incubated without stimulation and assessed for cell death using Annexin V labeling every 24 hrs for 96 hrs (data not shown). Thus, the reduction of CD4+ cell numbers in PKCθ-/- was not due to differential survival.
Figure 7.
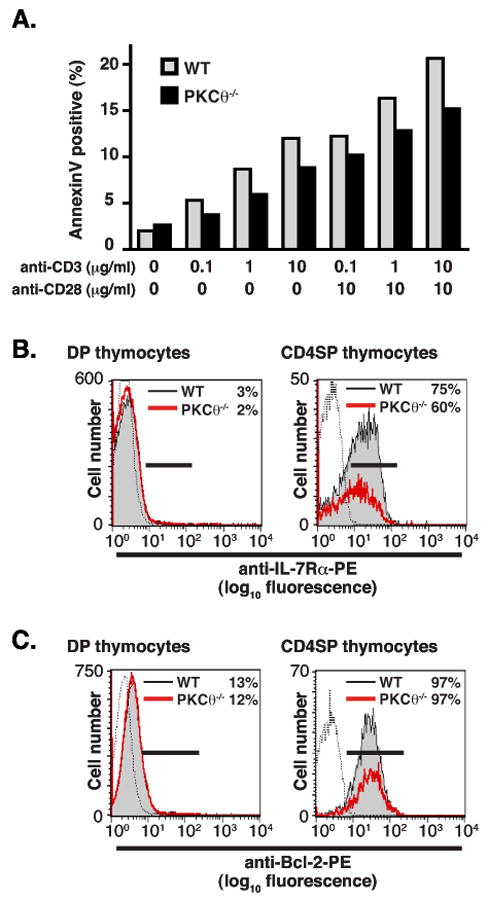
(A) No increase in apoptosis of PKCθ-/- thymocytes as compared to WT. Thymcoytes isolated from either non-transgenic PKCθ-/- (filled bars) or WT (grey bars) mice were stimulated overnight under the indicated conditions. Cells were then stained with Annexin V-Alexa488, anti-CD8PE, and anti-CD4-PerCP. Percentage of thymocytes positive for Annexin V shown. There was no difference in percentage of thymocytes positive for Annexin V when gating on DP thymocytes. There was no difference in cell death when thymocytes from n3.L2 transgenic mice were used. Data representative of two experiments, one with cells from non-transgenic mice and one with cells isolated from n3.L2 transgenic mice. (B, C) Thymocytes from PKCθ-/- (red line) and WT (filled histogram) mice were harvested and stained for (B) TCRβ, IL7-Rα, CD8 and CD4 or (C) TCRβ, Bcl-2, CD8 and CD4. The expression of (B) IL-7Rα on or (C) Bcl-2 on DP and TCRβhigh CD4SP thymocytes is shown. Isotype control shown as dotted line. The percentage of cells positive for either IL-7Rα or Bcl-2 is given in the upper right-hand corner of each histogram.
Additionally, positive selection has been shown to induce the upregulation of molecules required for survival of SP thymocytes, including IL-7Rα and Bcl-2 (27, 30). To determine whether altered expression of IL-7Rα or Bcl-2 contributed to decreased numbers of CD4SP PKCθ-/- thymocytes, the expression of IL-7Rα and Bcl-2 on thymocytes from PKCθ-/- and WT mice was examined (Fig. 7B, 7C). There was a slight decrease in the percentage of PKCθ-/- CD4SP cells that expressed IL-7Rα, but Bcl-2 expression was equivalent in CD4SP thymocytes from WT and PKCθ-/- mice. Similar results were obtained when thymocytes from n3.L2 WT and n3.L2 PKCθ-/- mice were assessed for IL-7Rα and Bcl-2 expression (data not shown). Thus, the 50% reduction of CD4SP thymocytes in PKCθ-/- mice was associated with a failure to progress from the DP to the SP stage, not with decreased survival or increased sensitivity to AICD.
It has been proposed that PKCθ may play a role in Notch signaling, which is required for progression through the DN to the DP stages of thymocyte development (31). To determine whether thymocyte development prior to positive selection was affected in PKCθ-/- mice, we assessed the expression of CD44 and CD25 on DN thymocytes isolated from n3.L2 PKCθ-/- and n3.L2 WT mice. There was no difference in the distribution of CD25 and CD44 DN populations between thymocytes isolated from n3.L2 WT and n3.L2 PKCθ-/- mice, nor between CD25 and CD44 DN populations in non-transgenic PKCθ-/- and B6.K mice (data not shown). Therefore, thymic maturation appears to progress normally in n3.L2 PKCθ-/- mice until the DP stage.
PKCθ-/- thymocytes are at a significant competitive disadvantage in mixed bone marrow chimeras
Positive selection of n3.L2 PKCθ-/- thymocytes was diminished compared to WT thymocytes but was not absent. Other PKC isoforms may have partially compensated for the loss of PKCθ and partially rescued positive selection of PKCθ-/- thymocytes. However, if PKCθ is the dominant PKC isoform required during positive selection, a more dramatic defect might be revealed by direct competition between PKCθ-/- and WT thymocytes. We therefore isolated bone marrow from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 RAG1-/- H-2k mice, mixed the bone marrow cells in a 1:1 ratio and transferred the cells into lethally irradiated non-transgenic H-2k recipients. Both n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 RAG1-/- H-2k DP thymocytes have equivalent chances to be positively selected in the recipient H-2k background; because the donor bone marrow cells were n3.L2 RAG1-/-, there was no risk for graft-versus-host disease. Thymuses and lymph nodes were harvested 5-6 weeks after transfer and analyzed for the percentage of n3.L2 DP and n3.L2 CD4SP cells that were positive for either H-2Kb, and thus derived from PKCθ-/- mice, or H-2Kk, and derived from PKCθ-sufficient mice.
There was an 80% decrease in the percentage of n3.L2 CD4SP thymocytes derived from PKCθ-/- mice compared to the percentage of PKCθ-/- n3.L2 DP thymocytes (Fig. 8), indicating that n3.L2 RAG1-/- PKCθ-sufficient thymocytes significantly outcompeted n3.L2 RAG1-/- PKCθ-/- thymocytes during positive selection. The observation that approximately 20% of n3.L2 DP thymocytes were derived from PKCθ-/- mice when 50% of transferred bone marrow cells were PKCθ-/- suggests that PKCθ may additionally function in bone marrow engraftment, thymocyte seeding, or thymocyte development prior to the DP stage. As there was no difference in the distribution of CD44 and CD25 populations in PKCθ-/- DN thymocytes (data not shown), it seems likely that the additional function of PKCθ occurs prior to the DN1 stage of thymocyte development. MHC class I molecules are not upregulated until the later stages of positive selection (17) and we therefore noted n3.L2 CD4SP thymocytes that expressed MHC class I at low levels for which it was not possible to accurately determine H-2Kk or H-2Kb expression (Fig. 8A). However, the proportion of H-2Kk and H-2Kb cells in the class I-low population would be predicted to be reflected in the proportion of H-2Kk and H-2Kb cells in the class I-high population of CD4SP thymocytes, as well as in the proportion of H-2Kk and H-2Kb cells in the mature CD4+ cells from the lymph nodes. As the proportion of H-2Kk and H-2Kb cells in n3.L2 CD4 mature T cells was not significantly different from the proportion of H-2Kk and H-2Kb cells in the n3.L2 CD4SP thymocytes, we do not think that the exclusion of MHC class I-low cells significantly altered our analysis. Comparison of the proportion of PKCθ-/--derived thymocytes in the CD4SP and DP populations allowed an analysis of the role of PKCθ during positive selection, independent of other possible functions of PKCθ. The large decrease in the percentage of PKCθ-/--derived cells in the n3.L2 CD4SP population, compared to the percentage of PKCθ-/--derived cells in the n3.L2 DP population suggests that PKCθ is the dominant PKC isoform required for TCR signaling during positive selection.
Figure 8.
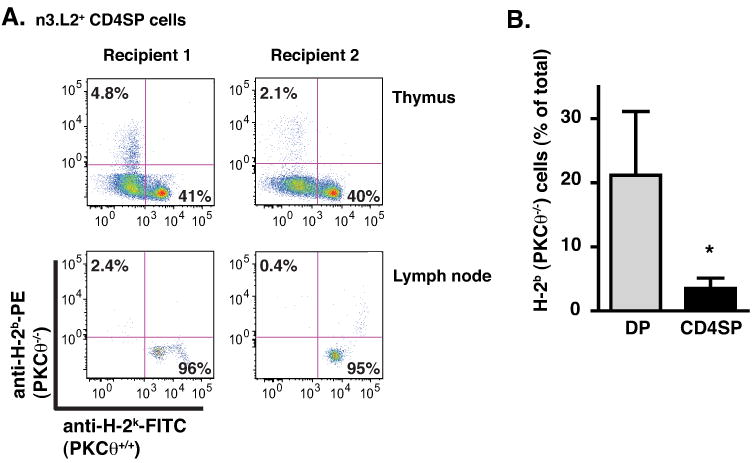
PKCθ-/- thymocytes are at a significant competitive disadvantage in mixed bone marrow chimeras. Bone marrow isolated from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 RAG1-/- H-2k mice was mixed in a 1:1 ratio and transferred into lethally irradiated non-transgenic H-2k recipients. Thymuses and lymph nodes were isolated 5-6 weeks following transfer and cells analyzed by flow cytometry for CD4, CD8, n3.L2, H-2k and H-2b. (A) The expression of H-2b and H-2k on n3.L2 CD4SP thymocytes and n3.L2 CD4+ mature T cells are shown from 2 representative mice. (B) The percentage of cells positive for H-2b from either n3.L2 DP (grey bar) or n3.L2 CD4SP thymocytes (filled bar) is shown. Data from 3 independent experiments using 2 recipients in each (mean ± S.E.M.; * p < 0.05). In 1 of 3 experiments, recipient mice were B6.K/CD45.1 and recipient T cells were excluded by flow cytometric analysis for CD45.1. The data was not different using B6.K or B6.K/CD45.1 mice as recipients.
Decreased production of Treg cells in n3.L2 PKCθ-/- mice
Defective production of CD25+, FoxP3+ Treg cells in PKCθ-/- mice has been previously reported (16). However, the authors had assumed an equivalent number of CD4SP thymocytes in PKCθ-/- and WT mice, and so did not account for a possible overall reduction in the number of CD4SP thymocytes when they assessed that PKCθ-/- mice generated fewer Treg cells. To determine whether reduced thymic production of Treg cells in PKCθ-/- mice could be explained by the observed decrease in production of CD4SP thymocytes, we determined the percentage of CD4SP thymocytes that were CD25+FoxP3+ from WT and PKCθ-/- mice (Fig. 9). Even when controlling for the reduction of CD4SP cells in PKCθ-/- mice, there was a dramatic (> 90%) decrease in the percentage and number of CD4SP thymocytes that were Treg cells (Fig. 9). There was also an approximate 50% decrease in the percentage and number of Treg cells isolated from the spleens and lymph nodes of PKCθ-/- mice (Fig. 9). Despite the decrease in Treg cell production, PKCθ-/- mice do not suffer from any spontaneous autoimmune disease. Possible explanations for this include the activation defect in mature PKCθ-/- T cells and that the numbers of Treg cells present in the periphery are sufficient to prevent disease. The decrease in thymic production of Treg cells cannot be explained by the observed decrease in generation of CD4SP thymocytes, as the reduction in Treg cell generation is profound even when controlling for the number of CD4SP thymocytes.
Figure 9.
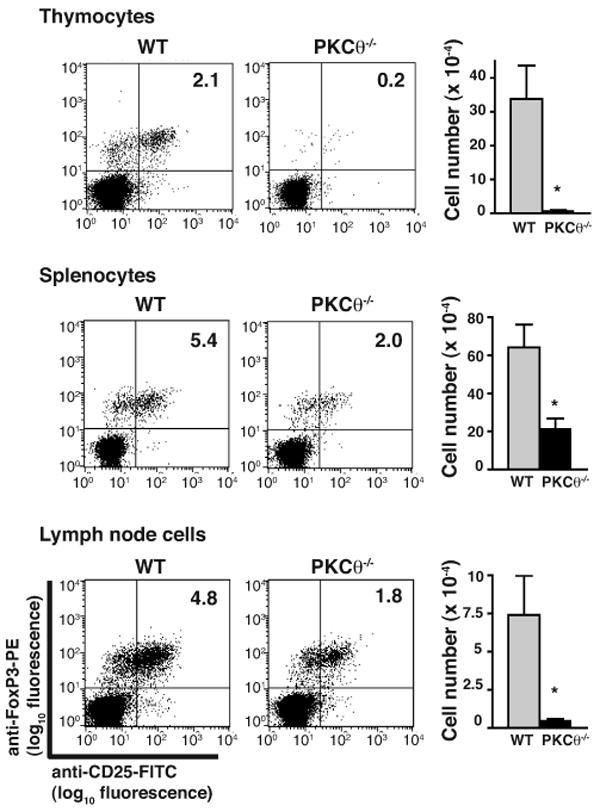
Profound reduction of CD25+FoxP3+ Treg cells in PKCθ-/- mice. Cells isolated from non-transgenic PKCθ-/- and WT mice were stained with anti-CD25-FITC, anti-FoxP3-PE, anti-CD8-PerCP and anti-CD4-APC. Representative flow cytometric analysis of CD25 and FoxP3 staining of CD4SP cells isolated from thymus, spleen and lymph nodes are shown. Absolute numbers of CD25+, FoxP3+ CD4SP cells isolated from WT (grey bars) and PKCθ-/- (filled bars) mice are given as mean ± SEM with n = 4. Reduction of numbers of Treg cells in PKCθ-/- mice was statistically significant with *p < 0.05.
Failure to sustain ERK activation in n3.L2 PKCθ-/- thymocytes
To further investigate TCR signaling events that underlie the dimunition of positive selection in n3.L2 PKCθ-/- mice, we examined n3.L2 RAG1-/- H-2b thymocytes. n3.L2 thymocytes are not positively selected on the H-2b background and accumulate in the DP stage.
Numerous reports have demonstrated the requirement of sustained ERK activation for positive selection of thymocytes (32-36). To determine whether the reduction of positive selection in PKCθ-/- thymocytes was associated with reduced ERK activation, non-selected DP thymocytes from n3.L2 PKCθ-/- RAG1-/- H-2b or n3.L2 WT RAG1-/- H-2b mice were stimulated on plate-bound anti-CD3 and anti-CD28 antibody (Fig. 10A). Activation of ERK was assessed by immunoblot for phosphorylated ERK. Phosphorylation of ERK was not sustained for as long in n3.L2 PKCθ-/- RAG1-/- H-2b thymocytes as in n3.L2 WT RAG1-/- H-2b thymocytes. Failure to sustain ERK activity thus correlated with diminished positive selection in PKCθ-/- thymocytes.
Figure 10.
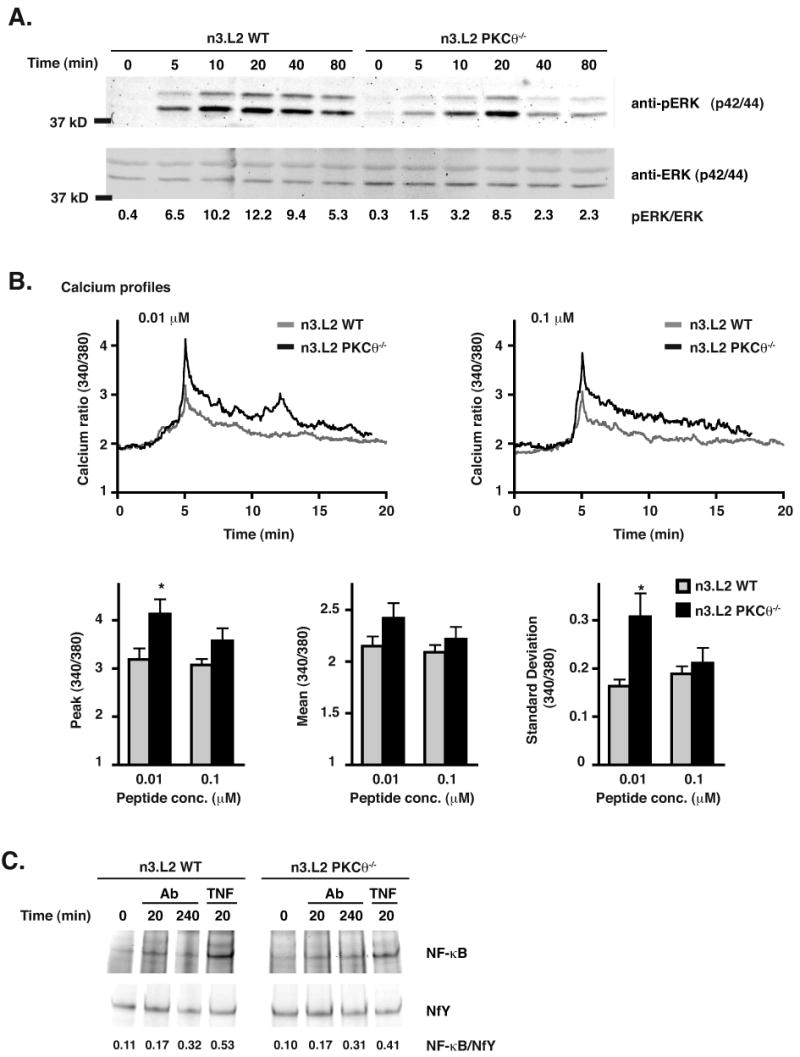
(A) Phosphorylation of ERK in stimulated thymocytes isolated from PKCθ-/- mice is reduced as compared to that of stimulated thymocytes from WT mice. Thymocytes isolated from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 WT RAG1-/- H-2b mice were stimulated by incubation on plate-bound anti-CD3 and anti-CD28 antibody (10 μg/ml) at 37°C for the indicated periods of time. Immunoblot for total ERK and phosphorylated ERK is shown. The intensity of each band was determined and the ratio of the phosphorylated form of ERK to total ERK present is given beneath each lane. Data representative of at least 3 experiments. (B). Calcium signaling is not inhibited in PKCθ-/- DP thymocytes as compared to WT DP thymocytes. Thymocytes isolated from n3.L2 PKCθ-/- RAG1-/- H-2b (solid line) and n3.L2 WT RAG1-/- H-2b (grey line) mice were loaded with Fura and incubated on APCs pulsed with the indicated concentrations of antigenic peptide for the indicated time. Intracellular calcium level is shown as the ratio of 340 to 380 fluorescence. Tracings represent average intracellular calcium levels with the peak calcium level of each cell set to 5 min. Peak calcium level at 5 min, mean calcium level from 6 to 20 min, and oscillatory behavior of calcium levels from 6 to 20 min shown in bar graphs below the tracings indicated. WT cells are shown as grey bars and PKCθ-/- cells shown as filled bars. Mean ± SEM shown. N = 17 cells from WT and PKCθ-/- mice stimulated with 0.01 μM peptide and n = 20 cells for WT and PKCθ-/- mice stimulated with 0.1 μM peptide, each from three independent experiments. Differences are statistically significant (*p < 0.05) as indicated. (C) Activation of NF-κB occurred normally in thymocytes isolated from n3.L2 PKCθ-/- RAG1-/- H-2b mice. Thymocytes isolated from n3.L2 PKCθ-/- RAG1-/- H-2B and n3.L2 WT RAG1-/- H-2b mice and stimulated with cross-linked anti-CD3 (10 μg/ml) and anti-CD4 (10 μg/ml) antibodies for the indicated periods of time. Stimulation of cells with TNF (50 ng/ml) was used as a positive control. Cells were lysed and nuclear extracts subjected to EMSA. EMSA of the housekeeping gene NfY was used as a loading control. The ratio of the intensity of the NF-κB band to NfY band is given beneath each lane.
Calcium signaling is also central to TCR signaling in thymocytes during positive selection (36, 37). A previous report has shown that calcium signaling can be regulated by PKCθ (13). Therefore, calcium signaling was assessed using single cell imaging (23). Non-selected DP thymocytes from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 RAG1-/- H-2b mice were loaded with Fura-2 and incubated on APCs pulsed with antigenic peptide at two concentrations (near-maximal stimulation and sub-optimal). At the lower concentration of agonist peptide, the calcium mobilization in PKCθ-/- thymocytes peaked at a higher level and was more oscillatory than that of the WT thymocytes (Fig. 10B). However, at an increased concentration of antigen the difference in peak calcium levels and oscillatory behavior was not statistically significant. Thus, TCR proximal signaling events leading to calcium signaling were not inhibited in PKCθ-/- thymocytes, and may have been enhanced at sub-optimal concentrations of agonist peptide. These results are consistent with findings from Manicassamy et al., in which PMA activation of PKCθ could inhibit calcium signaling in mature T cells (38).
In mature T cells, PKCθ is required for the activation of NF-κB (7, 9). In some systems, NF-κB has been linked to survival of DP thymocytes (39), though NF-κB activity may be dispensable for positive selection (40). To determine whether NF-κB activation was dependent upon PKCθ in thymocytes in our system, and to address the conflicting reports of the requirement for NF-κB in positive selection, we assessed the activation of NF-κB in PKCθ-/- DP thymocytes by EMSA. Non-selected DP thymocytes from n3.L2 PKCθ-/- RAG1-/- H-2b and n3.L2 WT RAG1-/- H-2b mice were stimulated with cross-linked anti-CD3 and anti-CD4, or TNF, which stimulates NF-κB activation independently of PKCθ (41). Activation of NF-κB occurred with the same kinetics in PKCθ-/- thymocytes as WT mice, indicating that in our system TCR-stimulation of NF-κB activation is not dependent upon PKCθ (Fig. 10C). These results are consistent with previous publications (7, 9). Furthermore, there was no correlation between diminished positive selection and activation of NF-κB, consistent with the hypothesis that NF-κB activation is not required for signaling to positive selection (40).
Discussion
PKCθ plays multiple roles in TCR signaling and T cell function (for reviews, see (3, 4, 42, 43). Given the central role of PKCθ in TCR signaling in mature T cells, it was surprising that no effect on thymocyte development was appreciated initially. However, after reexamining thymocyte development in PKCθ-/- mice using the class-II restricted transgenic TCR n3.L2, we now report that positive selection is less efficient in the absence of PKCθ. Both n3.L2 transgenic and non-transgenic PKCθ-/- mice produced approximately 50% fewer CD4SP thymocytes than did comparable WT mice. We also noted a statistically significant decrease in the number of CD8SP thymocytes generated in the non-transgenic PKCθ-/- mice, indicating that a dependence of thymocyte development on PKCθ was not restricted to the CD4 lineage or the n3.L2 transgenic system. Additionally, analysis of changes in the expression of markers associated with positive selection, such as upregulation of CD69 and downregulation of CD24, revealed that positive selection was less efficient in PKCθ-/- mice. Our results may differ from those previously published not only because of the use of a different transgenic TCR, but also because the strain examined here had been extensively backcrossed on the B6 background, whereas previous reports examined PKCθ-/- mice on mixed backgrounds (7, 9).
The decrease in numbers of CD4SP thymocytes in PKCθ-/- mice was not due to decreased cell survival, or to reduced expression of molecules required for survival, such as IL-7Rα or Bcl-2. IL-7Rα is not required for positive selection, but is upregulated as a consequence of selection (44). Although IL-7Rα expression was slightly reduced on CD4SP thymocytes from n3.L2 PKCθ-/- and PKCθ-/- mice, Bcl-2 expression was equivalent. As upregulation of Bcl-2 is dependent upon IL-7R signaling (45, 46), it seems unlikely that the slight diminution of IL-7Rα expression was physiologically relevant. Consistent with normal expression of Bcl-2, thymocytes from PKCθ-/- mice did not exhibit increased susceptibility to apoptosis, and in fact may have been slightly less likely than thymocytes from WT mice to undergo AICD (Fig. 7). Reduced AICD would be predicted to increase the number of CD4SP thymocytes in PKCθ-/- mice and may partially obscure the results of diminished positive selection in PKCθ-/- mice.
Partial compensation of PKCθ function by other PKC isoforms may also prevent a complete block of thymocyte development in PKCθ-/- mice (3). Functional redundancy of PKC isoforms appears to be different in thymocytes and mature T cells. For instance, PKCθ is specifically required for activation of NF-κB in mature T cells (7, 9), but not in thymocytes (Fig. 10 and (7)). This difference suggests that either another PKC isoform can substitute for PKCθ in the activation of NF-κB in thymocytes but not in mature T cells, or that NF-κB activation is PKC independent in thymocytes. Interestingly, PKCθ-/- thymocytes are at a significant competitive disadvantage with PKC-sufficient cells (Fig. 8), suggesting that even though other PKC isoforms may partially compensate for PKCθ, PKCθ is still the dominant isoform required for TCR signaling in thymocytes. Direct competition between PKCθ-/- and PKCθ –sufficient bone marrow stem cells in bone marrow chimeras also revealed a potential role for PKCθ in either bone marrow engraftment, early hematopoietic stem cell development, or possibly thymocyte seeding that could be further explored.
TCR signal strength determines cell fate during thymocyte development (47, 48). DP thymocytes that express TCRs incapable of recognizing self MHC molecules fail to receive any signal through pMHC-TCR engagement and are eliminated through death by neglect. DP thymocytes expressing α/β TCRs capable of interacting with self MHC molecules do receive a signal through pMHC-TCR engagement and are positively selected. Thymocytes bearing autoreactive TCRs receive a strong signal through pMHC-TCR engagement and are deleted by negative selection. To undergo positive but not negative selection, a T cell must therefore bear a TCR that receives enough of a signal from TCR-pMHC engagement to survive but not one strong enough to induce cell death—otherwise termed a “weak” signal. Complex intracellular signaling events must therefore be orchestrated to encode the strength of pMHC-TCR engagement in developing thymocytes.
Using defined APLs for the n3.L2 TCR, we tested whether the absence of PKCθ resulted in a “stronger” or “weaker” signal through the TCR. Because CD69 upregulation was reduced with all APLs tested, we concluded that TCR signaling was diminished in the absence of PKCθ. Furthermore, we demonstrated a decrease in TCR-stimulated ERK phosphorylation in PKCθ-/- thymocytes, as compared to WT thymocytes. Phosphorylation and activation of ERK appears to be one mechanism by which DP thymocytes encode TCR signal strength. Positively selecting, “weaker” ligands induce lower levels of ERK phosphorylation than do negatively selecting, “stronger” ligands (34, 49, 50). Multiple prior studies have demonstrated that sustained ERK activity is required for positive selection (32, 34, 51). A change in ERK phosphorylation would therefore be predicted to change the outcome of thymic development. PKCθ has been previously demonstrated to positively regulate ERK phosphorylation (12, 52-55). The mechanism of PKCθ regulation of ERK may be through activation of Ras-GRP (12). We demonstrate here that TCR-mediated phosphorylation of ERK is diminished in PKCθ-/- thymocytes. Reduced activation of ERK in PKCθ-/- thymocytes likely explains the observed decrease in positive selection.
However, not all TCR-mediated signaling events were diminished in PKCθ-/- DP thymocytes. We also examined calcium mobilization and NF-κB activation in PKCθ-/- thymocytes because both signaling pathways have been shown to be dependent upon PKCθ in mature T cells and both may be involved in signaling to positive selection (7, 9, 13, 36, 56-58). Since a prior study has examined calcium activation in PKCθ-/- thymocytes and found no inhibition in a bulk population of thymocytes (13), we examined calcium signaling at a single cell level to allow for a more detailed analysis. We also found no inhibition of calcium entry in n3.L2 PKCθ-/- DP thymocytes. Intriguingly, at a low concentration of antigenic peptide, calcium levels seemed to be slightly enhanced in PKCθ-/- thymocytes as compared to WT thymocytes. However, there was no statistically significant difference in calcium responses in PKCθ-/- and WT thymocytes at a higher concentration of peptide. As calcium entry was not inhibited in PKCθ-/- thymocytes, it seems unlikely that a slight change in calcium signaling explains the observed decrease in positive selection. Also consistent with previously published results, TCR-stimulated NF-κB activation was intact in PKCθ-/- DP thymocytes (7). The decreased TCR signaling in PKCθ-/- DP thymocytes therefore appears to be a specific defect in activation of ERK. The observation that not all TCR signaling pathways were PKCθ-dependent most likely explains the observation that upregulation of some markers associated with positive selection, such as IL-7Rα, were unchanged in PKCθ-/- thymocytes.
Development of CD25+FoxP3+ Treg cells is severely inhibited in PKCθ-/- mice. When the developmental defect of Treg cells in PKCθ-/- mice was initially described, the authors assumed that the generation of CD4SP thymocytes was unaffected. We therefore tested the possibility that the decrease in production of CD4SP thymocytes might explain the decrease in production of Treg cells in PKCθ-/- mice. However, even when controlling for the reduction of CD4SP thymocytes, there was still a profound decrease in the number of Treg cells produced in PKCθ-/- mice. The molecular mechanism for the differential requirement for PKCθ in the development of α/β T cells and FoxP3+ Treg cells is currently unclear. It has been proposed that defective activation of NF-κB in PKCθ-/- thymcoytes results in the defective production of Treg cells (16) but we find no inhibition of NF-κB in PKCθ-/- thymcoytes in our system. Activation of the Raf-MAPK pathway also appears to be required for full development of the Treg cell compartment (59). Thus, the defective activation of ERK in PKCθ-/- thymocytes may be sufficient to explain the diminished production of Treg cells as well as the inefficient positive selection of FoxP3 negative α/β thymocytes.
By using a class II-restricted transgenic TCR, a previously unrecognized dependence of thymocyte TCR signaling on PKCθ has been revealed. Diminished ERK activation following in vitro TCR ligation in PKCθ-/- thymocytes correlated with inefficient positive selection of PKCθ-/- thymocytes in vivo. Inefficient positive selection of PKCθ-/- thymocytes was not limited to either the n3.L2 transgenic system or to the CD4 lineage, as we found similar results in non-transgenic PKCθ-/- mice on a B6.K background for both CD4SP and CD8SP thymocytes. Therefore, PKCθ is important for both thymocyte and mature T cell TCR signal transduction.
Acknowledgments
PKCθ-/- mice were generously provided by Dan Littman. The authors thank Dan Littman and Wojciech Swat for critical reviews of the manuscript. The authors thank Darren Kreamalmeyer for technical assistance with management of the mouse colony, Stephen Horvath for technical assistance with genotyping the mice, Jennifer Racz for technical assistance with the generation of bone marrow chimeras, and Andrea Bredemeyer and Beth Helmink for assistance with the EMSAs.
Abbreviations
- PKCθ
Protein kinase C-θ
- Cab
clonotypic antibody
- APLs
altered peptide ligands
- APC
allophycocyanin
- pI-Ek
I-Ek with the antigenic peptide
- DP
double positive
- SP
single positive
- I10
IMDM with 10% serum
- MFI
mean fluorescence intensity
- AICD
activation-induced cell death
- Treg
regulatory T
Footnotes
S.C.M. is supported by the Pediatric Infectious Diseases Society-St. Jude's Fellowship Award for Basic Research. P.M.A. is supported by grants from the National Institutes of Health.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 2.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitaler M, Cantrell DA. Protein kinase C and beyond. Nat Immunol. 2004;5:785–790. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- 4.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-theta in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 5.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 6.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 8.Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O'Brien W, Thome M, Littman DR. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T, Penninger JM. The molecular adapter Carma1 controls entry of IkappaB kinase into the central immune synapse. J Exp Med. 2004;200:1167–1177. doi: 10.1084/jem.20032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL, Lin X. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential roles of PKC-theta in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy AM, Izmailova E, Fitzgerald M, Walker R, Hattersley M, Silva M, Siebert E, Terkelsen J, Picarella D, Pickard MD, LeClair B, Chandra S, Jaffee B. PKC-theta-deficient mice are protected from Th1-dependent antigen-induced arthritis. J Immunol. 2006;177:1886–1893. doi: 10.4049/jimmunol.177.3.1886. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mick VE, Starr TK, McCaughtry TM, McNeil LK, Hogquist KA. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J Immunol. 2004;173:5434–5444. doi: 10.4049/jimmunol.173.9.5434. [DOI] [PubMed] [Google Scholar]
- 18.Kersh GJ, Donermeyer DL, Frederick KE, White JM, Hsu BL, Allen PM. TCR transgenic mice in which usage of transgenic alpha- and beta-chains is highly dependent on the level of selecting ligand. J Immunol. 1998;161:585–593. [PubMed] [Google Scholar]
- 19.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 20.Kao H, Allen PM. An antagonist peptide mediates positive selection and CD4 lineage commitment of MHC class II-restricted T cells in the absence of CD4. J Exp Med. 2005;201:149–158. doi: 10.1084/jem.20041574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, Allen PM. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 22.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 23.Weber KS, Miller MJ, Allen PM. Th17 cells exhibit a distinct calcium profile from Th1 and Th2 cells and have Th1-like motility and NF-AT nuclear localization. J Immunol. 2008;180:1442–1450. doi: 10.4049/jimmunol.180.3.1442. [DOI] [PubMed] [Google Scholar]
- 24.Slavik JM, Hutchcroft JE, Bierer BE. CD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28. J Biol Chem. 1999;274:3116–3124. doi: 10.1074/jbc.274.5.3116. [DOI] [PubMed] [Google Scholar]
- 25.Weaver BK, Bohn E, Judd BA, Gil MP, Schreiber RD. ABIN-3: a molecular basis for species divergence in interleukin-10-induced anti-inflammatory actions. Mol Cell Biol. 2007;27:4603–4616. doi: 10.1128/MCB.00223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van De Wiele CJ, Marino JH, Murray BW, Vo SS, Whetsell ME, Teague TK. Thymocytes between the beta-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J Immunol. 2004;172:4235–4244. doi: 10.4049/jimmunol.172.7.4235. [DOI] [PubMed] [Google Scholar]
- 28.Nishida T, Matsuki Y, Ono T, Oguma T, Tsujimoto K, Sato M, Tadakuma T. The novel murine CD4+CD8+ thymocyte cell line exhibits lineage commitment into both CD4+ and CD8+ T cells by altering the intensity and the duration of anti-CD3 stimulation in vitro. J Immunol. 2004;172:6634–6641. doi: 10.4049/jimmunol.172.11.6634. [DOI] [PubMed] [Google Scholar]
- 29.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 30.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 31.Felli MP, Vacca A, Calce A, Bellavia D, Campese AF, Grillo R, Di Giovine M, Checquolo S, Talora C, Palermo R, Di Mario G, Frati L, Gulino A, Screpanti I. PKC theta mediates pre-TCR signaling and contributes to Notch3-induced T-cell leukemia. Oncogene. 2005;24:992–1000. doi: 10.1038/sj.onc.1208302. [DOI] [PubMed] [Google Scholar]
- 32.Delgado P, Fernandez E, Dave V, Kappes D, Alarcon B. CD3delta couples T-cell receptor signalling to ERK activation and thymocyte positive selection. Nature. 2000;406:426–430. doi: 10.1038/35019102. [DOI] [PubMed] [Google Scholar]
- 33.Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nat Immunol. 2004;5:289–298. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- 34.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettini ML, Kersh GJ. MAP kinase phosphatase activity sets the threshold for thymocyte positive selection. Proc Natl Acad Sci U S A. 2007;104:16257–16262. doi: 10.1073/pnas.0705321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo EM, Winslow MM, Cante-Barrett K, Radermacher AN, Ho L, McGinnis L, Iritani B, Neilson JR, Crabtree GR. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 38.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein kinase C-theta-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 39.Huang F, Kitaura Y, Jang I, Naramura M, Kole HH, Liu L, Qin H, Schlissel MS, Gu H. Establishment of the major compatibility complex-dependent development of CD4+ and CD8+ T cells by the Cbl family proteins. Immunity. 2006;25:571–581. doi: 10.1016/j.immuni.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Jost PJ, Weiss S, Ferch U, Gross O, Mak TW, Peschel C, Ruland J. Bcl10/Malt1 signaling is essential for TCR-induced NF-kappaB activation in thymocytes but dispensable for positive or negative selection. J Immunol. 2007;178:953–960. doi: 10.4049/jimmunol.178.2.953. [DOI] [PubMed] [Google Scholar]
- 41.Meichle A, Schutze S, Hensel G, Brunsing D, Kronke M. Protein kinase C-independent activation of nuclear factor kappa B by tumor necrosis factor. J Biol Chem. 1990;265:8339–8343. [PubMed] [Google Scholar]
- 42.Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003;192:64–79. doi: 10.1034/j.1600-065x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hare KJ, Jenkinson EJ, Anderson G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J Immunol. 2000;165:2410–2414. doi: 10.4049/jimmunol.165.5.2410. [DOI] [PubMed] [Google Scholar]
- 45.Wen R, Wang D, McKay C, Bunting KD, Marine JC, Vanin EF, Zambetti GP, Korsmeyer SJ, Ihle JN, Cleveland JL. Jak3 selectively regulates Bax and Bcl-2 expression to promote T-cell development. Mol Cell Biol. 2001;21:678–689. doi: 10.1128/MCB.21.2.678-689.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 48.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 49.Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 50.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 51.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 52.Hii CS, Costabile M, Mayne GC, Der CJ, Murray AW, Ferrante A. Selective deficiency in protein kinase C isoenzyme expression and inadequacy in mitogen-activated protein kinase activation in cord blood T cells. Biochem J. 2003;370:497–503. doi: 10.1042/BJ20021122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.del Rio R, Rincon M, Layseca-Espinosa E, Fierro NA, Rosenstein Y, Pedraza-Alva G. PKCtheta is required for the activation of human T lymphocytes induced by CD43 engagement. Biochem Biophys Res Commun. 2004;325:133–143. doi: 10.1016/j.bbrc.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Nika K, Charvet C, Williams S, Tautz L, Bruckner S, Rahmouni S, Bottini N, Schoenberger SP, Baier G, Altman A, Mustelin T. Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase C theta-mediated phosphorylation. Mol Cell Biol. 2006;26:1806–1816. doi: 10.1128/MCB.26.5.1806-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Despouy G, Joiner M, Le Toriellec E, Weil R, Stern MH. The TCL1 oncoprotein inhibits activation-induced cell death by impairing PKC{theta} and ERK pathways. Blood. 2007;110:4406–4416. doi: 10.1182/blood-2006-11-059501. [DOI] [PubMed] [Google Scholar]
- 56.Ochoa-Garay J, Kaye J, Coligan JE. Nuclear factor kappaB is required for peptide antigen-induced differentiation of a CD4+CD8+ thymocyte line. J Immunol. 1998;160:3835–3843. [PubMed] [Google Scholar]
- 57.Hettmann T, Leiden JM. NF-kappa B is required for the positive selection of CD8+ thymocytes. J Immunol. 2000;165:5004–5010. doi: 10.4049/jimmunol.165.9.5004. [DOI] [PubMed] [Google Scholar]
- 58.Raman V, Blaeser F, Ho N, Engle DL, Williams CB, Chatila TA. Requirement for Ca2+/calmodulin-dependent kinase type IV/Gr in setting the thymocyte selection threshold. J Immunol. 2001;167:6270–6278. doi: 10.4049/jimmunol.167.11.6270. [DOI] [PubMed] [Google Scholar]
- 59.Willoughby JE, Costello PS, Nicolas RH, Robinson NJ, Stamp G, Powrie F, Treisman R. Raf signaling but not the ERK effector SAP-1 is required for regulatory T cell development. J Immunol. 2007;179:6836–6844. doi: 10.4049/jimmunol.179.10.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]


