Abstract
We have investigated the ability of chlorophyllin (CHL) to interact with acridine mutagen ICR-191 (2-methoxy-6-chloro-9-(3-(2-chloroethyl)aminopropylamino)acridine) and also its ability to decrease binding of ICR-191 to DNA in a simple three-component competition system: CHL-ICR–DNA. Our data indicate a strong association of ICR-191 with CHL, stronger even than the association of ICR-191 with DNA. Calculations based on the measured affinity data show that a two- to three-fold excess of CHL reduces by about two-fold the concentration of the mutagen-DNA complex. We also exposed human leukemic HL-60 cells to ICR-191 in the absence and presence of CHL and measured the mutagen-induced DNA damage. The extent of DNA damage was assessed by analysis of histone H2AX phosphorylation. While ICR-191 induced significant increase in expression of phosphorylated H2AX (γH2AX), particularly in DNA replicating cells, this increase was totally abolished in the cells treated with ICR-191 in the presence of CHL.
Keywords: Chlorophyllin, DNA damage, Histone H2AX phosphorylation, Mutagen interceptor, Intercalation, Competitive interactions
1. Introduction
Chlorophyllin (CHL) is a water-soluble derivative of chlorophyll differing from the latter by having copper instead of magnesium as a cofactor. Because of anti-mutagenic and anti-oxidant properties both chlorophyll and CHL are in center of interest of many research laboratories. Several studies reported that CHL protects cells against mutagenic effects of benzo[a]pyrene [1–4], cyclophosphamide [3], heterocyclic amines [5–7], aflatoxin [8–12], heavy metals [13], and ionizing radiation [14]. Because of diversity of the mutagens neutralized by CHL and different modes of their genotoxicity it is quite apparent that more than a single mechanism may be responsible for the CHL-protective effects. One mechanism may involve the direct anti-oxidant properties of CHL. Indeed, the ability of CHL to scavenge reactive oxygen species (ROS) in vitro and in vivo was documented [15,16].
Another mechanism by which CHL neutralizes mutagens stems from its ability to form complexes with the aromatic mutagens [17–20]. The complexes are thought to be maintained via stacking (π–π) interactions between the flat aromatic molecules of the mutagen and the porphyrin rings of CHL. Because the mutagen is sequestered within the complex its concentration in monomeric, i.e. active form in solution, is reduced. Thus, by capturing mutagen molecules CHL neutralizes them preventing their uptake by the cells and subsequent interaction with DNA. It was proposed to define the agents with such properties as the interceptor molecules [21–24]. Similar to chlorophyllin, caffeine and pentoxifillin were shown to be interceptors of several aromatic compounds such as acridine orange, quinacrine mustard and acridine mutagens ICR-191 and ICR-170, attenuating their mutagenic and cytotoxic effects and preventing interaction with DNA [25–29].
In prior studies we explored molecular interactions that may be responsible for the interceptor properties of CHL vis-à-vis several intercalators [22,23]. Towards this end using optical spectroscopy we have studied interactions between the intercalators and DNA in the presence and absence of CHL. We have shown for example, that CHL forms complexes with acridine orange, quinacrine mustard and the antitumor drug doxorubicin, with association constants (Ka's) 7.0 × 105, 3.2 × 105 and 3.3 × 105 M−1, respectively [22]. Applying experimental analysis to the three-component interactive system: intercalator–DNA–CHL we observed that CHL attenuated binding of these intercalators to DNA thereby showing the interceptor activity [23]. In the present study using similar experimental approach we investigated interactions between the acridine mutagen ICR-191 and DNA in the absence and presence of CHL. ICR-191 is an aromatic mutagen having a three-ring structure, it intercalates into DNA and has also mustard-like alkylating properties [30,31]. We expected, therefore, that similar as in the case of intercalators studied by us before [23] CHL will also have the interceptor activity towards ICR-191.
Parallel to the biophysical studies we presently also assessed the potential of CHL to neutralize genotoxic activity of ICR-191. Towards this end we exposed human promyelocytic HL-60 cells to ICR-191 in the absence and presence of CHL and the extent of genotoxic effect of ICR-191 was measured by flow cytometry by means of immunocytochemical detection of phosphorylation of histone H2AX. Phosphorylation of histone H2AX on Ser-139 (phosphorylated H2AX has been named γH2AX) is considered to be a marker of DNA damage, particularly the induction of DNA double-strand breaks (DSBs) [32,33]. Cytometric detection of γH2AX was shown to provide a convenient tool to measure extent of DNA damage induced by variety of genotoxic agents including radiation [34,35], antitumor drugs [36,37], exogenous and endogenous oxidants [38] and environmental carcinogens [39].
2. Materials and methods
2.1. Spectral studies
2.1.1. Reagents
Chlorophyllin (sodium-copper salt), Acridine Mutagen ICR-191 (6-Chloro-9-[3-(2-chloroethylamino)propylamino]-2-methoxyacridine dihydrochloride), (both from Sigma Chemical Co, St. Louis, MO, USA), Tris (Tris-(hydroksymethyl)-aminomethane) (Fluka Chemie AG, Buchs, Switzerland). All solutions were prepared in Tris–HCl (30 mM), NaCl (50 mM) at pH 7.4.
2.1.2. Estimation of ICR–CHL association constant
Absorption and fluorescence emission measurement of ICR-191, CHL and mixtures of these compounds were done using Cary 300 spectrophotometer (Varian, Australia) and LS 50B spectrofluorimeter (Perkin Elmer, UK), respectively. Titrations were carried out at constant ICR-191 concentrations (8, 10 or 12 µM) while altering CHL concentration within the range between 0.9 to 60.0 µM. Absorption was measured in 3.0 cm width cuvettes while fluorescence emission was measured using 0.4 × 1.0 cm cuvettes with 5 nm excitation-, and 8 nm spectral width emission-slits, at λex=422 nm. The temperature of the cell-housing block was kept at 295±1 K. The data were processed using Prism 4 (GraphPad Software Inc, San Diego, CA, USA). Spectrophotometric and spectrofluorimetric titrations allowed us to estimate association constant Ka and molar absorption coefficient of the complex, assumed to have the components in 1:1 proportion, according to the method applied before [22].
Fluorescence spectrum was corrected with respect to the reabsorption and the inner filter effect as described before [40].
To estimate Ka from the spectrofluorimetric measurements we applied nonlinear regression for fluorescence intensity at λ=492 nm according to (Eq. (1)).
| (1) |
where:
total ICR-191 concentration
total CHL concentration
FAB fluorescence of ICR-191 totally bound to CHL
F0 fluorescence of ICR-191 in .
2.1.3. Estimation of ICR–DNA association constant
Absorption of ICR-191 and DNA separately as well as in mixture have been measured at constant ICR concentration within a range between 8.0 and 10.1 µM while DNA concentration was variable ranging between 1 to 60 µMbp. Absorption measurements were carried out in cuvettes of l=3 cm width while 0.4 × 1.0 cm width cuvettes were used for fluorescence measurement. The association constant Ki and binding site size in DNA (n) were estimated according to the McGhee-von Hippel model [41]. The Ki association constant and molar extinction coefficient of the DNA-bound intercalator ICR-191 were estimated by the least squares regression analysis as described [23]. Spectral fragments within 360–500 nm have been used in which ICR-191 has absorption band while DNA is not absorbing light. A correction for light scattering by DNA molecules was applied as described before [23].
2.1.4. Estimation of concentration of complexes in the three-component system
Concentrations of individual complexes in the three-component (ICR–DNA–CHL) system was estimated based on the model of simple competition as described in previously [23]. Assumption was made that Ka, Ki and n parameters estimated in the two-component systems remain unchanged in the three-component system. Further details pertaining this analytical approach are presented elsewhere [23].
2.2. Protection of DNA in HL-60 cells from ICR-191-induced damage by CHL
2.2.1. Cells and culture conditions
Human promyelocytic leukemic HL-60 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were grown in 25 ml FALCON flasks (Becton Dickinson Co., Franklin Lakes, NJ) in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine (all from GIBCO/BRL Life Technologies, Inc., Grand Island, NY, USA) at 37 °C in an atmosphere of 5% CO2 in air. At the onset of the experiments, there were fewer than 5 × 105 cells per ml in culture and the cells were in an exponential and asynchronous phase of growth. The cells were exposed in cultures to 1.2 µM of ICR-191 in the absence or presence of 1 µM CHL for 1 h. Cells in parallel cultures were either untreated or were treated with 1 µM of CHL alone for 1 h. Following the treatments the cells were fixed in ice-cold 1% methanol-free formaldehyde (Polysciences, Inc., Warrington, PA, USA) in PBS for 15 min in phosphate-buffered saline (PBS) for 15 min followed by suspension in 70% ethanol where they were stored at −20 °C for 2–24 h. The experiments were run in triplicate.
2.2.2. Immunocytochemical detection of γH2AX
The fixed cells were washed twice in PBS and suspended in 0.2% Triton X-100 (Sigma) in a 1% (w/v) solution of bovine serum albumin (BSA; Sigma) in PBS for 30 min to suppress nonspecific antibody (Ab) binding. The cells were then incubated in 100 µl of 1% BSA containing anti-phospho-histone H2A.X (Ser-139) mAb (Upstate, Lake Placid, NY, USA 1:100) for 2 h at room temperature, as described. [35,36]. The cells were then rinsed with 1% BSA in PBS (200 g, 5 min) and, after centrifugation, the cell pellets were resuspended in 100 µl of 1% BSA containing FITC-conjugated anti-mouse rabbit F(ab')2 fragment (DAKO, Carpinteria, CA, USA,1:30) for 30 min at room temperature in the dark. The cells were then counterstained with 5 µg/ml of propidium iodide (PI; Molecular Probes, Eugene, OR, USA) in the presence of 100 µg/ml of RNase A (Sigma). Cellular green (FITC) and red (PI) fluorescence (10,000 cells per sample) was measured using a FACScan flow cytometer (Becton-Dickinson, San Jose, CA, USA) and the data were analyzed using the CELLQuest software (Becton-Dickinson), as described [35,36].
3. Results
3.1. Interactions between ICR-191 and CHL
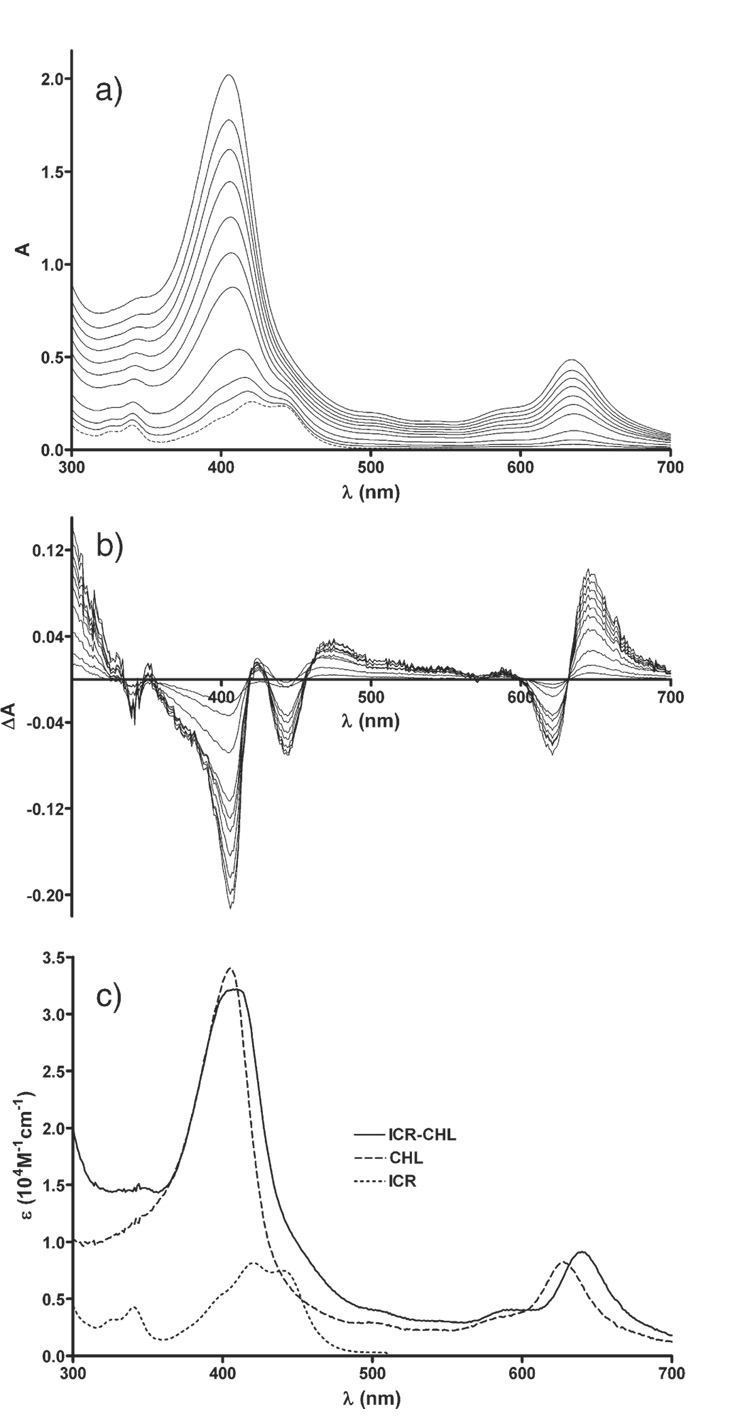
A shift of the main absorption bands of CHL and ICR-191 towards longer wavelength was observed during the titration of ICR-191 with CHL. This effect was not visually apparent by direct analysis of the measured spectra (Fig. 1a) because of a large difference in molar extinction coefficient of both measured components and an overlap of the absorption bands, with maximum of CHL at 405 nm and of ICR-191 at 422 nm. However, the bathochromic shift was distinctly evident from analysis of the differential spectra (Fig. 1b). The shift was also apparent after superimposition of the absorption spectrum of ICR-191–CHL complex on the spectra of individual components i.e. ICR-191 or CHL (Fig. 1c). The association constant Ka was estimated to be 2.8 × 105 M−1±0.5 × 105 M−1.
Fig. 1.
a) Absorption spectra of ICR-191 (broken line) and mixtures of ICR and CHL (solid lines), ICR was at 8 µM, while CHL concentration varied from 1 to 20 µM, from bottom to top; l=3 cm; b) respective differential spectra; c) absorption spectra of ICR (dotted line), CHL (dashed line), and computed spectrum of ICR–CHL complex (solid line).
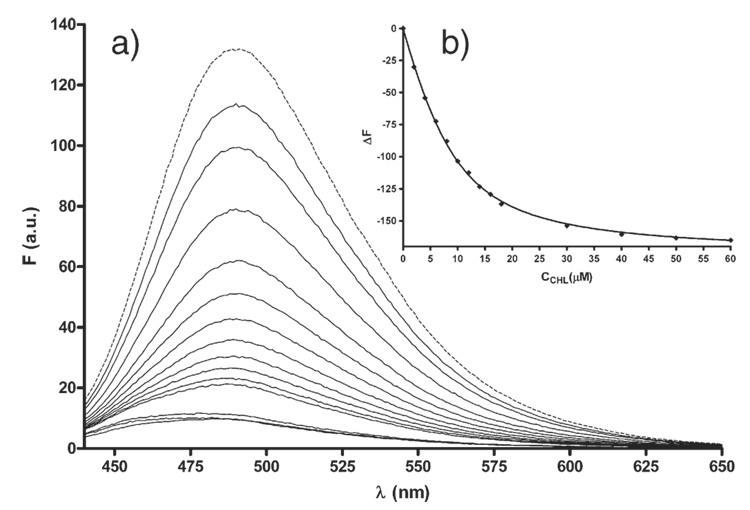
During spectrofluorimetric titration we observed progressive quenching of ICR-191 fluorescence with the increasing CHL concentration (Fig. 2a). At 60 µM concentration of CHL fluorescence of the complexwas very low. While chlorophyll is known to emit fluorescence upon excitation we did not observe fluorescence of CHL itself. After normalization of the fluorescence spectra a minor shift of the emission towards lower wavelength with an increases in CHL concentration become apparent. It appears therefore, that the ICR-191–CHL complex shows low intensity fluorescence. Fig. 2b presents the nonlinear regression analysis according to Eq. (1) at λem=526 nm. The association constant Ka was estimated to be 3.0 × 105 M−1±0.3 × 105 M−1.
Fig. 2.
a) Fluorescence spectra of ICR-191 (8 µM) alone (dashed line) and admixed during titration with different concentration (from 2 to 60 µM from top to bottom) of CHL (solid lines); b) non-linear regression calculated according to Eq. (1) at a wavelength of 492 nm, and concentration of ICR 9.5 µM.
3.2. Interactions between ICR-191 and DNA
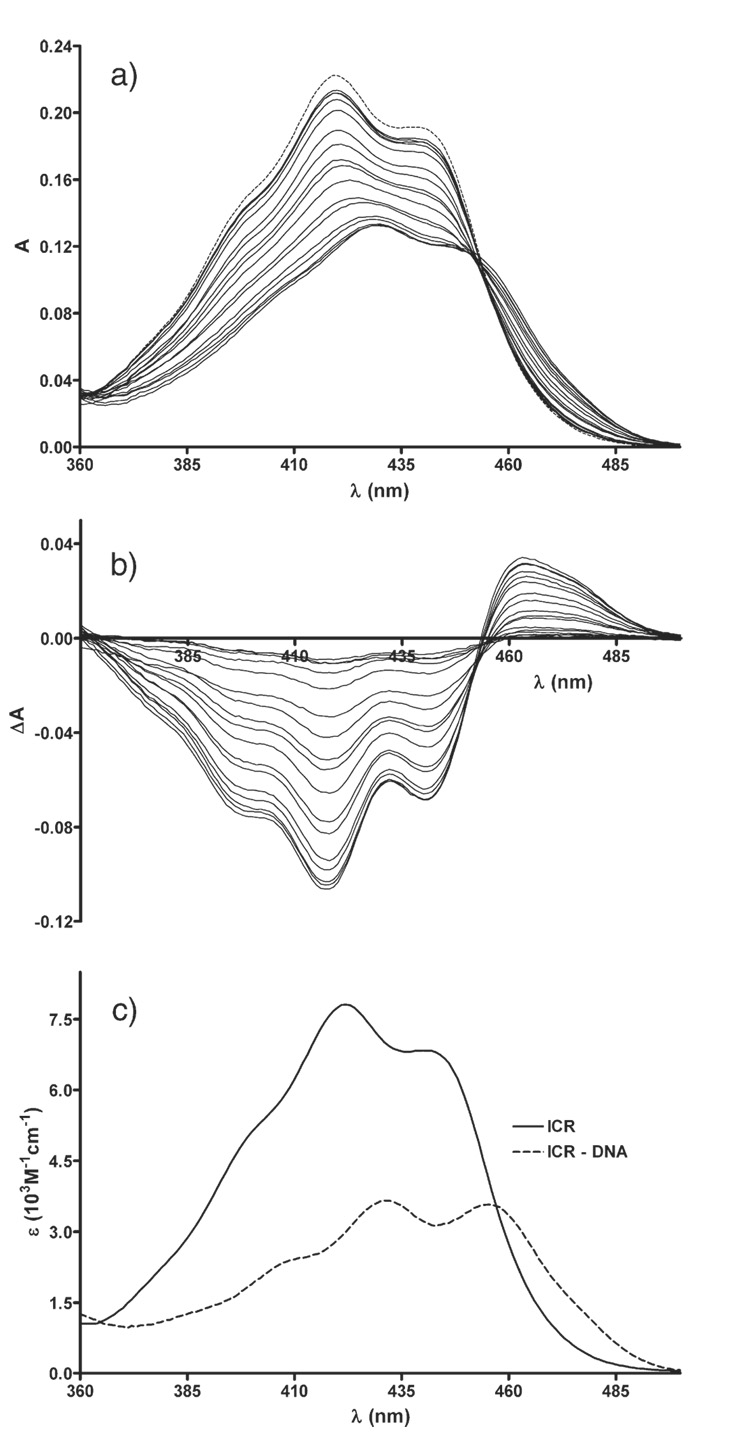
During spectrophotometric titration of ICR-191 with DNA we observed a shift of the absorption band of ICR-191 towards longer wavelength combined with the hypochromic effect. The isosbestic point was apparent at 453 nm (Fig. 3a, b). The spectrum of ICR-191–DNA complex is presented in Fig. 3c.
Fig. 3.
a) Absorption spectra of ICR-191 (broken line) and mixtures of ICR and DNA (solid lines), ICR was at 10 µM, while DNA concentration varied from 1 to 60 µMbp, from top to bottom; l=3 cm; b) respective differential spectra; c) absorption spectra of ICR, free in solution (dotted line), and in complex with DNA (solid line).
Similar to the titration with CHL the titration of ICR-191 with DNA also led to fluorescence quenching. Likewise, the emission band was shifted towards lower wavelength compared to the emission of ICR-191 alone. The association constant Ki characterizing ICR-191 binding to DNA was found to be 6.2 × 104 M−1±1.3 × 104 M−1 at n=1.5.
3.3. Interactions within the ICR–DNA–CHL mixture
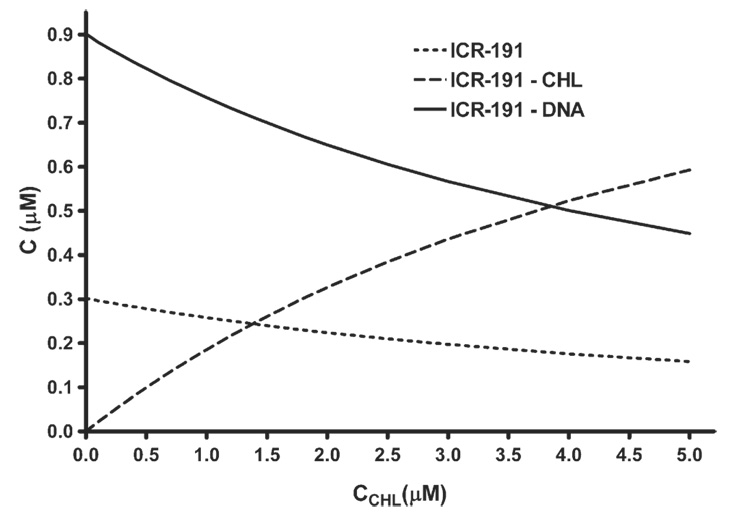
Themodel of simple competition as described by us before [23] was used to estimate concentration of individual components within the three-component mixture, based on the estimates of Ka association constants of ICR–CHL, parameters Ki and n and interactions between ICR-191 and DNA under assumed initial concentrations of the individual components. Fig. 4 illustrates changes in concentration of the complexes and of the free intercalator ICR-191 in the three-component mixture. Observed was a reduction in concentration of the DNA-bound ICR-191 paralleled by the increase in concentration of ICR–CHL complex and by minor decrease in concentration of the free intercalator.
Fig. 4.
Changes in concentration of individual constituents in the three-component mixture as a function of change in CHL concentration, determined assuming the simple competition model, on example of ICR-191 as the intercalator. Concentrations of ICR and DNA were 1.2 µM and 6 µMbp.
3.4. Protection of DNA in live cells
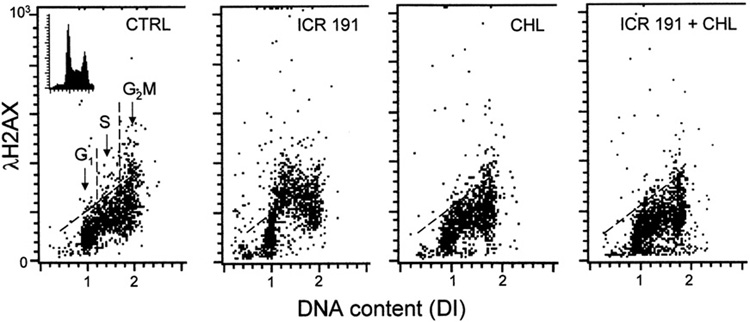
Exposure of HL60 cells to ICR-191 induced histone H2AX phosphorylation reflected as an increase in expression of its phosphorylated form (γH2AX) (Fig. 5; Table 1). In Fig. 5 the data are presented in form of bivariate distributions (scatterplots) in which individual cells, each represented by a single dot, are plotted on the coordinates where abscissa represents cellular DNA content and ordinate intensity of γH2AX immunofluorescence [36–38]. The DNA content abscissa is presented as DNA index (DI) and the intercellular differences in DNA content make it possible to identify cells in different phases of the cell cycle, namely G1 cells having DI=1.0±0.2, G2M cells having DI=2.0±0.2 and S-phase cells with DI between 1.2 and 1.8 (left panel). As it is evident the increase in intensity of γH2AX immunofluorescence, reporting H2AX phosphorylation, was most pronounced in S phase cells. In contrast, the treatment of cells with ICR-191 in the presence of CHL had no effect on expression of γH2AX, which remained essentially at the same level as in the untreated (CTRL) cultures. Likewise, exposure of cells to CHL alone had no apparent effect on H2AX phosphorylation.
Fig. 5.
Protection of DNA in HL-60 cells by CHL from the damage induced by ICR-191. The bivariate (DNA content versus expression of γH2AX) distributions (scatterplots) illustrating effect of ICR alone (1.2 µM), CHL alone (1.0 µM), and ICR (1.2 µM) together with CHL (1.0 µM), on phosphorylation of histone H2AX (expression of γH2AX). Based on differences in cellular DNA content the cells in G1, S and G2M phases of the cell cycle can be identified as shown in the left panel. Note an increase in expression of γH2AX detected immunocytochemically and measured by flow cytometry, particularly of the S-phase cells, upon treatment with ICR alone. This increase was prevented when the cells treated with ICR and CHL (right panel). CHL alone had no effect the level of H2AX phosphorylation. The skewed threshold lines show maximal level of γH2AX expression for 95% interphase untreated (CTRL) cells.
Table 1.
Effect of ICR-191 alone, CHL alone, and mixture of ICR and CHL, on level of expression of γH2AX in HL-60 cells
| Cell treatment | All cells | G1 | S | G2M |
|---|---|---|---|---|
| CTRL | 169±2.5 | 112±4.0 | 184±4.5 | 149±4.5 |
| CHL, 1 µM | 159±3.5 | 108±3.5 | 188±1.1 | 226±1.5 |
| ICR 191, 1.2 µM | 190±7.0 | 119±7.1 | 256±12.1 | 248±4.5 |
| ICR 191+CHL, 1 µM | 151±4.5 | 112±6.0 | 179±5.0 | 209±8.2 |
| ICR 191+CHL, 0.5 µM | 164±5.4 | 111±3.8 | 186±4.9 | 231±6.6 |
| ICR 191+CHL, 0.1 µM | 167±4.4 | 119±3.8 | 202±6.1 | 237±3.9 |
The cells were treated with these agents for 1 h and then intensity of γH2AX immunofluorescence concurrently with DNA content was measured in individual cells by flow cytometry as described in Material and methods section. The data show mean values of γH2AX expression (±SD) for all 10,000 cells measured in each sample as well as for cells in G1, S or G2M phases of the cell cycle, identified by the differences in cellular DNA content, as shown in Fig. 5 (left panel) and described in detail elsewhere [36–38]. Note an increase in γH2AX expression predominantly in S-phase cells in cells treated with ICR 191 alone, total elimination of the increase at 1 µM and 0.5 µM of CHL and partial suppression at 0.1 µM CHL. In all samples ICR 191 was present at 1.2 µM concentration.
The quantitative data representing mean values of the intensity of immunofluorescence of γH2AXcalculated either for all cells or separately for subpopulations of cells in G1, S or G2M phases of the cell cycle are shown in Table 1. These mean values were estimated by computer-assisted gating analysis of cells differing in DNA content (DI) as described above and reported before [36–38]. The data demonstrate that CHL, while alone had some effect increasing the level of γH2AX in G2M cell, at nearly equimolar concentration with ICR-191 (1.0 µM versus 1.2 µM) totally prevented the genotoxic effect of the mutagen as expressed by induction of H2AX phosphorylation in S-phase cells. Interestingly, the suppressive effect on induction of DNA damage by ICR-191 was also apparent at concentration of CHL as low as 0.5 µM and even 0.1 µM (Table 1).
4. Discussion
4.1. Interactions between ICR-191 and CHL
Our present data demonstrate that CHL forms complexes with ICR-191. The complex formation was reflected by the bathochromic shifts in the absorption spectra and strong quenching of ICR-191 fluorescence by CHL. The association constant, within the limits of the estimation errors, is high (2.8 × 105 M− 1±0.5 × 105 M− 1) when compared with complexes of other compounds considered as the interceptors with the mutagens. Thus, for example, the xanthines caffeine and pentoxifillin interact with ICR-191 and ICR-170 mutagens with association constants about 101 M−1 [28]. The association constant in the interaction between acridine orange and caffeine is 2 × 102 M−1 [42]. The interceptors in the green or black tea (catechins, epigallocatechins) interact with the aromatic mutagens with association constant within the range between 102 and 103 M−1 [43]. CHL thus can be considered much more effective interceptor of aromatic mutagens compared with the xanthines. In our earlier study we observed that CHL interacted with acridine orange, quinacrine mustard or doxorubicin with association constant approximately 105 M−1 [22]. Other authors report similar range of affinity of CHL in interaction with aflatoxin B1 [8], aflatoxin B2 [11] or dibenzo[a,1]pyrene [2,4]. This high affinity of CHL to intercept mutagens has already been recognized and CHL was applied in the clinic as a prophylactic agent for individuals having high level of aflatoxins in their diet [10,12] or in patients with trimethylaminuria [44].
4.2. Interactions between ICR-191 and DNA
The changes in absorption and fluorescence spectra of ICR-191 titrated with DNA are characteristic of intercalative mode of binding of this mutagen to DNA. The intrinsic association constant presently estimated (6.2 × 104 M−1±1.3 × 104 M−1) and parameter n=1.5 indicate on relatively low affinity. We have previously observed that interactions of DNA with acridine orange, quinacrine mustard or doxorubicin were stronger, with Ki being 2.4 × 105 M−1 (n=2.4), 2.7 × 105 M−1 (n=1.7) or 2.9 × 105 M−1 (n=1.8), respectively [23]. It should be noted, however, that these interactions are influenced by ionic strength and the binding affinity decreases with rise of salt concentration [45,46]. In the present study the ionic strength was higher (50 mM NaCl) compared with our previous studies in which we estimated binding affinity of other intercalators to DNA [23]. To some extent this may explain lower value of Ki in binding ICR-191 to DNA when compared to binding of quinacrine mustard [23], which is structurally similar do ICR-191. The difference, however, appears to be larger than expected if ionic strength would be the sole factor contributing to it. For example, a decrease of Ki from 1.5 × 105 M−1 to 5.0 × 104 M−1 was seen in binding of acridine orange to DNA when NaCl concentration was raised from 50 mM to 150 mM [45]. It should noted however that estimates of Ki and n for such large molecules such as DNA are hindered by difficulties in obtaining highly uniform solutions and by relatively small changes in absorption spectra. Thus even minor inaccuracies in the initial concentration of the prepared solutions may lower precision of the estimates.
4.3. Interactions within the mixture CHL–ICR–DNA
In the prior experiments we observed that CHL does not react with DNA, or if reacts, the interaction is so weak that is undetectable by the spectroscopy [23]. We adapted therefore the analytical model of simple competition between DNA and CHL for binding ICR-191. Theoretical calculations of concentrations of the individual components in the mixture DNA, CHL and one of the measured intercalators (acridine orange, quinacrine mustard or doxorubicin) were within the errors of the estimates based on spectrophotometric measurements [23]. Other authors also propose the model of simple competition in the interactions between DNA, mutagen and interceptor. This model was applied to study interactions between mutagen, caffeine and DNA [29] or mutagen, caffeine and vitamin B2 [47]. In the present study we applied theoretical calculations to estimate the role of changeable concentration of CHL on concentration of free ICR-191 and its binding to DNA. The calculations show that a two- to three- fold excess of CHL in relation to the this mutagen reduced by about two-fold concentration of the mutagen–DNA complex.
4.4. Protection of DNA in live cells
In the present study we also attempted to test whether CHL may protect DNA in live cells by exposing them to ICR-191 mutagen in the absence and presence of CHL. The extent of DNA damagewas assessed by measurement the induction of H2AX phosphorylation, the marker considered to report genotoxic changes in DNA molecule in chromatin [32–39]. Indeed in numerous studies phosphorylation of H2AX was shown to strongly correlate with other markers of DNA damage, such as assessed by single cell gel electrophoresis (comet assay), micronucleus assay or clonogenicity [33–37,48]. Our data show for the first time that exposure of cells to ICR-191 mutagen for 1 h was adequate to induce phosphorylation of H2AX. The phosphorylation was most pronounced in DNA replicating cells. Perhaps as in the case of cell treatment with certain intercalating agents such as DNA topoisomerase 2 inhibitors with aromatic structure resembling that of ICR-191, the interaction of the mutagen led to formation of the “cleavable complexes” and collision of DNA replication forks with these complexes converted them into DNA double-strand breaks, the potentially lethal lesions [49,50]. It is also possible that the mutagen caused local condensation of DNA in situ, preferentially in DNA replicating cells the cells, as described by us before [51], and this led to formation of DSBs. Whatever is the mechanismof induction of DNA damage by ICR-191 in live cells our data distinctly show that the presence of CHL at approximately equimolar concentration totally precluded the damage, at least as reported by H2AX phosphorylation. The protective effect most likely was due to the fact that ICR-191 once “intercepted” by CHL in solution of the culture media was unable to penetrate plasma membrane and thereby react with DNA. It is less likely, although perhaps not totally impossible, that such large molecule as CHL could easily penetrate through plasma membrane and intercept the mutagen within the cell. In conclusion, thus, our data provide further evidence that CHL, and by analogy, its insoluble form chlorophyll, are effective interceptors of flat aromatic mutagens that are able to induce DNA damage.
We recently reported that the background, constitutive, expression of γH2AX observed in untreated cells reports oxidative DNA damage caused by metabolically generated reactive species [38]. Because CHL is an antioxidant [7,8] it may have an ability to scavenge the radicals and thus potentially may attenuate constitutive DNA damage. In the present study, however, we observed that exposure of HL-60 cells to CHL alone had no significant effect on expression of γH2AX (Table 1). This lack of CHL effect on the level of constitutive DNA damage by endogenous oxidants may be due to the fact that the treatment was of short duration (1 h), much below the time required to see such effect [38]. Also, because of large size the CHL molecules could not effectively penetrate through plasma membrane of the cells to serve as scavenger of intracellular radicals.
Acknowledgments
Supported by BST 0706-802 (ZW) and NCI CA RO1 28 704 (ZD).
References
- 1.Arimoto S, Kan-yama K, Rai H, Hayatsu H. Inhibitory effect of hemin, chlorophyllin and related pyrrole pigments on the mutagenicity of benzo[a]pyrene and its metabolites. Mutat. Res. 1995;345:127–135. doi: 10.1016/0165-1218(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 2.Reddy AP, Harttig U, Barth MC, Baird WM, Schimerlik M, Hendricks JD, Bailey GS. Inhibition of dibenzo[a,l]pyrene-induced multi-organ carcinogenesis by dietary chlorophyllin in rainbow trout. Carcinogenesis. 1999;20:1919–1926. doi: 10.1093/carcin/20.10.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim MA, Elbehairy AM, Ghoneim MA, Amer HA. Protective effect of curcumin and chlorophyllin against DNA mutation induced by cyclophosphamide or benz[a]pyrene. Z. Naturforsch. 2007;62:215–222. doi: 10.1515/znc-2007-3-410. [DOI] [PubMed] [Google Scholar]
- 4.Pratt MM, Reddy AP, Hendricks JD, Pereira C, Kensler TW, Bailey GS. The importance of carcinogen dose in chemoprevention studies: quantitative inter-relationships between, dibenzo[a,1] pyrene dose, chlorophyllin dose, target organ DNA adduct biomarkers and final tumor outcome. Carcinogenesis. 2007;28:611–624. doi: 10.1093/carcin/bgl174. [DOI] [PubMed] [Google Scholar]
- 5.Dashwood RH, Guo D. Inhibition of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)- DNA binding by chlorophyllin: studies of enzyme inhibition and molecular complex formation. Carcinogenesis. 1992;13:1121–1126. doi: 10.1093/carcin/13.7.1121. [DOI] [PubMed] [Google Scholar]
- 6.Dashwood RH, Guo D. Antimutagenic potency of chlorophyllin in the Salmonella assay and its correlation with binding constants of mutagen — inhibitor complexes. Environ. Mol. Mutagen. 1993;22:164–171. doi: 10.1002/em.2850220309. [DOI] [PubMed] [Google Scholar]
- 7.Hernaez J, Xu M, Dashwood RH. Effects of tea and chlorophyllin on the mutagenicity of N-hydroxy — IQ: studies of enzyme inhibition, molecular complex formation, and degradation/scavenging of the active metabolites. Environ. Mol. Mutagen. 1997;30:468–474. doi: 10.1002/(sici)1098-2280(1997)30:4<468::aid-em12>3.0.co;2-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breinholt V, Hendricks J, Pereira C, Arbogast D, Bailey G. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995;55:57–62. [PubMed] [Google Scholar]
- 9.Dashwood RH, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1 a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 10.Kensler TW, Groopman JD, Roebuck BD. Use of aflatoxin adducts as intermediate endpoints to assess the efficacy of chemopreventive interventions in animals and man. Mutat. Res. 1998;402:165–172. doi: 10.1016/s0027-5107(97)00294-7. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Schimerlik M, Bailey G. Mechanisms of chlorophyllin anticarcinogenesis: dose-responsive inhibition of aflatoxin uptake and biodistribution following oral co-administration in rainbow trout. Toxicol. Appl. Pharmacol. 1999;158:132–140. doi: 10.1006/taap.1999.8695. [DOI] [PubMed] [Google Scholar]
- 12.Egner PA, Muñoz A, Kensler TW. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. 2003;523–524:209–216. doi: 10.1016/s0027-5107(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 13.García-Rodríguez MC, López-Satniago V, Altamirano-Lozano M. Effect of chlorophyllin on chromium trioxide-induced micronuclei in polychromatic erythrocytes in mouse peripheral blood. Mutat. Res. 2001;496:145–151. doi: 10.1016/s1383-5718(01)00225-x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SS, Chaubey RC, Devasagayam TP, Priyadarsini KI, Chauhan PS. Inhibition of radiation-induced DNA damage in plasmid pBR322 by chlorophyllin and possible mechanism(s) of action. Mutat. Res. 1999;425:71–79. doi: 10.1016/s0027-5107(98)00250-4. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SS, Shankar B, Sainis KB. Effect of chlorophyllin against oxidative stress in splenic lymphocytes in vitro and in vivo. Biochim. Biophys. Acta. 2004;1673:100–111. doi: 10.1016/j.bbagen.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Park KK, Park JH, Jung YJ, Chung WY. Inhibitory effect of chlorophyllin, hemin and tetrakis(4-benzoicacid)porphyrin on oxidative DNA damage and mouse skin inflammation induced by 12-O-tetradecanoylphorbol-13-acetate as a possible antitumor promoting mechanism. Mutat. Res. 2003;542:89–97. doi: 10.1016/j.mrgentox.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Arimoto S, Fukuoka S, Itome C, Nakano H, Rai H, Hayatsu H. Binding of polycyclic planar mutagens to chlorophyllin resulting in inhibition of the mutagenic activity. Mutat. Res. 1993;287:293–305. doi: 10.1016/0027-5107(93)90022-8. [DOI] [PubMed] [Google Scholar]
- 18.Arimoto-Kobayashi S, Harada N, Tokunaga R, Odo J, Hayatsu H. Adsorption of mutagens to chlorophyllin-chitosan, an insoluble form of chlorophyllin. Mutat. Res. 1997;381:243–249. doi: 10.1016/s0027-5107(97)00188-7. [DOI] [PubMed] [Google Scholar]
- 19.Hayatsu H, Sugiyama C, Arimoto-Kobayashi S, Negishi T. Porphirins as possible preventers of heterocyclic amine carcinogenesis. Cancer Lett. 1999;143:185–187. doi: 10.1016/s0304-3835(99)00122-6. [DOI] [PubMed] [Google Scholar]
- 20.Hartman PE, Shankel D. Antimutagens and anticarcinogens: a survey of putative interceptor molecules. Environ. Mol. Mutagen. 1990;5:145–182. doi: 10.1002/em.2850150305. [DOI] [PubMed] [Google Scholar]
- 21.Ardelt B, Kunicki J, Traganos F, Darzynkiewicz Z. Chlorophyllin protects cells from the cytostatic and cytotoxic effects of quinacrine mustard but not nitrogen mustard. Int. J. Oncol. 2001;18:849–853. doi: 10.3892/ijo.18.4.849. [DOI] [PubMed] [Google Scholar]
- 22.Pietrzak M, Wieczorek Z, Stachelska A, Darzynkiewicz Z. Interaction of chlorophyllin with acridine orange, quinacrine mustard and doxorubicin analyzed by light absorption and fluorescence spectroscopy. Biophys. Chemist. 2003;104:305–313. doi: 10.1016/s0301-4622(02)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrzak M, Wieczorek Z, Wieczorek J, Darzynkiewicz Z. The “interceptor” properties of chlorophyllin measured within the three-component system: intercalator-DNA-chlorophyllin. Biophys. Chemist. 2006;123:11–19. doi: 10.1016/j.bpc.2006.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferruzzi MG, Blakeslee J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007;27:1–12. [Google Scholar]
- 25.Kapuscinski J, Ardelt B, Piosik J, Zdunek M, Darzynkiewicz Z. The modulation of the DNA-damaging effect of polycyclic aromatic agents by xanthines. Part I. Reduction of cytostatic effects of quinacrine mustard by caffeine. Biochem. Pharmacol. 2002;63:625–634. doi: 10.1016/s0006-2952(01)00904-2. [DOI] [PubMed] [Google Scholar]
- 26.Traganos F, Kapuscinski J, Gong JP, Ardelt B, Darzynkiewicz RJ, Darzynkiewicz Z. Caffeine prevents apoptosis and cell cycle effects induced by camptothecin or topotecan in HL-60 cells. Cancer Res. 1993;53:4613–4618. [PubMed] [Google Scholar]
- 27.Bedner E, Du L, Traganos F, Darzynkiewicz Z. Caffeine dissociates complexes between DNA and intercalating dyes. Application for bleaching fluorochrome-stained cells for their subsequent re-staining and analysis by laser scanning cytometry. Cytometry. 2001;43:38–45. [PubMed] [Google Scholar]
- 28.Piosik J, Ulanowska K, Gwizdek-Wiśniewska A, Czyż A, Kapuściński J, Węgrzyn G. Alleviation of mutagenic effects of polycyclic aromatic agents (quinacrine mustard, ICR-191 and ICR-170) by caffeine and pentoxifiline. Mutat. Res. 2003;530:47–57. doi: 10.1016/s0027-5107(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 29.Evstigneev MP, Khomich VV, Davies DB. Complexation of anthracycline drugs with DNA in the presence of caffeine. Eur. Biophys. J. 2006;36:1–11. doi: 10.1007/s00249-006-0071-9. [DOI] [PubMed] [Google Scholar]
- 30.Sahasrabudhe SR, Luo X, Humayun MZ. Specificity of base substitutions induced by the acridine mutagen ICR-191: mispairing by guanine N7 adducts as a mutagenic mechanism. Genetics. 1991;129:981–989. doi: 10.1093/genetics/129.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman RK, Dworkin NB. Effect of gene induction on the rate of mutagenesis by ICR-191 in Escherichia coli. J. Bacteriol. 1971;106:543–550. doi: 10.1128/jb.106.2.543-550.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 33.Sedelnikova OA, Rogakou EP, Panuytin IG, Bonner W. Quantitative detection of 125IUdr-induced DNA double-strand breaks with γ-H2AX antibody. Radiat. Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.MacPhail SH, Banath JP, Yu TY, Chu EH, Lambur H, Olive PL. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int. J. Radiat. Biol. 2003;79:351–358. doi: 10.1080/0955300032000093128. [DOI] [PubMed] [Google Scholar]
- 35.Halicka HD, Huang X, Traganos F, King MA, Dai W, Darzynkiewicz Z. Histone H2AX phosphorylation after cell irradiation with UV-B: relationship to cell cycle phase and induction of apoptosis. Cell Cycle. 2005;4:339–345. [PubMed] [Google Scholar]
- 36.Huang X, Okafuji M, Traganos F, Luther E, Holden E, Darzynkiewicz Z. Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by DNA crosslinking agent cisplatin. Cytometry A. 2004;58A:99–110. doi: 10.1002/cyto.a.20018. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Halicka HD, Traganos F, Seiter K, Darzynkiewicz Z. Induction of ATM activation, histone H2AX phosphorylation and apoptosis by etoposide: relation to the cell cycle phase. Cell Cycle. 2007;6:371–376. doi: 10.4161/cc.6.3.3835. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry A. 2007;71A:905–914. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albino AP, Huang X, Yang J, Gietl D, Jorgensen E, Traganos F, Darzynkiewicz Z. Induction of histone H2AX phosphorylation in A549 human pulmonary epithelial cells by tobacco smoke and in human bronchial epithelial cells by smoke condensate: a new assay to detect the presence of potential carcinogens in tobacco. Cell Cycle. 2004;3:1062–1068. [PubMed] [Google Scholar]
- 40.Wieczorek Z, Stepinski J, Darzynkiewicz E, Lönnberg H. Association of nucleosides and their 5′-monophosphates with a tryptophan-containing tripeptide, Trp-Leu-Glu, by fluorescence spectroscopy. Biophys. Chemist. 1993;47:233–240. doi: 10.1016/0301-4622(93)80048-n. [DOI] [PubMed] [Google Scholar]
- 41.McGhee JD, von Hippel PH. Theoretical aspects of DNA — protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J. Mol. Biol. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- 42.Larsen RW, Jasuja R, Heltzer RK, Muraoka PT, Andrada VG, Jameson DM. Spectroscopic and molecular modeling study of caffeine complexes with DNA intercalators. Biophys. J. 1996;70:443–452. doi: 10.1016/S0006-3495(96)79587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernaez J, Meirong X, Dashwood R. Effects of tea and chlorophyllin on the mutagenicity of N-hydroxy — IQ: studies of enzyme inhibition molecular complex formation, and degradation /scavenging of the active metabolites. Environ. Mol. Mutagen. 1997;30:468–474. doi: 10.1002/(sici)1098-2280(1997)30:4<468::aid-em12>3.0.co;2-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamazaki H, Fujieda M, Togashi M, Saito T, Preti G, Cashman JR, Kamataki T. Effects of the dietary supplements, activated charcoal and copper chlorophyllin, on urinary excretion of trimethylamine in Japanese trimethylaminuria patients. Life Sci. 2004;74:2739–2747. doi: 10.1016/j.lfs.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Kapuscinski J, Darzynkiewicz Z. Interactions of acridine orange with double stranded nucleic acids. Spectral and affinity studies. J. Biomol. Struct. Dyn. 1987;5(1):127–143. doi: 10.1080/07391102.1987.10506381. [DOI] [PubMed] [Google Scholar]
- 46.Chaires JB, Dattagupta N, Crothers DM. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry. 1982;21:3933–3940. doi: 10.1021/bi00260a005. [DOI] [PubMed] [Google Scholar]
- 47.Evstigneev MP, Mykhina YV, Davies DB. Complexation of daunomycin with DNA oligomer in the presence of an aromatic vitamin (B2) determined by NMR spectroscopy. Biophys. Chemist. 2005;118:118–127. doi: 10.1016/j.bpc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Halicka HD, Traganos F, Darzynkiewicz Z. Phosphorylation of histone H2AX on Ser 139 and activation of ATM during oxidative burst in phorbol ester-treated human leukocytes. Cell Cycle. 2006;5:2671–2675. doi: 10.4161/cc.5.22.3472. [DOI] [PubMed] [Google Scholar]
- 49.Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 50.Del Bino G, Lassota P, Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp. Cell Res. 1991;193:27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- 51.Kapuscinski J, Darzynkiewicz Z. Relationship between the pharmacological activity of antitumor drugs Ametantrone and Mitoxantrone (Novantrone) and their ability to condense nucleic acids. Proc. Natl. Acad. Sci. U. S. A. 1986;83:6302–6306. doi: 10.1073/pnas.83.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]