Summary
Introduction
Tuberculosis (TB) has emerged as a serious public health problem in the country of Georgia. However, there have been little or no data on rates and risk factors for drug resistant TB including multidrug-resistant (MDR)-TB in Georgia.
Objective
To assess the prevalence and risk factors for drug resistant TB.
Methodology
A cross-sectional prospective survey of patients with suspected pulmonary TB was carried out at four sentinel sites (Tbilisi, Zugdidi, Kutaisi, and Batumi) in Georgia to in 2001-2004.
Results
Among 1,422 patients with suspected pulmonary TB, 996 (70.0%) of 1,422 patients were culture positive; 931 (93.5%) of 996 had drug susceptibility testing performed. Overall, 64% of patients (48.3% of new and 85.3% of retreatment cases) had positive cultures for Mycobacterium tuberculosis resistant to ≥1 first line antituberculosis drugs. The overall prevalence of MDR-TB was 28.1% (10.5% of newly diagnosed patients and 53.1% of retreatment cases). In multivariate analysis, risk factors for MDR-TB included: being a retreatment case (prevalence ratio [PR]=5.28, 95% CI 3.95-7.07); history of injection drug use (PR=1.59, 95% CI 1.21-2.09); and female gender (PR=1.36, 95% CI 1.12-1.65).
Conclusion
MDR-TB has emerged as a serious public health problem in Georgia and will greatly impact TB control strategies.
Keywords: Tuberculosis, multidrug-resistance, prevalence
Introduction
Tuberculosis (TB) has emerged as an enormous public health problem in former Soviet republics due to economic decline and the general failure of tuberculosis control and other health services following the break up of the Soviet Union1,2. The country of Georgia, an independent nation that was previously part of the former Soviet Union (Figure 1), has one of the highest rates of tuberculosis among former Soviet republics3,4. In 2005, a total of 6,448 TB cases were reported in Georgia; the incidence and prevalence of TB was 97 cases and 147 cases per 100,000 population, respectively5. In 2003 cohort treatment success rate was 67%, an additional 2% died, 4% failed, and 15% defaulted, 8% were transferred and 4% had missing data.3 The situation in Georgia was exacerbated by a civil war in 1992-1993 following independence, that resulted in several hundred thousand internally displaced persons who have been noted to have high rates of tuberculosis1.
Figure 1. Map of the country of Georgia.
Red dots show the four sentinel sites where the study was conducted (Tbilisi, Zugdidi, Kutaisi, and Batumi)
Despite high rates of TB in Georgia, there have been little or no data on the prevalence of or risk factors for drug resistant tuberculosis. High rates of drug resistant TB have been reported among persons being incarcerated in Georgian prisons in the late 1990s6, but there have been little or no previous data available about rates or risk factors for drug resistant tuberculosis or multi-drug resistant (MDR) tuberculosis (defined as resistance to at least isoniazid and rifampin) among the Georgian civilian population. Drug-resistant TB, especially MDR-TB, is associated with significantly higher morbidity and mortality than drug susceptible disease7-9. Drug resistance rates also reflect current and prior effectiveness of TB control programs10. Drug-resistant TB may threaten TB control efforts by reducing the effectiveness of short-course antituberculosis regimens delivered under the World Health Organization (WHO) recommended directly observed therapy, short course (DOTS) strategy7,11 and by disproportionately absorbing TB control program resources.
The purpose of our prospective study was to assess rates of and risk factors for drug resistant TB, including MDR-TB in Georgia. This information is important for developing effective TB control strategies in Georgia and assessing the need for implementation of the DOTS-Plus strategy for the treatment of MDR-TB12.
Methods
Study Population
A cross-sectional prospective survey was carried out at 4 sentinel sites in Georgia. Patients aged 15 years or older with highly suspected pulmonary tuberculosis (i.e. patients with clinical symptoms and chest radiograph findings suggestive of TB) presenting to four sentinel sites (inpatient facilities in four Georgian cities which included Tbilisi, Zugdidi, Kutaisi and Batumi) between January 1, 2001 and December 31, 2004 in the country of Georgia were eligible for enrollment into the study. In Georgia, patients are offered hospitalization for the intensive phase of TB treatment. Written informed consent was obtained from all patients prior to enrollment. The study was approved by the Georgian National Center for Tuberculosis and Lung Disease (NCTLD) Ethics Committee, the Georgian National Center for Disease Control Ethics Committee, and the Emory University Institutional Review Board (IRB).
Data collection and definitions
Clinical, demographic, and epidemiologic data were collected through interview of patients and review of medical records. Demographic data included patient age, gender, nationality (Georgian, Armenian, Azeri, Russian and other), region of residence, status of an internally displaced person (IDP), incarceration history, employment status, and history of tobacco, alcohol, and injection drug use. In addition, data on comorbid illnesses including hepatitis, HIV infection, diabetes mellitus, gastritis, and peptic ulcer disease was collected. The case status for each patient with TB was recorded (newly diagnosed vs. previously treated) as well as the primary reason for culture examination (initial diagnosis vs. follow-up visit). The study focused primarily on patients with TB who had positive cultures.
New cases were defined as patients who had never had treatment for TB or who received antituberculosis drugs for less than one month; retreatment cases were defined as patients who had a prior history of treatment with antituberculosis drugs for more than one month13. Retreatment cases included relapses, treatment after failure, treatment after default, and chronic cases (i.e., a patient with TB who is sputum-positive at the end of a standard retreatment regimen). Multi-drug resistance (MDR) was defined as resistance to at least both isoniazid and rifampin14. Monoresistance was defined as resistance exclusively to one of the four first-line antituberculosis drugs tested14. Polyresistance was defined as resistance to two or more of the five first-line antituberculosis drugs, but not both isoniazid and rifampin.
Laboratory Methods
Three sputum samples for AFB smear microscopy and culture were obtained from each patient enrolled into the study. Specimens were obtained at each sentinel site and then transported to the Georgian National Reference Laboratory in Tbilisi where AFB cultures were performed with Lowenstein-Jensen media using standard methodologies15. Drug susceptibility testing (DST) to first line antituberculosis drugs (isoniazid, rifampin, ethambutol, and streptomycin) was performed on Mycobacterium tuberculosis isolates using the absolute concentration method16. The concentrations of the antituberculosis drugs tested were as follows: isoniazid 0.1 μg/ml, rifampin 40.0 μg/ml, streptomycin 10.0 μg/ml, and ethambutol 2.0 μg/ml.
Statistical Analysis
All statistical analyses were performed using SAS, version 9.0 (SAS Institute Inc., Cary, NC, USA). Trends in the prevalence of drug resistance over time were assessed using the Chi-square test for trends. Risk factors for having any resistance and multidrug-resistance among culture-confirmed TB cases was assessed. Univariate analysis was performed to determine unadjusted association of TB drug resistance with patients' clinical and demographic characteristics. For dichotomous variables prevalence ratios (PR) with 95% confidence intervals (CI) were calculated using PROC GENMOD in SAS. To obtain the adjusted estimates, two multivariate log-binomial regression models were fitted with DST results (any resistance vs. fully susceptible and MDR-TB vs. non-MDR-TB, respectively) as outcome variables. Variables significantly associated with outcome of interest in univariate analysis as well as potential confounders and effect modifiers based on literature review were included in the final multivariate model. Interaction and confounding were assessed. A p-value of ≤0.05 was considered statistically significant.
Results
A total of 2,212 patients underwent culture examination during the 4-year period. Patients with tuberculosis who had sputum collected at these sites for reasons other than diagnosis of TB (n=607, e.g., follow up specimens for patients undergoing current treatment or unknown reason for sputum examination) were excluded from the analysis. A total of 1,605 patients with suspected tuberculosis who had sputum specimens collected for diagnostic purposes as the primary reason for culture examination at four sentinel sites in Georgia were enrolled into the study. Among the 1,605 patients with suspected TB enrolled into the study, 1,422 (89%) had valid culture results (either positive or negative cultures) and constituted the sample for culture confirmation analysis (Figure 2). Of these 1,422 patients, 996 (70%) had a positive culture for M. tuberculosis. Drug susceptibility testing results were available for 931 (94%) of 996 patients with positive cultures. AFB smear results were available for 1,188 patients with positive or negative culture results; of these 925 (78%) were AFB smear positive, and 263 (22%) were smear negative. A total of 66 patients with negative smear (5.6% of all with available smear results) had positive culture.
Figure 2.
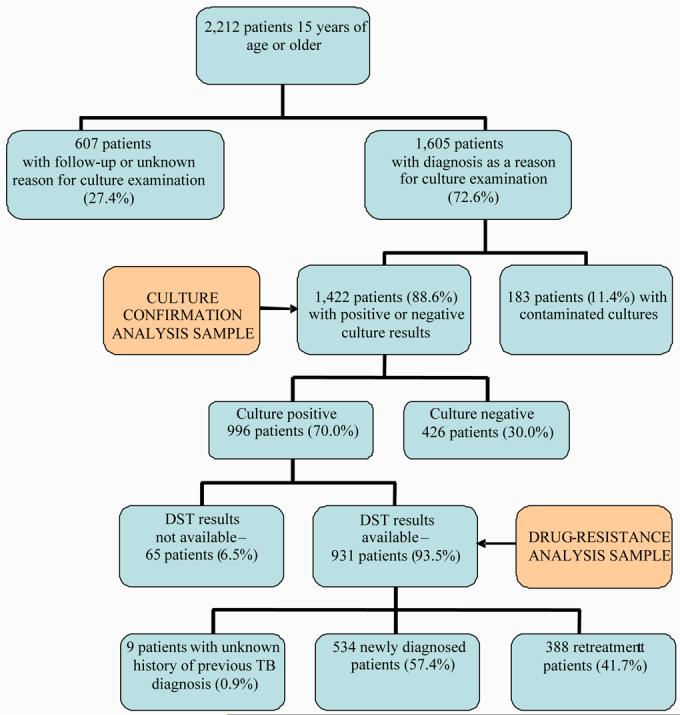
Study sample of patients with suspected pulmonary tuberculosis at four sentinel sites in the country of Georgia
The mean age of the 1,422 patients with culture results was 38 years (range 15-81 years); 73.5% were male, 87.1% had Georgian nationality, and 54.3% resided in Tbilisi (Table 1). The history of previous treatment was known for 1,154 of 1,422 (81.2%) patients, and of those 682 (59.1%) were newly diagnosed cases and 472 (40.9%) were retreatment cases (Table 1). Having a positive culture for M. tuberculosis was significantly associated with male gender and residence outside Tbilisi (Table 1). Patients of age groups 55-64 years and >65 were significantly less likely to have had a positive culture compared to those aged 15-24 years. Patients with unknown treatment history significantly less often had positive cultures, compared to new cases.
Table 1.
Demographic characteristics of suspected pulmonary TB cases at Four Sentinel Sites in Georgia, 2001-2004.
| Variable | All Suspected TB Cases (N=1,422) |
Culture Positive (N=996) |
Culture Negative (N=426) |
PRa (95% CI) |
|---|---|---|---|---|
| Age group | ||||
| 15-24 25-34 35-44 45-54 55-64 >65 |
237 (16.7%) 408 (28.7%) 376 (26.4%) 216 (15.2%) 111 (7.8%) 74 (5.2%) |
174 (17.5%) 301 (30.2%) 285 (28.6%) 146 (14.7%) 65 (6.5%) 25 (2.5%) |
63 (14.8%) 107 (25.1%) 91 (21.4%) 70 (16.4%) 46 (10.8%) 49 (11.5%) |
REF 1.00 (0.91-1.11) 1.03 (0.94-1.14) 0.92 (0.82-1.04) 0.80 (0.67-0.95) 0.46 (0.33-0.64) |
| Gender | ||||
| Male | 1,045 (73.5%) | 749 (75.2%) | 296 (69.5%) | 1.09 (1.01-1.19) |
| Female | 377 (26.5%) | 247 (24.8%) | 130 (30.5%) | REF |
| Case Status | ||||
| New | 682 (48.0%) | 579 (58.1%) | 103 (24.2%) | REF |
| Retreatment | 472 (33.2%) | 408 (41.0%) | 64 (15.0%) | 0.98 (0.94-1.03) |
| Unknown | 268 (18.8) | 9 (0.9) | 259 (60.8) | 0.04 (0.02-0.07) |
| Residence (994 b/417c) | ||||
| Tbilisi | 766 (54.3%) | 513 (51.6%) | 253 (60.7%) | 0.88 (0.84-0.96) |
| Other | 645 (45.7%) | 481 (48.4%) | 164 (39.3%) | REF |
| Nationality | ||||
| Georgian | 1,239 (87.1%) | 872 (87.6%) | 367 (86.2%) | 1.04 (0.93-1.16) |
| Other | 183 (12.9%) | 124 (12.5%) | 59 (13.8%) | REF |
Unadjusted Prevalence Ratio (PR) for comparison of culture positive to culture negative patients
Number of patients with positive cultures for whom data were available
Number of patients with negative cultures for whom data were available.
Risk of Drug Resistant Tuberculosis
Drug susceptibility test (DST) results were available on M. tuberculosis isolates recovered from 931 patients (534 newly diagnosed cases, 388 retreatment cases, and 9 cases with unknown treatment history). The prevalence of different patterns of resistance is shown in Table 2. Overall, 596 (64.0%) patients (258 [48.3%] new and 331 [85.3%] retreatment cases) had isolates resistant to one or more first-line antituberculosis drugs. Risk factors for resistance to one or more antituberculosis drugs in univariate analysis are shown in Table 3. When 9 patients with unknown treatment history were excluded, analysis yielded similar results (data not shown). In multivariate analysis, having any resistance to 1 or more first line antituberculosis drugs was independently associated with being a retreatment case (PR=1.75, 95% CI 1.58-1.94), and being an internally displaced person (PR=1.10, 95% CI 1.00-1.21).
Table 2.
Prevalence of drug resistance to first line antituberculosis at 4 sentinel sites in Georgia, 2001-2004
| Resistance Profile | All Cases | New Cases | Retreatment Cases |
Unknown Treatment History |
||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total number of strains tested |
931 |
100.0 |
534 |
100.0 |
388 |
100.0 |
9 |
100.0 |
| I. Any drug resistance a | 596 | 64.0 | 258 | 48.3 | 331 | 85.3 | 7 | 77.8 |
| Isoniazid (INH) | 442 | 47.5 | 159 | 29.8 | 280 | 72.2 | 3 | 33.3 |
| Rifampin (RIF) | 287 | 30.8 | 66 | 12.4 | 220 | 56.7 | 1 | 11.1 |
| Streptomycin (SM) | 490 | 52.6 | 199 | 37.3 | 284 | 73.2 | 7 | 77.8 |
| Ethambutol (EMB) | 210 | 22.7 | 56 | 10.5 | 154 | 39.7 | 0 | 0 |
| II. Monoresistance b | 188 | 20.2 | 126 | 23.6 | 59 | 15.2 | 3 | 33.3 |
| INH only | 59 | 6.3 | 39 | 7.3 | 20 | 5.2 | 0 | 0 |
| RIF only | 7 | 0.8 | 3 | 0.6 | 4 | 1.0 | 0 | 0 |
| SM only | 108 | 11.6 | 75 | 14.0 | 30 | 7.7 | 3 | 33.3 |
| EMB only | 14 | 1.5 | 9 | 1.7 | 5 | 1.3 | 0 | 0 |
| III. MDR-TBa | 262 | 28.1 | 56 | 10.5 | 206 | 53.1 | 0 | 0 |
| INH + RIF | 15 | 1.6 | 6 | 1.1 | 9 | 2.3 | 0 | 0 |
| INH + RIF + EMB | 6 | 0.6 | 0 | 0 | 6 | 1.6 | 0 | 0 |
| INH + RIF + SM | 98 | 10.5 | 26 | 4.9 | 72 | 18.5 | 0 | 0 |
| INH + RIF + SM + EMB | 143 | 15.4 | 24 | 4.5 | 119 | 30.7 | 0 | 0 |
| IV. Polyresistance c | 146 | 15.7 | 76 | 14.2 | 66 | 17.0 | 4 | 44.4 |
| INH + EMB | 2 | 0.2 | 1 | 0.2 | 1 | 0.3 | 0 | 0 |
| INH + SM | 86 | 9.2 | 48 | 9.0 | 35 | 9.0 | 3 | 33.3 |
| INH + EMB + SM | 33 | 3.5 | 15 | 2.8 | 18 | 4.6 | 0 | 0 |
| RIF + EMB | 3 | 0.3 | 1 | 0.2 | 2 | 0.5 | 0 | 0 |
| RIF + SM | 13 | 1.4 | 5 | 0.9 | 7 | 1.8 | 1 | 11.1 |
| RIF + EMB + SM | 2 | 0.2 | 1 | 0.2 | 1 | 0.3 | 0 | 0 |
| EMB + SM | 7 | 0.7 | 5 | 0.9 | 2 | 0.5 | 0 | 0 |
|
Number of drugs resistant to: |
||||||||
| Susceptible to 4 drugs | 335 | 36.0 | 276 | 51.7 | 57 | 14.7 | 2 | 22.2 |
| Resistance to 1 drug | 188 | 20.2 | 126 | 23.6 | 59 | 15.2 | 3 | 33.3 |
| Resistance to 2 drugs | 126 | 13.5 | 66 | 12.3 | 56 | 14.4 | 4 | 44.4 |
| Resistance to 3 drugs | 139 | 14.9 | 42 | 7.9 | 97 | 25.0 | 0 | 0 |
| Resistance to 4 drugs | 143 | 15.4 | 24 | 4.5 | 119 | 30.7 | 0 | 0 |
INH=isoniazid; RIF=rifampin; EMB=ethambutol; SM=streptomycin MDR-TB=multidrug-resistant tuberculosis (resistance to at least both isoniazid and rifampin) Polyresistance=resistance to two or more drugs but not both isoniazid and rifampin
p<0.001 in Chi-square test for comparison of prevalence of resistance pattern among retreatment cases to that among new cases
p=0.002 in Chi-square test for comparison of prevalence of resistance pattern among retreatment cases to that among new cases
p=0.25 in Chi-square test for comparison of prevalence of resistance pattern among retreatment cases to that among new cases.
Table 3.
Univariate analysis of association of having resistance to at least one first line antituberculosis drug with patient demographic and clinical characteristics (N=931)
| Variable | Any Drug Resistance (N=596) |
Drug Susceptible (N=335) |
Prevelance Ratios [PR] (95% CI) |
|
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age Group, years | 15-24 | 83 (13.9) | 78 (23.3) | REF |
| 25-34 | 197 (33.0) | 84 (25.1) | 1.36 (1.15-1.61) | |
| 35-44 | 187 (31.4) | 87 (25.9) | 1.32 (1.12-1.57) | |
| 45-54 | 76 (12.8) | 58 (17.3) | 1.10 (0.89-1.36) | |
| 55-64 | 41 (6.9) | 16 (4.8) | 1.40 (1.12-1.74) | |
| >65 | 12 (2.0) | 12 (3.6) | 0.97 (0.63-1.49) | |
| Gender | Female | 147 (24.7) | 81 (24.2) | 1.01 (0.90-1.13) |
| Male | 449 (75.3) | 254 (75.8) | REF | |
| Case Status | New | 258 (43.3) | 276 (82.4) | REF |
| Retreatment | 331 (55.5) | 57 (17.0) | 1.77 (1.60-1.95) | |
| Unknown | 7 (1.2) | 2 (0.6) | 1.61 (1.12-2.31) | |
| Residence (595 a/334b) | Tbilisi | 285 (47.9) | 193 (57.8) | 0.87 (0.79-0.96) |
| Other | 310 (52.1) | 141 (42.2) | REF | |
| Nationality | Georgian | 510 (85.6) | 305 (91.0) | 0.84 (0.75-0.95) |
| Other | 86 (14.4) | 30 (9.0) | REF | |
|
Internally Displaced Person (537 a/284b) |
Yes | 37 (6.9) | 8 (2.8) | 1.28 (1.10-1.48) |
| No | 500 (93.1) | 276 (97.2) | REF | |
|
History of Incarceration (537 a/283b) |
Yes | 87 (16.2) | 16 (5.6) | 1.35 (1.22-1.49) |
| No | 450 (83.8) | 267 (94.4) | REF | |
|
Unemployed (509 a/271b) |
Yes | 427 (83.9) | 206 (76.0) | 1.21 (1.04-1.41) |
| No | 82 (16.1) | 65 (24.0) | REF | |
|
Tobacco Use (538 a/284b) |
Yes | 268 (49.8) | 141 (49.6) | 1.00 (0.91-1.11) |
| No | 270 (50.2) | 143 (50.4) | REF | |
| Alcohol Use (537 a/283b) | Yes | 145 (27.0) | 76 (26.9) | 1.00 (0.90-1.12) |
| No | 392 (73.0) | 207 (73.1) | REF | |
|
History of Injection Drug Use (477 a/272b) |
Yes | 19 (4.0) | 3 (1.1) | 1.37 (1.15-1.63) |
| No | 458 (96.0) | 269 (98.9) | REF | |
| Hepatitis (508 a/271b) | Yes | 40 (7.9) | 14 (5.2) | 1.15 (0.97-1.36) |
| No | 468 (92.1) | 257 (94.8) | REF | |
| HIV | Yes | 5 (0.8) | 0 (0) | Undefined |
| No | 135 (22.7) | 92 (27.5) | REF | |
| Unknown | 465 (76.5) | 243 (72.5) | 1.06 (0.97-1.17) | |
|
Diabetes mellitus (518 a/273b) |
Yes | 25 (4.8) | 11 (4.0) | 1.06 (0.88-1.33) |
| No | 493 (95.2) | 262 (96.0) | REF | |
|
History of Gastritis/Peptic Ulcer Disease (517 a/273b) |
Yes | 17 (3.3) | 11 (4.0) | 0.93 (0.68-1.25) |
| No | 500 (96.7) | 262 (96.0) | REF | |
Number of patients with any drug resistance for whom data were available
Number of patients with drug susceptible cultures for whom data were available.
Two hundred sixty-two (28.1%) of 931 patients were demonstrated to have MDR-TB. The prevalence of MDR-TB was significantly higher among retreatement cases than among newly diagnosed cases (206/388 [53.1%] vs. 56/534 [10.5%], p<0.001) (Table 2). More than half of MDR-TB cases (143 [54.6%] of 262) were resistant to all four first-line antituberculosis drugs tested (Table 2). Retreatment cases were significantly more likely to have MDR-TB than non-MDR-TB (206 [78.6%] of 262 patients with MDR-TB were retreatment cases compared to 182 [27.2%] of 669 non-MDR-TB cases (PR=5.06, 95% CI 3.88-6.60) (Table 4). Other significant predictors of MDR-TB in univariate analysis included patient age groups 25-34, and 35-44 (compared to age group 15-24 years), living outside of Tbilisi, history of incarceration, unemployment, and history of injection drug use (Table 4). When 9 patients with unknown treatment history were excluded from analysis, the results were similar (data not shown). In multivariate analysis, independent risk factors for MDR-TB included being a retreatment case (PR=5.28, 95% CI 3.95-7.07), history of injection drug use (PR=1.59, 95% CI 1.21-2.09), and female gender (PR=1.36, 95% CI 1.12-1.65) (Table 5).
Table 4.
Univariate analysis of association of having MDR-TB and patient demographic and clinical characteristics (N=931)
| Variable | MDR-TB Case (N=262) |
Non-MDR-TB Case (N=669) |
Prevalence Ratios [PR] (95% CI) |
|
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age Group, years | 15-24 | 33 (12.6) | 128 (19.1) | REF |
| 25-34 | 90 (34.4) | 191 (28.6) | 1.56 (1.10-2.21) | |
| 35-44 | 83 (31.7) | 191 (28.6) | 1.48 (1.04-2.10) | |
| 45-54 | 36 (13.7) | 98 (14.6) | 1.31 (0.87-1.98) | |
| 55-64 | 15 (5.7) | 42 (6.3) | 1.28 (0.76-2.18) | |
| >65 | 5 (1.9) | 19 (2.8) | 1.02 (0.44-2.35) | |
| Gender | Female | 70 (26.7) | 158 (23.6) | 1.12 (0.89-1.41) |
| Male | 192 (73.3) | 511 (76.4) | REF | |
|
Case Status by History of TB Treatment |
New | 56 (21.4) | 478 (71.4) | REF |
| Retreatment | 206 (78.6) | 182 (27.2) | 5.06 (3.88-6.60) | |
| Unknown | 0 (0) | 9 (1.4) | Undefined | |
| Residence (261a/668b) | Tbilisi | 110 (42.2) | 368 (55.1) | 0.69 (0.56-0.85) |
| Other | 151 (57.8) | 300 (44.9) | REF | |
| Nationality | Georgian | 235 (89.7) | 580 (86.7) | 1.24 (0.88-1.75) |
| Other | 27 (10.3) | 89 (13.3) | REF | |
|
Internally Displaced Person (246a/575b) |
Yes | 17 (6.9) | 28 (4.9) | 1.28 (0.87-1.89) |
| No | 229 (93.1) | 547 (95.1) | REF | |
|
History of Incarceration (246a/574b) |
Yes | 53 (21.5) | 50 (8.7) | 1.91 (1.53-2.39) |
| No | 193 (78.5) | 524 (91.3) | REF | |
| Unemployed (235a/545b) | Yes | 203 (86.4) | 430 (78.9) | 1.47 (1.06-2.04) |
| No | 32 (13.6) | 115 (21.1) | REF | |
| Tobacco Use (247a/575b) | Yes | 122 (49.4) | 287 (49.9) | 0.99 (0.80-1.21) |
| No | 125 (50.6) | 288 (50.1) | REF | |
| Alcohol Use (246a/574b) | Yes | 65 (26.4) | 156 (27.2) | 0.97 (0.77-1.23) |
| No | 181 (73.6) | 418 (72.8) | REF | |
|
History of Injection Drug Use (216a/533b) |
Yes | 15 (6.9) | 7 (1.3) | 2.47 (1.81-3.36) |
| No | 201 (93.1) | 526 (98.7) | REF | |
|
History of Hepatitis (237a/542b) |
Yes | 20 (8.4) | 34 (6.3) | 1.24 (0.86-1.78) |
| No | 217 (91.6) | 508 (93.7) | REF | |
| HIV | Yes | 2 (0.8) | 3 (0.5) | 1.40 (0.47-4.17) |
| No | 65 (24.8) | 162 (24.2) | REF | |
| Unknown | 195 (74.4) | 504 (75.3) | 0.97 (0.77-1.24) | |
| Diabetes (243a/548b) | Yes | 13 (5.4) | 23 (4.2) | 1.19 (0.76-1.85) |
| No | 230 (94.6) | 525 (95.8) | REF | |
|
History of Gastritis/Peptic Ulcer Disease (243a/547b) |
Yes | 11 (4.5) | 17 (3.1) | 1.29 (0.80-2.07) |
| No | 232 (95.5) | 530 (96.9) | REF | |
Number of patients with positive cultures for whom data were available
Number of patients with negative cultures for whom data were available.
Table 5.
Multivariate analysis of risk factors for multidrug-resistant tuberculosis (MDR-TB) in Georgia.
| Variable | Adjusted Prevelance Ratios [PR] (95% CI) |
P value |
|---|---|---|
| Retreatment Case | 5.28 (3.95-7.07) | <0.001 |
| History of Injection Drug Use | 1.59 (1.21-2.09) | <0.001 |
| Female Gender | 1.36 (1.12-1.65) | 0.002 |
Resistance to rifampin was a significant predictor of resistance to isoniazid and streptomycin: 262 (91.3%) of 287 isolates resistant to rifampin were also resistant to isoniazid (PR=11.59, 95% CI 7.85-17.12), and 256 (89.2%) isolates were also resistant to streptomycin (PR=7.43, 95% CI 5.24-10.55).
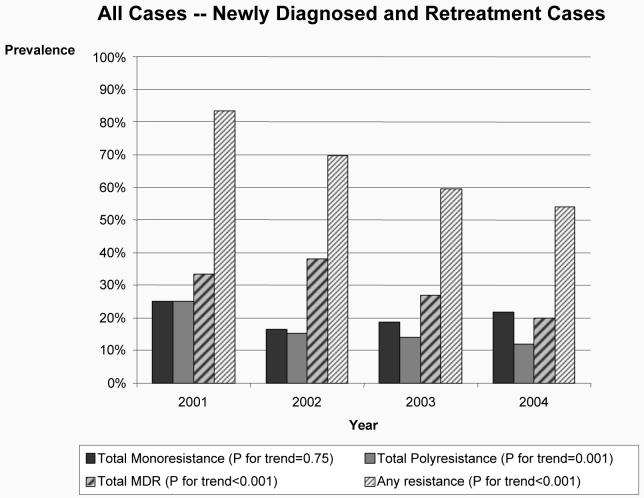
The prevalence of any drug resistance among TB cases significantly decreased during the study period from 83.5% in 2001 to 54.1% in 2004 (p-value for trend <0.001) (Figure 3). The prevalence of MDR-TB decreased from 33.3% in 2001 to 20.0% in 2004 (p-value for trend <0.001) (Figure 3). Similar trends were observed for both new and retreatment cases (data not shown).
Figure 3.
Dynamics of the prevalence of antituberculosis drug resistance over time in the country of Georgia, 2001-2004.
Discussion
Our study demonstrated high rates of drug resistance including MDR-TB in Georgia among both newly diagnosed and retreatment cases in the civilian population. Overall, 64% of the TB cases had resistance to ≥1 antituberculosis drug (48.3% of new and 85.3% of retreatment cases). The overall prevalence of MDR-TB in Georgia in our study was 28.1%, with very high prevalence both in newly diagnosed (10.5%) patients and in retreatment cases (53.1%). Resistance to any first-line drug was independently associated with being a retreatment case, and being an internally displaced person. Independent risk factors for MDR-TB included being a retreatment case, history of injection drug use, and female gender.
Our study is the first to our knowledge to report rates of drug resistance among TB cases in a civilian population in Georgia. High rates of MDR-TB (4-5% in new cases and 19-25% in retreatment cases) have been reported from Abkhazia an autonomous republic of Georgia which functions independently from Georgia.17,18 Our findings of high rates of drug resistant TB including MDR-TB in Georgia are similar to what has been reported from other former Soviet republic. This region has among the highest prevalence of drug resistant TB19. In countries of the former Soviet Union, MDR-TB rates have been reported to range from 4% (Dashoguz, Turkmenistan) to 22.7% (Samara, Russia) in new cases20,21, and from 18% (Dashoguz, Turkmenistan) to 54-60% (Republic of Lithuania; Arkhangelsk, Russia, respectively) in retreatment cases20,22,23. High rates of MDR-TB have also been reported found in Kazakhstan - 14.2%, Tomsk, Russian Federation - 13.7%, and Estonia - 12.2%19. The median prevalence of resistance to the four first-line drugs among retreatment cases in the countries of the former Soviet Union was as high as 30%, compared to a median of 1.3% in all other settings19.
In general, high rates of MDR-TB among newly diagnosed patients suggest problems with TB control in the past, while high rates of MDR-TB among retreatment cases is suggestive of existing problems in TB control program including suboptimal treatment regiments and poor compliance with treatment10. TB has emerged as a major public health problem in Georgia following the dissolution of the Soviet Union in 1991. Following independence, there was a civil war in 1992-1993 which resulted in large number of internally displaced persons, living conditions in Georgia deteriorated for a large proportion of the population, healthcare infrastructure was destroyed and there was increased poverty and migration. Inadequate management of TB services, significant shortage or absence of TB drugs, institutional spread, and poor mechanisms to ensure adherence to treatment1,2,6 are factors that have likely contributed to the high rates of drug resistant TB found in our study. High default rates which have been reported to be as much as 23-25% among new cases and 39% among retreatment cases in study in 1995-1996, and 15% among new cases and 23% among retreatment cases in 20032,3 have likely contributed to the emergence of MDR-TB as well. While high rates of drug resistant TB including MDR-TB have been previously reported from correctional facilities in Georgia6, our study is the first to report rates the prevalence and risk factors for drug resistant TB in the civilian population in Georgia.
The Georgian National Tuberculosis Program was established in 1995. After its formation, Georgia started implementing pilot projects based on WHO-recommended DOTS strategy with WHO support2, and currently Georgia has 100% of DOTS coverage3. However, implementation of DOTS in Georgia was limited until the past few years when robust DOTS programs in selected regions (including Gori and Tbilisi) were achieved. Temporal trends between 2001-2004 in our study demonstrated significant decrease in overall prevalence of MDR-TB, any resistance, and polyresistance (resistance to 2 or more drugs but not both isoniazid and rifampin) during the study period. These decreases likely reflect enhanced TB control efforts in Georgia including implementation of standardized TB treatment (including the provision of directly observed therapy), improved drug supply system, improved diagnostics and laboratory capacities, implementation of proper recording and reporting, and training of healthcare staff2,24. Despite these improvements, MDR-TB rates among new and retreatment cases remain high which provide important challenges for TB control in Georgia. In settings with existing high rates of MDR-TB, the current WHO standard treatment policy of administration of first line drugs to re-treatment cases should be revised.7 About one third of the study population would not have responded to the standard WHO retreatment regimen with first line drugs (5 drugs with the addition of streptomycin) and drug resistance could be amplified by receiving such regimens. Better access to DST results including rapid DST methods at the time of TB diagnosis would facilitate appropriate selection of treatment regimens. Limited resources and laboratory capacity remains a limitation to implement this strategy. However, increasing laboratory capacity to deal with MDR-TB as recommend by the Global Plan to Stop TB (e.g., at a regional level), better referral of specimens from patients living in remote areas to regional/national reference laboratories should be encouraged to meet this challenge. The application of Georgian National TB Program to the Green Light Committee was approved and treatment of TB patients with drug resistant tuberculosis using second line drugs started in 2007.
The strongest predictor of having both any drug resistance and multidrug resistance in our study was being a retreatment case. Previous treatment is well-known risk factor for development of drug resistance6,19,25. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance demonstrated in multivariate analysis that the proportion of retreatment cases among the total number of cases was significantly associated with both MDR and any drug resistance19. Previous treatment was also demonstrated as the strongest determinant of MDR-TB in Europe in a systematic review based on studies from twelve European countries26. The likelihood of having MDR-TB has linear increase with increasing of as the total time of prior treatment27. The current WHO recommendations of treatment of relapses and failures in category II, which includes administration of 5 already used drugs may result in monotherapy and lead to further amplification of resistance7.
In our study, internally displaced persons (IDP) had an increased risk of having any resistance to first-line antituberculous drugs. IDPs are ethnic Georgians from the northwest province of Abkhazia that were forced to flee their homes during the civil war of 1992-1993 and reside in other parts of Georgia, often in places such as former hotels or hospitals where overcrowding is common. IDPs have been reported to be at increased risk for TB infection and disease1. These conditions may favor transmission of TB including drug resistant strains. Internally displaced status and risk of drug resistance could be influenced by previous treatment. Injection drug use previously has been reported to be a risk factor for MDR-TB in newly diagnosed cases in some studies21,28, while other studies failed to find such association29. Association of injection drug use with MDR-TB could be related to close contacts of drug users and transmission of resistant stains within this group. Also it was demonstrated that injection drug use is a significant predictor of TB treatment nonadherence30, which may impact development of resistance.
Tuberculosis is more common among men, including MDR-TB. In a systematic review by Faustini et al26, MDR-TB cases were more likely to be male in Western Europe, while in Eastern Europe male gender was not associated with MDR-TB. These authors assumed that male gender could modify the association between previous treatment and MDR-TB since men are believed to be less adherent to treatment than women26. Interestingly, we found that female gender was an independent risk factor for MDR-TB in Georgia. Preliminary results of a population based study in Georgia, also found that female gender is independently associated with increased risk for MDR-TB31. Our findings in Georgia that women are at increased risk for MDR-TB if they have TB is similar to that reported in two studies conducted in former Soviet republics (Arkhangelsk, Russia, and Estonia)23,32. The reasons for the association between female gender and MDR-TB are unclear and deserves further study. We hypothesize that this association could be related to the fact that women care for men and others with MDR-TB both in households and in healthcare settings in Georgia where the majority of health care workers are female. There previously has been no treatment available in Georgia for MDR-TB and such patients therefore likely remain infectious for long periods of time increasing risk of household and institutional transmission for MDR-TB. We found that a higher proportion of women had new cases of MDR-TB, compared to males.
Our study is the subject to several limitations. One of these is potential misclassification of the new and retreatment cases when some cases registered as new actually may have had TB treatment in the past. Classification was based on patient history of prior treatment for TB and review of medical records (which were not available for all patients enrolled). Some patients with suspected TB had contaminated cultures and were excluded from analysis which has the potential for introducing selection bias. In addition, further bias is possible because our study was carried out at selected sites in Georgia and was not population-based. The high rate of missing data among those with a negative AFB culture was due to the fact that the study focused primarily on patients with positive culture. However, our study provides important initial data on drug resistance in Georgia and enhanced infrastructure development which allowed for the subsequent development of a population based study on drug resistance which is ongoing31.
In summary, drug-resistant tuberculosis including MDR-TB has emerged as a serious public health problem in Georgia and will greatly impact TB control strategies. The overall prevalence of MDR-TB was 28.1% (10.5% of newly diagnosed patients and 53.1% of retreatment cases). In multivariate analysis, risk factors for MDR-TB included: being a retreatment case (PR=5.28); history of injection drug use (PR=1.59); and female gender (PR=1.36). This study provides important implications for TB control in Georgia. It highlights the need to fully implement the Georgia National Tuberculosis Program's new five year plan for TB control which is based on The Global Plan to Stop TB (2006-2015)12. This includes rapid DOTS expansion so DOTS is fully implemented throughout Georgia in order to prevent further emergence of drug resistance. Other measures include development of the capacity to treat those with MDR-TB in Georgia, through implementation of DOTS-Plus (provision of diagnosis, treatment and management for all patients with MDR-TB through the DOTS-Plus strategy); implementation of TB infection control measures to prevent institutional spread of tuberculosis; engaging the overall health system in TB related activities; empowering patients and communities to support TB control and reducing TB-related stigma; and enabling and promoting TB-related research (including research in simplified and easy to use tests to detect drug resistance).
Acknowledgements
Funding source
This study was supported in part by funding from the U.S. Department of Health and Human Services Biotechnology Exchange Program (BTEP), the U.S. Civilian and Research Development Foundation (CRDF), and the National Institutes of Health (NHLBI [K07 HL03078] and Fogarty International Center [D43 TW007124 and D43 TW01042]).
Footnotes
Conflict of Interest Statement.
Nobody of authors had any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.Weinstock DM, Hahn O, Wittkamp M, Sepkowitz KA, Khechinashvili G, Blumberg HM. Risk for tuberculosis infection among internally displaced persons in the Republic of Georgia. Int J Tuberc Lung Dis. 2001;5(2):164–9. [PubMed] [Google Scholar]
- 2.Zalesky R, Abdullajev F, Khechinashvili G, Safarian M, Madaras T, Grzemska, et al. Tuberculosis control in the Caucasus: successes and constraints in DOTS implementation. Int J Tuberc Lung Dis. 1999;3(5):394–401. [PubMed] [Google Scholar]
- 3.Global tuberculosis control: surveillance, planning, financing. WHO report 2006. World Health Organization; Geneva: WHO/HTM/TB/2006.362. [Google Scholar]
- 4.Richards D, Mikiashvili T, Parris J, Kourbatova E, Wilson J, Shubladze N, Tertsvadze T, Khechinashvili G, del Rio C, Blumberg HM. High prevalence of hepatitis C virus infection but not HIV co-infection among patients with tuberculosis in the Republic of Georgia. Int J Tuberc Lung Dis. 2006;10(4):396–401. [PubMed] [Google Scholar]
- 5.Salakaia A. US-Caucasus workshop on operational research in HIV and TB. Tbilisi; Georgia: Oct 25, 2005. TB epidemiology in Georgia: achievements and challenges [presentation] 2005. [Google Scholar]
- 6.Aerts A, Habouzit M, Mschiladze L, Malakmadze N, Sadradze N, Menteshashvili O, Portaels F, Sudre P. Pulmonary tuberculosis in prisons of the ex-USSR state Georgia: results of a nation-wide prevalence survey among sentenced inmates. Int J Tuberc Lung Dis. 2000;4:1104–1109. [PubMed] [Google Scholar]
- 7.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283(19):2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 8.Pablos-Mendez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276(15):1223–8. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Garcia Mde L, Ponce-De-Leon A, Garcia-Sancho MC, Ferreyra-Reyes L, Palacios-Martinez M, Fuentes J, et al. Tuberculosis-related deaths within a well-functioning DOTS control program. Emerg Infect Dis. 2002;8(11):1327–33. doi: 10.3201/eid0811.020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Tuberculosis Programme and International Union Against Tuberculosis and Lung Disease . Guidelines for surveillance of drug resistance in tuberculosis. Geneva: 1994. WHO/TB/94.178. [Google Scholar]
- 11.Quy HT, Lan NT, Borgdorff MW, Grosset J, Linh PD, Tung LB, et al. Drug resistance among failure and relapse cases of tuberculosis: is the standard re-treatment regimen adequate? Int J Tuberc Lung Dis. 2003 Jul;7(7):631–6. [PubMed] [Google Scholar]
- 12.Stop TB Partnership and World Health Organization . Global Plan to Stop TB 2006–2015. World Health Organization; Geneva: 2006. WHO/HTM/STB/2006.35. [Google Scholar]
- 13.Treatment of tuberculosis: guidelines for national programmes. World Health Organization; Geneva: 2003. WHO/CDS/TB/2003.313. [Google Scholar]
- 14.Crofton J, Chaulet P, Maher D. Guidelines for the management of drug-resistant tuberculosis. World Health Organization; Geneva: 1997. WHO/TB/96.210 (Rev.1) [Google Scholar]
- 15.World Health Organization . Laboratory services in tuberculosis control. Part III: culture. Geneva: 1998. WHO/TB/98.258. [Google Scholar]
- 16.Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, Mahler HT, et al. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ. 1963;29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet M, Sizaire V, Kebede Y, Janin A, Doshetov D, Mirzoian B, Arzumanian A, Muminov T, Iona E, Rigouts L, Rüsch-Gerdes S, Varaine F. Does one size fit all? Drug resistance and standard treatments: results of six tuberculosis programmes in former Soviet countries. Int J Tuberc Lung Dis. 2005;9(10):1147–54. [PubMed] [Google Scholar]
- 18.Pardini M, Iona E, Varaine F, Karakozian H, Arzumanian H, Brunori L, Orefici G, Fattorini L, LONG-DRUG Study Group Mycobacterium tuberculosis drug resistance, Abkhazia. Emerg Infect Dis. 2005;11(3):501–3. doi: 10.3201/eid1103.040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Third Global Report. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance 1999-2002. World Health Organization; Geneva: 2004. Anti-tuberculosis drug resistance in the world. WHO/HTM/TB/2004.343. [Google Scholar]
- 20.Cox HS, Orozco JD, Male R, Ruesch-Gerdes S, Falzon D, Small I, et al. Multidrug-resistant tuberculosis in central Asia. Emerg Infect Dis. 2004;10(5):865–72. doi: 10.3201/eid1005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruddy M, Balabanova Y, Graham C, Fedorin I, Malomanova N, Elisarova E, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax. 2005 Feb;60(2):130–5. doi: 10.1136/thx.2004.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewan P, Sosnovskaja A, Thomsen V, Cicenaite J, Laserson K, Johansen I, et al. High prevalence of drug-resistant tuberculosis, Republic of Lithuania, 2002. Int J Tuberc Lung Dis. 2005;9(2):170–4. [PubMed] [Google Scholar]
- 23.Toungoussova S, Caugant DA, Sandven P, Mariandyshev AO, Bjune G. Drug resistance of Mycobacterium tuberculosis strains isolated from patients with pulmonary tuberculosis in Archangels, Russia. Int J Tuberc Lung Dis. 2002;6(5):406–14. [PubMed] [Google Scholar]
- 24.Khechinashvili G, Mdivani N, Blumberg HM. Lessons learned from implementation of DOTS (Directly Observed Therapy, short course) tuberculosis control strategy in the Republic of Georgia. 42nd Annual Meeting of Infectious Diseases Society of America; Infectious Diseases Society of America; Boston. October 2004. [Google Scholar]
- 25.Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998;338(23):1641–9. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 26.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–63. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5(10):887–93. [PubMed] [Google Scholar]
- 28.Kourbatova EV, Borodulin BE, Borodulina EA, Leonard MK, Blumberg HM. High rates and risk factors for primary drug resistant tuberculosis in Samara, Russia. 43rd Annual Meeting of Infectious Diseases Society of America; Infectious Diseases Society of America; San Francisco. October 2005. [Google Scholar]
- 29.Kimerling ME, Slavuckij A, Chavers S, Peremtin GG, Tonkel T, Sirotkina O, et al. The risk of MDR-TB and polyresistant tuberculosis among the civilian population of Tomsk city, Siberia, 1999. Int J Tuberc Lung Dis. 2003;7(9):866–72. [PubMed] [Google Scholar]
- 30.Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997;102(2):164–70. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- 31.Lomtadze N, Salakaia A, Aspindzelashvili R, Janjgava M, Blumberg HM. High rates of Multidrug Resistant Tuberculosis in the Country of Georgia: A Population Based Study; 11th Annual Meeting of the International Union Against Tuberculosis and Lung Disease – North American Region; Vancouver, Canada. February 2007; [PMC free article] [PubMed] [Google Scholar]
- 32.Lockman S, Kruuner A, Binkin N, Levina K, Wang Y, Danilovitsh M, Hoffner S, Tappero J. Clinical outcomes of Estonian patients with primary multidrug-resistant versus drug-susceptible tuberculosis. Clin Infect Dis. 2001;32(3):373–80. doi: 10.1086/318489. [DOI] [PubMed] [Google Scholar]