Abstract
The Human Embryonic Kidney (HEK) 293 cell line is widely used in cell biology research. Although HEK293 cells have been meticulously studied, our knowledge about endogenous G protein-coupled receptors (GPCR) in these cells is incomplete. While studying the effects of bradykinin (BK), a potent growth factor for renal cells, we unexpectedly discovered that BK activates extracellular signal-regulated protein kinase 1 and 2 (ERK) in HEK293 cells. Thus, we hypothesized that HEK293 cells possess endogenous BK receptors. RT-PCR demonstrated the presence of mRNAs for BK B1 and BK B2 receptors in HEK293 cells. Western blotting with BK B1 and BK B2 receptor antibodies confirmed this result at the protein level. To establish that BK receptors are functional, we employed fluorescent measurements of intracellular Ca2+, measured changes in extracellular acidification rate (ECAR) as a reflection of the Na+/H+ exchange (NHE) with a Cytosensor™ microphysiometer, and assessed ERK activation by Western blotting with a phospho-specific ERK antibody. Exposure of HEK293 cells to BK produced a concentration-dependent rise in intracellular Ca2+ (EC50 = 36.5 ± 8.0 10−9 M), a rapid increase in tyrosine phosphorylation of ERK (EC50 = 9.8 ± 0.4 10−9 M), and elevation in ECAR by ~20%. All of these signals were blocked by HOE-140 (B2 receptor antagonist) but not by des-Arg10-HOE-140 (B1 receptor antagonist). We conclude that HEK293 cells express endogenous functional BK B2 receptors, which couple to the mobilization of intracellular Ca2+, increases in ECAR and increases in ERK phosphorylation.
Keywords: G protein-coupled receptors, signal transduction, intracellular Ca2+, Na+/H+ exchange, extracellular signal-regulated protein kinase 1 and 2
1. INTRODUCTION
The Human Embryonic Kidney (HEK293) cell line was generated by adenoviral transformation of cultured normal human embryonic kidney cells in the early 1970s [1], and have become very widely used in cell biology research. This cell line has been used extensively as an expression tool for recombinant proteins, mainly due to its ease of propagation and maintenance, high efficiency of transfection and protein production, and correct translation and processing of proteins [2]. The bradykinin B1 (BK B1) and bradykinin B2 (BK B2) receptor subtypes are prototypical G-protein-coupled receptors (GPCRs) [3,4] that mediate the actions of kinins. The endogenous intrarenal kinin hormone, BK, plays a significant role as a modulator of a renal function acting as a vasodilator [5]. In addition to its vasoactive properties, BK plays roles in renal cell growth and proliferation [6,7,8]. Therefore, studies on BK receptors are imperative to better understanding of the molecular mechanisms by which BK affects renal cells.
Multiple studies on the structural and functional properties of the BK B2 receptor have been performed in the HEK293 cell model. Indeed, HEK293 cells transfected with green fluorescent protein (GFP)-tagged BK B2 receptors have been used to study BK B2 receptor endocytosis, recycling and down-regulation [9], and nuclear localization [10]. The agonist-promoted trafficking of human BK B1 and BK B2 receptors also was analyzed in HEK293 cells transfected with wild type and GFP-tagged receptors [11]. HEK293 cells transfected with cDNAs for wild-type BK B2 receptor and series of mutants with truncated COOH-terminal tail, with deletion of integral regions of the COOH-terminal tail, and with serine and/or threonine point mutations were used to study the role of the COOH-terminal tail of the human renal BK B2 receptor in receptor internalization [12]. Although most of those studies did not show results using non-transfected HEK293 cells, one study did show a lack of responsiveness to BK [13]. Therefore, we did not expect that HEK293 cells express endogenous BK B2 receptors, and chose this model as a “negative control” while studying the mechanisms of BK-induced signaling in kidney cells. To our surprise, we discovered that non-transfected HEK293 cells respond to BK. The expression of functional endogenous BK receptors in HEK293 cells was not established, although a microarray analysis detected the presence of mRNA specific for the BK B2 receptor, as well as many other GPCRs [14]. Because we are interested in studying signaling pathways that lead to mitogenic effects of BK in kidney cells [8,15], and because the presence of endogenous BK receptors in HEK293 cells could confound transfection studies, we determined whether BK has effects in non-transfected HEK293 cells.
The current study supports the presence of the functional endogenous BK B2 receptors in HEK293 cells. These results should be taken into consideration when performing experiments that involve HEK293 cells over-expressing wild type or mutant BK receptors since constitutively expressed endogenous proteins can interfere with exogenous protein function.
2. MATERIALS AND METHODS
2.1. Materials
Tetramethylammonium (TMA) chloride, probenecid, ethidium bromide, bradykinin, des-Arg9 BK, HOE-140, des-Arg10-HOE-140, and various salts were from Sigma (St Louis, MO). 5-(N-methyl-N-isobutyl)-amiloride (MIA) was purchased from RBI (Natick, MA). U-73122 (1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole 2,5-dione) and U-73343 (1-[6-[((17β)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-2,5-pyrrolidinedione) were from Calbiochem (Gibbstown, NJ). BK B1 antibody was from Abcam Inc., (Cambridge, MA) and BK B2 antibody was from BD Transduction Laboratories™ (Franklin Lakes, NJ). All cell culture media and supplements were from Invitrogen, (Carlsbad, CA). Polycarbonate cell culture inserts for microphysiometry were from Corning Costar (Cambridge, MA), and lysine-coated 96 well clear-bottom black plates needed for the FLIPR were from Greiner Bio-One (Longwood, FL). Fluo-3AM and black pipette tips were from Molecular Devices Corporation (Sunnyvale, CA).
2.2. Cell Culture
HEK293 cells were obtained from American Type Culture Collection (Rockville, MD). Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2.
2.3. RT-PCR
Total RNA was isolated using the SAT-60 reagent (Tel-Test Inc., Friendswood, TX) and RT-PCR was performed using the one step RT-PCR kit (Qiagen, Valencia, CA). Both methods were performed following the manufacturer’s instructions. Specific sequences for sense (S) and antisense (AS) primers for BK B1 and BK B2 receptors that were previously published [16], were synthesized (IDT Technologies Inc., Coralville, IA). Primers for BK B1 receptor were: 5′-TTC TTA TTC CAG GTG CAA GCA G-3′ (S) and 5′-CTT TCC TAT GGG ATG AAG ATA T-3′ (AS), yielding a 213 bp fragment. Primers for BK B2 receptor were: 5′-TGC TGC TGC TAT TCA TCA TC-3′ (S) and 5′-CCA GTC CTG CAG TTT GTG AA-3′ (AS), yielding a 335 bp product. GAPDH primer sets, 5′-ACC ACA GTC CAT GCC ATC AC-3′ (S) and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (AS), yielding a 452 bp product were obtained from IDT Technologies Inc.(Coralville, IA) and used as an internal control. PCR conditions for both BK B1 and BK B2 primer sets were: cDNA synthesis at 50°C for 30 min and 95°C for 15 min (one cycle), followed by 94°C for 30 s, 52.4°C for 30 s, and 72°C for 1 min during 35 cycles, and 72°C for 10 min (one cycle). Amplified products were separated by electrophoresis on 4–12% polyacrylamide gel (Invitrogen, Carlsbad, CA), and visualized following staining with ethidium bromide (1 μg/ml) under UV with Fluorochem Image (Alpha Innotech corp., San Leandro, CA).
2.4. Measurement of Intracellular Ca2+
We used a FLIPR (Molecular Devices Corporation, Sunnyvale, CA) Fluorometric Imaging Plate Reader [17] to non-ratiometrically measure increases in intracellular free calcium as previously described [18], with the use of the calcium-sensitive dye Fluo-3AM. Cells were plated into lysine-coated 96-well clear-bottom black plates at a density of 40,000 cells/well. Subsequent to reaching confluence, cells were deprived of serum for either 4 hours or overnight (0.1% BSA/MEM). Cells were incubated with Fluo-3AM (“No Wash”, Molecular Devices) according to manufacturer’s directions. At the end of the incubation, cells were placed into the FLIPR, and exposed to various concentrations of BK (10−9 to 10−5 M). Increases in intracellular free calcium were reflected by increases in detected fluorescence. Calculations for EC50 were made from log-logit transformations of obtained concentration response curves.
2.5. Microphysiometry
Extracellular acidification rates (ECAR) were measured in real time in intact cells placed in an eight chamber Cytosensor™ microphysiometer (Molecular Devices Corporation, Sunnyvale, CA) as described previously [19,20]. The microphysiometer uses a light-addressable silicon sensor to detect extracellular protons, which can be derived primarily from Na+/H+ exchange and glycolysis, and from other metabolic pathways [21]. Rate data transformed by a personal computer running Cytosoft™ version 2.0 (Molecular Devices Corporation, Sunnyvale, CA) were presented as ECAR in μVolts/sec, which roughly correspond to millipH units/min. In order to facilitate comparison of data between two channels, values were expressed as percent increases of the baseline as determined by computerized analysis of the three data points after exposure of the cell monolayers to test substances.
The day prior to experimentation, HEK293 cells were plated onto polycarbonate membranes (3 micron pore size, 12 mm size) at a density of 300,000 cells per insert. The day of the study, cells were washed with serum-free, bicarbonate-free Ham’s F-12 medium, placed into the microphysiometer chambers, and perfused at 37°C with the same medium or with balanced salt solutions containing NaCl or TMA substituted mM/mM for sodium. The pump cycle was set to perfuse cells for 60 seconds, followed by a 30 second “pump-off” phase, during which proton efflux was measured from the sixth through the 28th seconds. Cells were exposed to the test agents for four cycles (360 sec). Valve switches (to add or remove test agents) were performed at the middle of the pump cycle. Data points were then acquired every 90 seconds. The peak effects during stimulation were expressed as percentage increases from average basal ECAR from five rate measurements prior to application of the test agent(s).
2.6. ERK Assays
ERK phosphorylation was assessed using a phosphorylation state specific ERK antibody (Cell Signaling, Beverly, MA), which specifically recognizes threonine202 and tyrosine204-phosphorylated ERK as previously described [8,15]. Briefly, cells were cultured in 12-well plates, serum starved for 4 hours, and stimulated with vehicle or BK. After treatment, cells were scraped into Laemmli buffer, and subjected to SDS-PAGE using 4–20% pre-cast gels (Invitrogen, Carlsbad, CA), and semi-dry transferred to PVDF membranes (Millipore Corporate Headquarters, Billerica, MA). The membranes were blocked with a BLOTTO buffer and incubated with the phospho-ERK antibody (at 1:1000 dilutions), followed by incubation with goat anti-rabbit alkaline phosphatase-conjugated IgG (Chemicon International, Inc., Temecula, CA). Immunoreactive bands were visualized by a chemiluminescent method (CDP Star™, New England Biolabs, Ipswich, MA) using pre-flashed Kodak X-AR film, and quantified using a GS-670 densitometer and Molecular Analyst software (Bio-Rad Life Science Research, Hercules, CA). The membranes were stripped using Re-Blot Plus antibody stripping solution (Chemicon International, Inc., Temecula, CA) and re-probed with the control ERK antibody (Cell Signaling, Beverly, MA), which recognizes equally well phosphorylated and non-phosphorylated ERK to quantify total ERK. Results are presented as intensities of phospho-ERK bands relative to total ERK bands and expressed as percent of control phosphorylation (non-treated cells).
2.7. Data Analysis
ERK assays were performed in duplicate and repeated at least three times. Data are presented as mean ± S.E.M. (standard error of the mean) and were analyzed for repeated measures by unpaired 2-tailed Student’s t-test. Differences were considered significant at p< 0.05.
3. RESULTS
3.1. HEK293 cells express endogenous BK B1 and B2 receptors
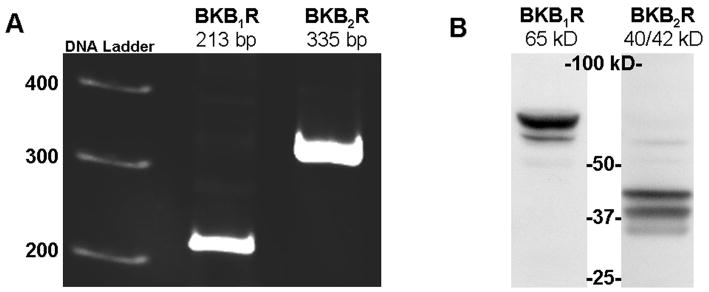
Total RNA extracted from HEK293 cells was subjected to RT-PCR using specific primers for BK B1 and BK B2 mRNA. As shown in Figure 1A, products with the expected sizes of 213 bp for BK B1 receptor, and of 335 bp for BK B2 receptor were detected. To confirm the expression of BK receptors on the protein level, we performed Western blotting of HEK293 lysates with BK B1 and BK B2 receptors antibodies. Western blot analysis shows a major band at 65 kDa that is immunoreactive for BK B1 receptor, as well as a duplet at 40/42 kDa immunoreactive for BK B2 (Figure 1B).
Figure 1.
HEK293 cells express BK B1 and BK B2 receptors. Panel A. RT-PCR demonstrates the presence of BK B1 and BK B2 receptors mRNA in HEK293 cells. Panel B. Western blot analyses of HEK293 cell lysates (40 μg of total protein) with BK B1 and with BK B2 receptor antibodies support the expression of BK receptors on a protein level. Antibodies were used at 1:1000 dilution according to the manufacture’s recommendations.
3.2. BK increases intracellular Ca2+ through a BK B2 receptor in HEK293 Cells
We used a FLIPR™ to simultaneously measure intracellular Ca2+ in HEK293 cells plated into 96-well microtitre plates. Figure 2A shows representative raw data from a single experiment demonstrating that BK induced a rapid and sustained elevation of intracellular Ca2+ in HEK293 cells pre-loaded with 2 μM Fluo-3 AM. The BK signal was dependent on concentration, with a half-maximal effect (EC50) at 36.5 ± 8.0 10−9 M (n=8) (Figure 2B). Pre-incubation with the specific phospholipase C (PLC) inhibitor, U-73122 (10−5 M for 30 minutes) completely prevented BK-induced intracellular Ca2+ mobilization supporting that PLC activation is required for BK-induced Ca2+ signal. Pre-incubation with 10−5 M of HOE-140 (BK B2 receptor antagonist) completely eliminated the Ca2+ signal, whereas pre-incubation with the BK B1 receptor antagonist, des-Arg10-HOE-140 (10−5 M), had no effect (Figure 2C) providing strong evidence that the effect is mediated by BK B2 (and not BK B1) receptors.
Figure 2.
BK induces elevations in intracellular free Ca2+ in HEK293 cells via BK B2 receptor subtype. Panel A. Representative raw data from two wells showing changes in Fluo-3 AM fluorescence in response to 10−7 M BK. Cells pretreated with 10−5 M U-73122 for 30 minutes prior to addition of BK did not respond. Panel B. Concentration-response curve for BK-induced elevations in intracellular free Ca2+. HEK293 cells were loaded with the intracellular fluorescent Ca2+ probe Fluo-3 AM and exposed to the indicated concentrations of BK in the absence or presence of the BK B2 receptor antagonist HOE-140 (10−5 M) or the specific PLC inhibitor U-73122 (10−5 M). Calcium fluxes were measured using the FLIPR to detect changes in Fluo-3 AM fluorescence as described under Methods. Values are average from studies done in triplicate ± S.E.M. n = 8 (BK alone); n = 5 (BK + HOE-140); n = 3 (BK + U-73122). RFU - relative fluorescence units. Panel C: Maximum fluorescence stimulated by BK (10−7 M) in the absence or presence of the BK B1 receptor antagonist des-Arg10-HOE-140, or the BK B2 receptor antagonist HOE-140, or the specific PLC inhibitor, U-73122. Cells were preincubated with 10−5 M of each BK receptor antagonist for 15 minutes, or with 10−5 M of U-73122 for 30 minutes prior to addition of BK. Presented are average values from at least 7 experiments (BK in the presence or absence of BK receptor antagonists) and from 3 experiments (BK + U-73122), all performed in triplicate. ‡ P < 0.05 vs. BK alone; * P < 0.05 vs. BK alone. Error bars represent the S.E.M.
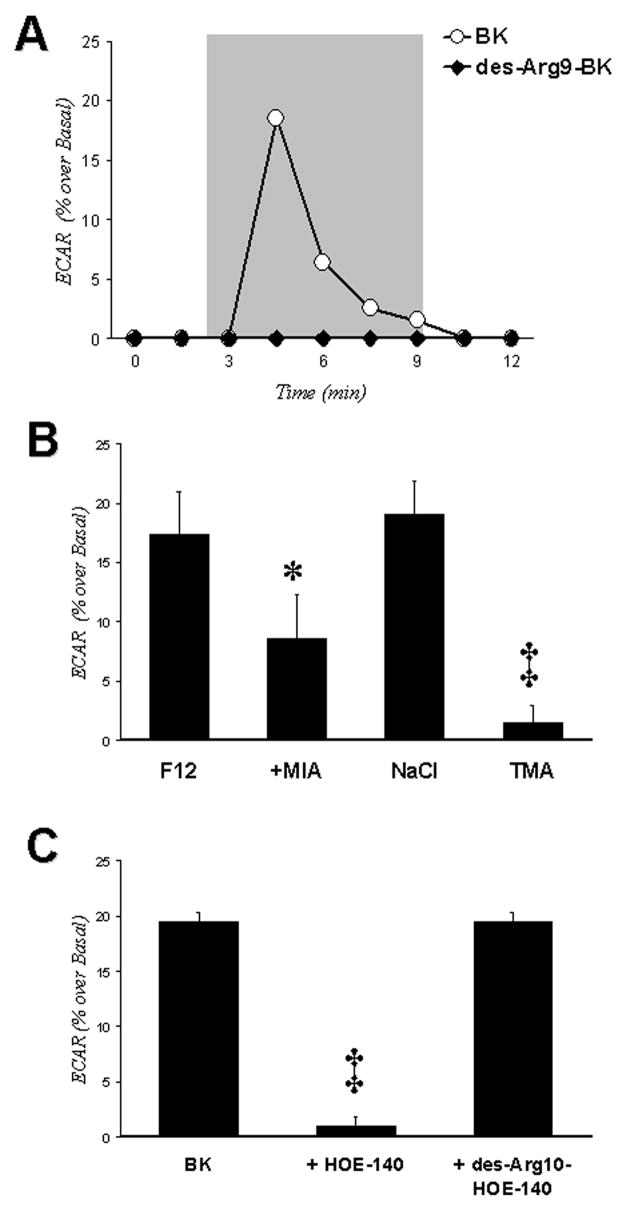
3.3. BK stimulates a sodium-dependent proton efflux in HEK293 cells through a BK B2 receptor
Figure 3A shows that cells treated with 10−6 M BK (open circles) had a rapid increase in extracellular acidification rates that did not occur when cells were exposed to the 10−6 M of BK B1 receptor agonist, des-Arg9 BK (black circles). Figure 3B shows that the stimulatory effects of BK occurred in sodium-containing Ham’s F12 medium or in sodium containing balanced salt solution, but not in a solution in which tetramethylammonium (TMA) was substituted for sodium. The effect could be blocked partially (~50%) by 10−5 M of 5-(N-methyl-N-isobutyl)-amiloride (MIA), an inhibitor of sodium-proton exchangers types 1 and 2 (NHE-1 and -2). Figure 3C shows studies in which two specific BK receptor antagonists were examined for inhibition of BK-stimulated ECAR in HEK293 cells. These studies showed that the receptor is BK B2 receptor because proton efflux was blocked by the BK B2 receptor antagonist HOE-140, but not by the BK B1 receptor antagonist des-Arg10-HOE-140. Thus, Figure 3 presents evidence that the BK B2 receptor in HEK293 cells activates proton efflux through stimulation of a NHE.
Figure 3.
BK stimulates ECAR in HEK293 cells. Panel A. ECAR measurements were obtained as described in Methods. BK (white circles) stimulates ECAR, whereas the BK B1 receptor agonist des-Agr9-BK (dark circles) does not. Cells were exposed to perfusate containing drug during the time span encompassed by gray box. Panel B. ECAR stimulated by 10−6 M of BK in various buffers, including Ham’s F12 medium, without and with 10−5 M MIA, a balanced salt solution containing NaCl or TMA substituted mM per mM for sodium. * P < 0.05 vs. BK alone; ‡ P < 0.01 vs. BK in balanced salt solution with NaCl. Panel C. Effects of BK B2 (HOE-140) and BK B1 (des-Arg10-HOE-140) receptor antagonists on BK-stimulated ECAR. Antagonists (10−5 M) were added 30 min prior to addition of BK. All experiments were performed at least 4 times. ‡ P < 0.01 vs. BK alone. Error bars in Panels B and C represent the S.E.M.
3.4. Activation of ERK by BK
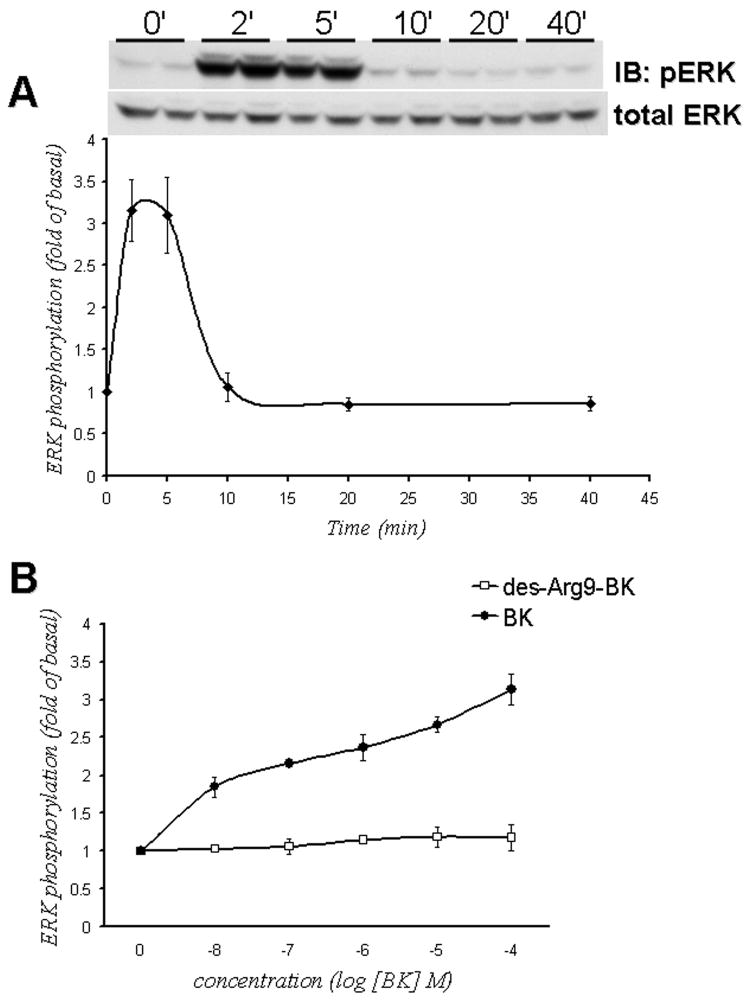
Treatment of HEK293 cells with 10−6 M of BK resulted in a time-dependent increase in threonine/tyrosine phosphorylation of ERK (Figure 4A). BK treatment produced a 2- to 3-fold increase in ERK phosphorylation over basal activity. ERK phosphorylation peaked at 2–5 minutes, and returned to the basal levels after 10 minutes. BK stimulated ERK activation in a concentration-dependent manner with a half-maximal effect (EC50) at 9.8 ± 0.4 10−9 M (n=5). At the same time, the selective BK B1 receptor agonist, des-Arg9 BK was not able to stimulate ERK phosphorylation suggesting that BK B1 receptor probably does not couple to activation of ERK (Figure 4B).
Figure 4.
BK activates ERK in HEK293 cells. Panel A. BK induces ERK phosphorylation in a time-dependent manner. HEK293 cells were stimulated with 10−6 M BK for the indicated periods of time. ERK phosphorylation was measured by immunoblotting with anti-phospho-ERK antibodies as described in Methods. Data points represent intensities of phospho-ERK bands relative to total-ERK expressed as percent of control (samples without BK treatment). Inset shows a representative immunoblot (phospho-ERK). The same blot was stripped and re-probed with antibodies for total ERK that recognize ERK independently of phosphorylation state to assure an equal loading of a protein samples on a gel (total ERK). Panel B. Concentration-dependence of BK-induced ERK phosphorylation. HEK293 cells were stimulated with the indicated concentrations of BK or des-Agr9-BK for 5 minutes. Data points represent intensities of phospho-ERK bands relative to total ERK measured by densitometry and expressed as percent of control (samples without BK treatment). Experiments were performed at least 3 times. Data are shown as mean+/− S.E.M.
3.5. BK stimulates ERK through a BK B2 receptor
HEK293 cells were pretreated for 30 minutes with the BK B1 receptor antagonist des-Arg10-HOE-140 (10−5 M) or with the BK B2 receptor antagonist HOE-140 (10−5 M), and then stimulated with 10−6 M of BK for 5 minutes. Figure 5 shows that pretreatment with HOE-140 prevented the BK-induced activation of ERK, whereas des-Arg10-HOE-140 was without effect. Thus, BK stimulates ERK activity via BK B2 receptor.
Figure 5.
BK-induced ERK phosphorylation is mediated by BK B2 receptor. HEK293 cells were pretreated for 30 minutes with the BK B1 receptor antagonist des-Arg10-HOE-140 (10−5 M) or with the BK B2 receptor antagonist HOE-140 (10−5 M), then were stimulated with 10−6 M of BK for 5 minutes. ERK phosphorylation was detected by immunoblotting with anti-phospho-ERK antibodies as described in Methods. Bars represent intensities of phospho-ERK relative to total ERK expressed as percent of control (cells without BK treatment) phosphorylation. Experiments were performed 3 times in duplicates. Data are presented as mean+/− S.E.M; ** P < 0.01 vs. control; ‡ P < 0.01 vs. BK alone. Inset shows a representative phospho-ERK blot and the same blot, stripped and re-probed with antibody for total ERK.
4. DISCUSSION
What is new about this work is that we unexpectedly have demonstrated the presence of endogenous functional BK B2 receptors in HEK293 cells. We also detected the presence of mRNA specific for the BK B1 receptor in these cells.
Although an extensive microarray study in HEK293 cells by Shaw et al. suggested the presence of mRNA for 28 GPCRs, including mRNA specific for the BK B2 receptor [14], we did not come across any reports in the literature of functional endogenous BK receptors in HEK293 cells. Possible explanations for this discrepancy are that HEK293 cells either do not express endogenous BK receptors on a protein level or do not provide accessory proteins required for BK receptors to be functional.
While studying the mechanism of BK-induced signaling, we unexpectedly discovered that BK activates ERK in non-transfected HEK293 cells. After having verified our results in several separate batches of HEK293 cells, we hypothesized that these cells posses endogenous BK receptors. We found the presence of BK B1 and BK B2 receptor mRNAs in cultured HEK293 cells, and demonstrated the presence of these receptors on the protein level by Western blotting (Figure 1). We chose to investigate whether these receptors are functional, and tested three signaling pathways that are known to be stimulated by BK in other cell types. The BK B2 receptor usually couples to the GTP-binding protein Gq, and stimulates phospholipase C activity, causing phosphoinositide hydrolysis, that leads to generation of inositol triphosphate and to subsequent mobilization of intracellular Ca2+ from the endoplasmic reticulum [22, 23]. BK-induced intracellular Ca2+ mobilization has been described in several kidney cell lines including rat mesangial cells [24], the mIMCD-3 murine inner medullary collecting duct cell line [20], Kirsten murine sarcoma-virus transformed rat kidney KNRK cells, [25] and the TKPTS mouse proximal tubule epithelial cell line [26]. BK produced a concentration-dependent elevation of intracellular Ca2+ in HEK293 cells (Figure 2) with a EC50 value of 36.5 ± 8.0 10−9 M which is somewhat higher than EC50 values reported for other renal cell lines that demonstrate nanomolar potency of BK for stimulating Ca2+ mobilization [20,24,25,26]. Intracellular Ca2+ mobilization was completely blocked by the specific phospholipase C (PLC) inhibitor, U-73122 supporting the requirement for PLC activation in the BK-induced Ca2+ signal. The response to BK was blocked by the BK B2 receptor antagonist (HOE-140), but not by the BK B1 receptor antagonist des-Arg10-HOE-140, indicating a role of the BK B2 receptor. This was confirmed by the inability of the selective BK B1 receptor agonist des-Arg9-BK to stimulate Ca2+ mobilization in HEK293 cells (data not shown).
Next we decided to test potential roles for BK receptors in the regulation of sodium-proton exchange in HEK293 cells, because BK B2 receptors have been shown to activate sodium-proton exchange in mIMCD-3 cells [20], KNRK cells [25], and in renal tubular epithelial cells [27]. In our study, HEK293 cells treated with 10−6 M BK had a rapid increase in ECAR as measured by microphysiometry (Figure 3). The burst of Na+-dependent H+ efflux in response to BK was partially blocked by preincubation with 10−5 M MIA, which is an inhibitor of NHE-1 and NHE-2. Because MIA was able to block only ~50% of BK-induced increase in ECAR, we cannot exclude roles for other isoforms of NHE besides NHE-1 and/or NHE-2. The selective BK B1 receptor agonist des-Arg9-BK was not able to stimulate ECAR, and the response to BK was blocked by the BK B2 receptor antagonist (HOE-140), but not by the BK B1 receptor antagonist des-Arg10-HOE-140, supporting a role of the BK B2 receptor.
Activation of ERK by BK in kidney cells has been demonstrated for cultured mesangial cells [6,7], rabbit cortical collecting duct cells [28], and mIMCD-3 cells [8,15]. In the current study we showed that in HEK293 cells, BK induces time- and concentration-dependent threonine/tyrosine phosphorylation of ERK, which peaked at 2–5 minutes after BK application (Figure 4). A half-maximal effect (EC50) for BK-induced ERK activation was at 9.8. ± 0.4 10−9 M, which was comparable to EC50 values or estimates reported for BK-induced ERK activation in cultured mesangial [6,7] and mIMCD-3 cells [8]. BK-induced ERK activation was blocked by a BK B2 bradykinin receptor antagonist but not by a BK B1 receptor antagonist, indicating that BK activates ERK via a BK B2 receptor (Figure 5).
In this study we provide evidence that BK B2 receptor in HEK293 cells is functionally coupled to PLC-dependent calcium mobilization, increases in sodium-dependent proton efflux, and ERK phosphorylation. Because the pharmacological evidence does not support a role for BK B1 receptor in stimulation the tested pathways, the functions of the BK B1 receptor in HEK293 cells remains unclear. One possibility is that this receptor couples to some other signaling pathways as it has been described in smooth muscle cells, e.g. eicosanoid production [29] or arachidonic acid release [30]. Another possible function of the BK B1 receptor in HEK293 cells could be a crosstalk between BK B1 and BK B2 receptors. In that regard, formation of a complex between BK B1 and BK B2 receptors has been described in prostate cancer PC3 cells [31], and in HEK293 cells transfected to over-express these receptors [32].
In summary, our data support the presence of endogenous BK B1 and BK B2 receptors in HEK293 cells. These results should be taken under consideration because the HEK293 cell line is widely used as a model for studying signaling enzymes, ion channels, and transfected receptors including BK receptors. Thus, HEK293 cells transfected with the BK B2 receptor cDNAs (wild type, mutant, and GFP conjugates) have been used to study BK B2 receptor internalization [12], sequestration [33], endocytosis, recycling and down-regulation [9], nuclear localization [10], and the agonist-promoted trafficking [11]. Faussner et al. compared HEK293 cells and Chinese hamster ovary (CHO) cells expressing wild-type BK B2 receptors, and demonstrated that ligand-induced receptor-specific sequestration and internalization strongly depends on the expression level of receptors [33]. Interestingly, the authors mentioned that they were not able to produce HEK293 cells with low BK B2 receptor expression [33]. A possible explanation would be the presence of endogenous BK B2 receptors in HEK293 cells. Therefore, it is essential that HEK293 cells should be assessed prior to performing experiments that involve over-expression of wild type or mutant BK receptors. Because constitutively expressed endogenous proteins can interfere with exogenously expressed protein function, it is imperative to control the level of expression and activity of endogenous BK B1 and BK B2 receptors in HEK293 cells, while performing experiments that involve over-expression of wild type or mutant BK receptors.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (Merit Awards and a REAP Award to MNG and JRR), AHA (GIA 0655445U to MNG), the National Institutes of Health (DK52448 and GM63909 to JRR), and a laboratory endowment jointly supported by the M.U.S.C. Division of Nephrology and Dialysis Clinics, Incorporated (JRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P, Smart TG. HEK293 cell line: A vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Hess JF, Borowski JA, Young GS, Strader CD, Ransom RW. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992;184:260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- 4.Menke JG, Borkowski JA, Bierilo KK, MacNeil T, Derrick AW, Schneck KA, et al. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994;269:21583–21586. [PubMed] [Google Scholar]
- 5.Bagate K, Grima M, Imbs JL, Jong WD, Helwig JJ, Barthelmebs M. Signal transduction pathways involved in kinin B(2) receptor-mediated vasodilation in the rat isolated perfused kidney. Br J Pharmacol. 2001;132:1735–1752. doi: 10.1038/sj.bjp.0704027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Dahr SS, Dipp S, Baricos WH. Bradykinin stimulates the ERK Elk-1 Fos/AP-1 pathway in mesangial cells. Am J Physiol Renal Physiol. 1998;275:F343–F352. doi: 10.1152/ajprenal.1998.275.3.F343. [DOI] [PubMed] [Google Scholar]
- 7.Jaffa AA, Miller BS, Rosenzweig SA, Naidu PS, Velarde V, Mayfield RK. Bradykinin induces tubulin phosphorylation and nuclear translocation of MAP kinase in mesangial cells. Am J Physiol Renal Physiol. 1997;273:F916–F924. doi: 10.1152/ajprenal.1997.273.6.F916. [DOI] [PubMed] [Google Scholar]
- 8.Mukhin YV, Garnovsky EA, Ullian ME, Garnovskaya MN. Bradykinin B2 receptor activates extracellular signal-regulated protein kinase in mIMCD-3 cells via epidermal growth factor receptor transactivation. J Pharmacol Expt Ther. 2003;304:968–977. doi: 10.1124/jpet.102.043943. [DOI] [PubMed] [Google Scholar]
- 9.Bacharov DR, Houle S, Bacharova M, Bouthillier J, Adam A, Marceau F. Bradykinin B2 receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Expt Ther. 2001;297:19–26. [PubMed] [Google Scholar]
- 10.Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, et al. Agonist-independent nuclear localization of the apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 11.Lamb ME, de Weerd WFC, Leeb-Lundberg LMF. Agonist-promoted trafficking of human bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in HEK293 cells. Biochem J. 2001;355:741–750. doi: 10.1042/bj3550741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizard A, Blaukat A, Muller-Esterl W, Alhenc-Gelas F, Rajerison RM. Bradykinin-induced internalization of the human B2 receptor requires phosphorylation of three Serine and two Threonine residues at its carboxyl tail. J Biol Chem. 1999;274:12738–12747. doi: 10.1074/jbc.274.18.12738. [DOI] [PubMed] [Google Scholar]
- 13.Houle S, Molinaro G, Adam A, Marceau F. Tissue Kallikrein actions at the rabbit natural or recombinant kinin B2 receptors. Hypertension. 2003;41:611–617. doi: 10.1161/01.HYP.0000054971.03046.9B. [DOI] [PubMed] [Google Scholar]
- 14.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK293 cells. FASEB Journal. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 15.Mukhin YV, Gooz M, Raymond JR, Garnovskaya MN. Collageneses 2 and 3 mediate epidermal growth factor receptor transactivation by Bradykinin B2 receptor in kidney cells. J Pharmacol Expt Ther. 2006;318:1033–1043. doi: 10.1124/jpet.106.104000. [DOI] [PubMed] [Google Scholar]
- 16.Bertram CM, Baltic S, Misso NL, Bhoola KD, Foster PS, Thompson PJ, et al. Expression of kinin B1 and B2 receptors in immature, monocyte-derived dentritic cells and bradykinin-mediated increase in intracellular Ca2+ and cell migration. J Leukoc Biol. 2007;81:1445–1454. doi: 10.1189/jlb.0106055. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder KS, Neagle BD. FLIPR: A New Instrument for Accurate, High Throughput Optical Screening. J Biomol Screen. 1996;1:75–80. [Google Scholar]
- 18.Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee M, Luttrell L, Ullian ME. Identification of a putative nuclear localization sequence within ANG II AT1A receptor associated with nuclear activation. Am J Physiol Cell Physiol. 2007;292:C1398–C1408. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- 19.Garnovskaya MN, Gettys TW, van Biesen T, Chuprun JK, Prpic V, Raymond JR. G protein-coupled 5-HT1A receptor activates Na+/H+ exchange in CHO-K1 cells through Gia2 and Gia3. J Biol Chem. 1997;272:7770–7776. doi: 10.1074/jbc.272.12.7770. [DOI] [PubMed] [Google Scholar]
- 20.Mukhin YV, Vlasova T, Jaffa AA, Collinsworth G, Bell JL, Tholanikunnel BG, et al. Bradykinin B2 receptors activate Na+/H+ exchange in mIMCD-3 cells via Janus kinase 2 and Ca2+/calmodulin. J Biol Chem. 2001;276:17339–17346. doi: 10.1074/jbc.M010834200. [DOI] [PubMed] [Google Scholar]
- 21.McConnell HM, Owicki JC, Parce JW, Miller DL, Baxter GT, Wada HG, et al. The cytosensor microphysiometer: biological applications of silicon technology. Science. 1992;257:1906–1912. doi: 10.1126/science.1329199. [DOI] [PubMed] [Google Scholar]
- 22.Wiernas TK, Davis TL, Griffin BW, Sharif NA. Effects of bradykinin on signal transduction, cell proliferation, and cytokine, prostaglandinE2 and collagenase-1 release from human corneal epithelial cells. Brit J Pharmacol. 1998;123:1127–1137. doi: 10.1038/sj.bjp.0701700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon B, Sharma R, Dickerson T, Fortune J. Bradykinin and angiotensin II: activation of protein kinase C in arterial smooth muscle. Am J Physiol Cell Physiol. 1994;266:C1406–C1420. doi: 10.1152/ajpcell.1994.266.5.C1406. [DOI] [PubMed] [Google Scholar]
- 24.Bascands JL, Emond C, Pecher C, Regoli D, Girolami JP. Bradykinin stimulates production of inositol (1,4,5) triphosphate in cultured mesangial cells of the rat via a B2 receptor. Brit J Pharmacol. 1991;102:962–966. doi: 10.1111/j.1476-5381.1991.tb12284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefler D, Mukhin YV, Pettus T, Leeb-Lundberd LMF, Garnovskaya MN, Raymond JR. Jak2 and Ca2+/calmodulin are key intermediates for bradykinin B2 receptor-mediated activation of Na+/H+ exchange in KNRK and CHO cells. ASSAY and Drug Development Technologies. 2003;1:281–289. doi: 10.1089/15406580360545099. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari MM, Prather PL, Mayeux PR. Mechanism of bradykinin-induced Ca2+ mobilization in murine proximal tubule epithelial cells. J Pharmacol Expt Ther. 2005;313:798–805. doi: 10.1124/jpet.104.080408. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura K, Singer WD, Cano A, Miller RT. G alpha q and G alpha13 regulate NHE-1 and intracellular calcium in epithelial cells. Am J Physiol Cell Physiol. 1995;268:C101–C110. doi: 10.1152/ajpcell.1995.268.1.C101. [DOI] [PubMed] [Google Scholar]
- 28.Lal M, Proulx P, Hebert RL. A role for PKC and MAP kinase in bradykinin-induced arachidonic acid release in rabbit CCD cells. Am J Physiol Renal Physiol. 1998;274:F728–F735. doi: 10.1152/ajprenal.1998.274.4.F728. [DOI] [PubMed] [Google Scholar]
- 29.Levesque L, Larrivée JF, Bachvarov DR, Rioux F, Drapeau G, Marceau F. Regulation of kinin-induced contraction and DNA synthesis by inflammatory cytokines in the smooth muscle of the rabbit aorta. Br J Pharmacol. 1995;116:1673–1679. doi: 10.1111/j.1476-5381.1995.tb16390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tropea MM, Gummelt D, Herzig MS, Leeb-Lundberg LMF. B1 and B2 kinin receptors on cultured rabbit superior mesenteric artery smooth muscle cells: receptor-specific stimulation of inositol phosphate formation and arachidonic acid release by des-Arg9-bradykinin and bradykinin. J Pharmacol Exp Ther. 1993;264:930–937. [PubMed] [Google Scholar]
- 31.Barki-Harrington L, Bookout AL, Wang G, Lamb ME, Leeb-Lundberg LMF, Daaka Y. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem J. 2003;371:581–587. doi: 10.1042/BJ20021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang DS, Ryberg K, Mörgelin M, Leeb-Lundberg LMF. Spontaneous Formation of a Proteolytic B1 and B2 Bradykinin Receptor Complex with Enhanced Signaling Capacity. J Biol Chem. 2004;279:22102–22107. doi: 10.1074/jbc.M402572200. [DOI] [PubMed] [Google Scholar]
- 33.Faussner A, Bauer A, Kalatskaya I, Jochum M, Fritz H. Expression levels strongly affect ligand-induced sequestration of B2 bradykinin receptors in transfected cells. Am J Physiol Heart Circ Physiol. 2003;284:H1892–H1898. doi: 10.1152/ajpheart.01147.2002. [DOI] [PubMed] [Google Scholar]