Abstract
Aims
S100A8/A9 is expressed in activated monocytes/macrophages and assumed to be heavily involved in the pathogenesis of acute inflammation. Although several studies have asserted that S100A8/A9 has a proinflammatory function, the exact biological function of S100A8/A9 is yet to be described. We examined the anti-inflammatory effects of S100A8/A9 on experimental autoimmune myocarditis (EAM) in rats.
Methods and results
Experimental autoimmune myocarditis was induced in Lewis rats by immunization with porcine cardiac myosin. The recombinant (R-) S100A8/A9 was injected intraperitoneally into EAM rats. R-S100A8/A9 attenuated the severity of myocarditis, as evidenced by echocardiographic and histological findings. In addition, we found that not only the mRNA expression of proinflammatory cytokines [interleukin (IL)-1β, IL-6, and tumour necrosis factor (TNF)-α] in the myocardium, but also their serum concentrations were suppressed in EAM rats treated with R-S100A8/A9. Nuclear factor-kappa B expression in inflammatory cells was also suppressed in the treated rats. To elucidate the mechanistic function of S100A8/A9 on proinflammatory cytokines in vivo, we used an ELISA on the supernatant of homogenized heart tissue treated with R-S100A8/A9. The findings revealed high-affinity binding of R-S100A8/A9 with IL-1β, IL-6, and TNF-α in the myocardium, suggesting the trapping of proinflammatory cytokines by R-S100A8/A9.
Conclusion
S100A8/A9 attenuates EAM through modulation of the proinflammatory cytokine network.
Keywords: S100A8/A9, Autoimmune myocarditis, Cytokines, Inflammation
Introduction
S100A8/A9, consisting of two low molecular mass subunits (10.6 and 13.5 kDa), is a member of the S100 family and contains two calcium-binding sites per molecule.1,2 The two subunits are expressed in activated human granulocytes and macrophages in the inflammatory process.2,3 In activated human neutrophils and macrophages, S100A8 and S100A9 are non-covalently associated to rapidly form the heterodimer S100A8/A9 in a calcium-dependent manner.2–4 Translocation of the heterodimer from cytoplasm to membrane is induced in the presence of increased intracellular calcium concentration and correlates with the inflammatory action of activated granulocytes and/or macrophages. This protein is known to be involved in several infections and immune diseases, including advanced arthritis, transplant rejection, and sarcoidosis,5–7 suggesting that it plays an important role in the pathogenesis of the inflammatory process. Although several studies have suggested that S100A8/A9 has a proinflammatory function, we recently demonstrated that S100A8/A9 indirectly suppresses the overproduction of nitrous oxide in activated neutrophils and/or macrophages in rats with lipopolysaccharide-induced liver injury and revealed the binding of S100A8/A9 with proinflammatory cytokines in vitro.8 However, the presence of the S100A8/A9-proinflammatory cytokine complexes was not confirmed in vivo in that study.
Experimental autoimmune myocarditis (EAM) in rat models is characterized by severe myocardial damage with inflammatory cell infiltration. The EAM model has been widely used as a disease model of human myocarditis,9 and experimental data have documented that macrophages play a pivotal role in the inflammatory process of EAM in rats.10
To verify our hypothesis that S100A8/A9 has an anti-inflammatory effect and to clarify the mechanistic function of S100A8/A9 on proinflammatory cytokines in vivo, we treated the EAM rat model with newly prepared recombinant S100A8/A9.
Methods
Preparation of recombinant S100A8/A9
Expression of cDNA for S100A8 or S100A9 in Escherichia coli cells
cDNAs with the histidine tag sequence for the human S100A8 and S100A9 subunits were synthesized using PCR.11 The resulting cDNAs were inserted into pCold I vectors (Takara Bio, Shiga, Japan). The two different expression vectors were then separately transfected into E. coli cells. The S100A8 or S100A9 cDNA-transfected E. coli cells were cultivated in Miller’s LB Broth for 3 h at 37°C. When the absorbance of the culture medium at 600 nm ranged between 0.5 and 0.8, the culture bottle was quickly cooled to 15°C and cultivated for 24 h after adding a final concentration of 1 mmol/L IPTG. The cultivated cells were harvested and then frozen at −80°C until use.
Purification of S100A8 and S100A9 by Ni-agarose affinity column
The E. coli cells expressing S100A8 or S100A9 were suspended in 200 mL of binding buffer and then treated by an ultrasonic generator for 10 min at 4°C. After centrifugation at 17 400 g for 20 min at 4°C, the supernatant obtained was applied to a Ni-agarose affinity column equilibrated with the binding buffer. After washing the column with washing buffer, S100A8 and S100A9 were eluted from the column with the elution buffer. The fractions containing S100A8 or S100A9 were concentrated to an adequate volume using an Amicon Ultra centrifugal filter device (MW 5000; Millipore, Billerica, MA, USA), and their protein concentrations were obtained by measuring absorbance at 280 nm.
Synthesis and purification of S100A8/A9
Equal molar concentrations of S100A8 and S100A9 were mixed and the mixture was poured into an MW 5000 and incubated overnight in 2.0 mol/L Tris–NaOH solution (pH 12.0) at 4°C. It was dialyzed against 0.1 M Tris–HCl buffer (pH 10.0) containing 300 mM NaCl for 3–4 h at 4°C. After confirming the protein band of recombinant S100A8/A9 by SDS–PAGE, S100A8/A9 was partially purified on a gel filtration column (Sephacryl S-300 HR). Major fractions containing S100A8/A9 were pooled and concentrated to an adequate volume using the same filter device. The above procedures were repeated until the product was adequately concentrated.
Animals and immunization
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee, Osaka Medical College. Male Lewis rats (7 weeks old; body weight 200–250 g) were purchased from Japan SLC (Shizuoka, Japan). Before initiating the experiments, they were kept in cavity for 1 week with free access to food and water. The rats were immunized subcutaneously twice with 0.7 mg purified porcine cardiac myosin (Sigma Chemical Co., St Louis, MO, USA) in an equal volume of complete Freund’s adjuvant supplemented with Mycobacterium tuberculosis H37RA (Difco, Sparks, MD, USA) on Days 0 and 7.12
Administration of recombinant S100A8/A9
Immunized rats were randomly assigned to two groups: Group S (recombinant S100A8/A9, n = 20) and Group C (saline as vehicle, n = 20). Recombinant S100A8/A9 (1 mg/day) or saline was injected intraperitoneally into the immunized rats each day from Days 8 to 13. On Day 14 or 21, 10 rats in each group (S14, S21, C14, and C21) were sacrificed under ether anaesthesia. Rats that were neither immunized nor received S100A8/A9 were used as normal controls (N14 and N21, n = 5, respectively).
Echocardiography
Rats were lightly anaesthetized with pentobarbital sodium (1 mg/kg body weight i.p.) on Day 14 or 21. Echocardiography was performed with an echocardiographic apparatus equipped with a 10-MHz transducer (Vivid Five, General Electric-Vingmed, Milwaukee, WI, USA). Two-dimensional targeted M-mode echocardiograms were obtained along the short axis of the left ventricle at the level of the papillary muscles. Left ventricular dimensions at end-diastole (LVDd) and end-systole (LVDs) were measured. Left ventricular ejection fraction (LVEF) was calculated as follows: [(LVDd3 − LVDs3)/LVDd3] × 100.
Histological assessment
For the microscopic evaluation, apex, mid-ventricular, and basal level slices were stained with haematoxylin–eosin. The entire heart and the regions affected by myocarditis (i.e. regions showing inflammation with inflammatory cells and myocardial necrosis) were examined as described previously,10,13,14 using a computer-assisted analyzer (Scion Image Beta 4.03; Scion Corp, Frederick, MD, USA). The area ratio (percentage value of affected area/entire area) was calculated by two investigators (Y.K. and T.K.) who were blinded to slide identification; the inter- and intra-observer variance was <5%.
Immunohistochemistry
Anti-human S100A8/A9 (rabbit) antibody6 was used to determine the localization of endogenous S100A8/A9 in Group C14, with anti-rabbit immunogloblins/FITC swine polyclonal antibody (Dako, Denmark) as secondary antibody. Mouse monoclonal antibodies against CD68 were used as a marker for macrophages (AbD serotec, Raleigh, NC, USA), with labelled goat anti-mouse IgG antibody (10 µg/mL, Invitrogen, Carlsbad, CA, USA) as secondary antibody. The resulting fluorescent signal was detected by a fluorescence microscopy.
Rabbit monoclonal antibodies against nuclear factor-kappa B (NF-κB) p65 (Cell Signaling Technology, Danvers, MA, USA) were used to evaluate the activation of NF-κB. The primary antibodies were reacted to sections and immunoreactivity was evaluated using the avidin–biotin horseradish peroxidase complex method (ScyTek Laboratories, Logan, UT, USA). The reactions were optimized with diaminobenzidine chromogen.
Measurement of mRNA levels for proinflammatory cytokines in the heart
RNA isolation and cDNA preparation
RNA was extracted from the excised LV using the RNeasy Mini Kit (Qiagen). The concentration and purity of RNA was determined by measuring the optical density at 260 and 280 nm prior to use. Total RNA (1 µg) from each sample was used for reverse transcription. First strand cDNA was synthesized with random primers and Moloney murine leukaemia virus reverse transcriptase (SuperScript III; Invitrogen).
Quantitative RT–PCR analysis
Quantitative RT–PCR analysis was performed using the LightCycler Real-Time PCR System (Roche Molecular Biochemicals, Indianapolis, IN, USA) to detect the mRNA expression of interleukin (IL)-1β, IL-6, and tumour necrosis factor (TNF)-α. The expression of glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA was measured as the internal control. Assays were designed using the Roche Universal Probe Library (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp). For each reaction, the LightCycler Taqman Master Kit (Roche Molecular Biochemicals) was used according to the manufacturer’s instructions. PCR cycling was conducted under the following conditions: preheating for 1 cycle at 95°C for 10 min, amplification for 45 cycles at 95°C for 10 s and 60°C for 25 s, and final cooling to 40°C. mRNA levels were quantified and normalized against levels of GAPDH. The averaged and normalized levels of mRNA in each control group were expressed as 1.0.
Measurement for serum concentrations of proinflammatory cytokines
Serum concentrations of IL-1β, IL-6, and TNF-α were determined by enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Detection of S100A8/A9–proinflammatory cytokine complexes in heart tissue
The S100A8/A9–proinflammatory cytokine complexes were detected as follows: Heart tissue from EAM rats treated with recombinant S100A8/A9 (Group S14) was quickly homogenized after sacrifice and centrifuged at 7700 g at 4°C. Supernatant was assayed by ELISA in which anti-S100A8/A9 monoclonal antibody was used as the first antibody. S100A8/A9 of the complexes reacted first to the antibody. Subsequently, biotinylated anti-rat IL-1β, IL-6, and TNF-α IgGs were added to the wells and incubated for 1 h at room temperature. After washing with washing buffer, 100 µL of diluted streptavidin–horseradish peroxidase conjugate was added and further incubated for 30 min at room temperature. After washing, colour development of the plate was achieved by adding 100 µL of colour reagent (30 mg o-PD/10 µL of 30% hydrogen peroxide/20 mL of citrate buffer, pH 5.0), and terminated by adding 100 µL of 0.75 mol/L sulfuric acid. The concentrations of the S100A8/A9–IL-1β, IL-6, and TNF-α complexes were obtained using a standard curve for S100A8/A9.
Statistical analysis
Data are expressed as the mean ± SE for continuous variables and as numbers (%) for categorical variables. Multiple comparisons among three groups were performed by ANOVA followed by Scheffe’s test (SPSS; Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
Results
Infiltration of macrophages and S100A8/A9
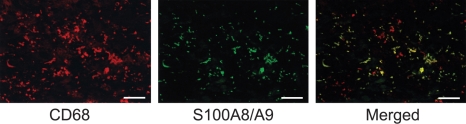
We examined the localization of endogenous S100A8/A9 in the inflamed myocardium of EAM rats by a double staining method using the fluorescent immunohistochemistry. Most of the infiltrating mononuclear cells were positive for CD68, and all cells positive for S100A8/A9 were positive for CD68 (Figure 1).
Figure 1.
Infiltration of macrophages and endogenous S100A8/A9 in cardiac tissue of rats with EAM. Most of the infiltrating mononuclear cells were positive for CD68, and all cells positive for S100A8/A9 were positive for CD68 (merged). Bar indicates 25 µm.
Purification of recombinant S100A8/A9
Recombinant S100A8/A9 was purified successfully. As determined by densitometry, the purity of recombinant S100A8/A9 was ∼93% protein concentration (Figure 2). The recombinant S100A8/A9 was used in the subsequent study.
Figure 2.
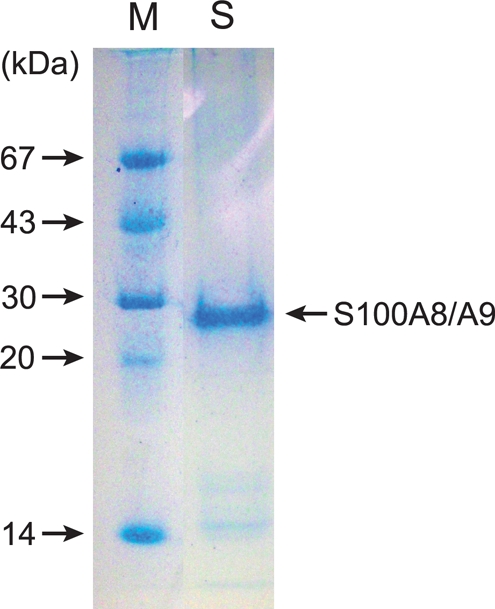
Purification of recombinant S100A8/A9. SDS–PAGE was performed as described in the Methods section. Lane S: purified recombinant S100A8/A9. As determined by densitometry, the purity of recombinant S100A8/A9 as a protein concentration was ∼93%. Lane M: this lane contains molecular mass markers.
Effect of treatment with recombinant S100A8/A9 on the severity of myocarditis
Group S14 vs. Group C14
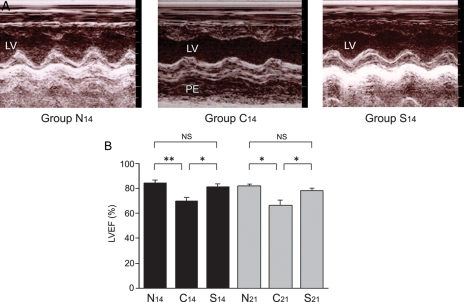
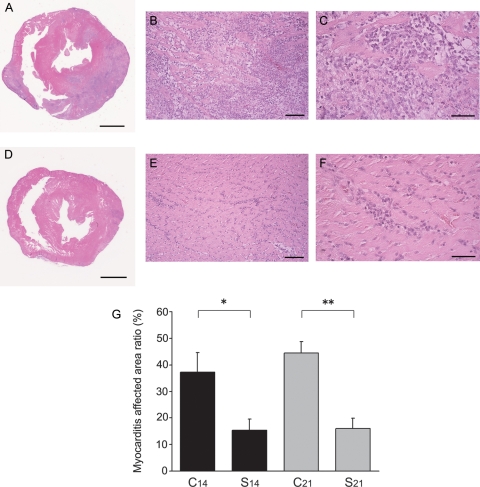
On macroscopic observation, the hearts in Group C14 were enlarged and contained large, dark-blue–greyish areas with massive pericardial effusion. The hearts in Group S14, however, were slightly enlarged, had small, dark-blue–greyish areas, and showed almost no pericardial effusion. Echocardiography revealed that LVEF in Group S14 was significantly higher than that in Group C14 (81 ± 2% vs. 70 ± 3%, P = 0.017) (Figure 3A and B). There was no significant difference in LVEF between Groups N14 and S14 (85 ± 2% vs. 81 ± 2%). The heart weight (1.02 ± 0.03 g vs. 1.21 ± 0.06 g, P = 0.015) as well as the heart weight/body weight ratio (0.41 ± 0.02% vs. 0.55 ± 0.03%, P = 0.002) in Group S14 were significantly lower than that in Group C14 (Table 1). The area ratio of myocarditis in microscopic grading was significantly lower in Group S14 than that in Group C14 (15.4 ± 4.2% vs. 37.2 ± 7.3%, P = 0.036) (Figure 4A–G).
Figure 3.
Echocardiographic findings. (A) Representative M-mode echocardiographic findings. Non-treated EAM rats (Group C14) showed reduced left ventricular ejection fraction (LVEF) and pericardial effusion on Day 14. Recombinant S100A8/A9 administration from Days 8 to 13 (Group S14) improved LVEF and suppressed pericardial effusion. (B) EF (%) in Groups N14, C14, S14, N21, C21, and S21. *P < 0.05; **P < 0.01.
Table 1.
Effect of S100A8/A9 on heart weight, body weight, and heart/body weight ratio
| Day 14 |
Day 21 |
|||||
|---|---|---|---|---|---|---|
| Normal control (Group N14, n = 5) | EAM (Group C14, n = 10) | EAM with S100A8/A9 (Group S14, n = 10) | Normal control (Group N21, n = 5) | EAM (Group C21, n = 10) | EAM with S100A8/A9 (Group S21, n = 10) | |
| Heart weight (g) | 0.89 ± 0.02 | 1.21 ± 0.06# | 1.02 ± 0.03* | 0.97 ± 0.01 | 1.16 ± 0.04# | 0.99 ± 0.05* |
| Body weight (g) | 279 ± 6 | 221 ± 5## | 248 ± 6#,** | 297 ± 4 | 242 ± 4## | 256 ± 6## |
| Heart/body weight ratio (%) | 0.32 ± 0.004 | 0.55 ± 0.03## | 0.41 ± 0.02** | 0.33 ± 0.004 | 0.48 ± 0.02## | 0.39 ± 0.02** |
#P < 0.05 (vs. Group N).
##P < 0.01 (vs. Group N).
*P < 0.05 (vs. Group C).
**P < 0.01 (vs. Group C).
Figure 4.
Representative cross-sections of heart. (A) Histopathological findings of specimen obtained from the midportion of the left ventricle of a vehicle rat (Group C14). (B and C) Severe infiltration of relatively large mononuclear cells was observed in myocardium (Group C14). (D) Cross-section of heart from a rat treated with recombinant S100A8/A9 (Group S14). (E and F) Less severe infiltration of inflammatory cells is revealed (Group S14). (G) Myocarditis-affected area ratio in the respective groups. Bar indicates 5 mm in (A and D), 100 µm in (B and E), and 50 µm in (C and F). *P < 0.05; **P < 0.001.
Group S21 vs. Group C21
Echocardiography revealed a significant difference in LVEF between Groups S21 and C21 (78 ± 2% vs. 67 ± 4%, P = 0.049) (Figure 3B). The heart weight/body weight ratio in Group S21 was significantly lower than that in Group C21 (0.39 ± 0.02% vs. 0.48 ± 0.02%, P = 0.009) (Table 1). The area of myocarditis was significantly smaller in Group S21 than that in Group C21 (16.0 ± 3.8% vs. 44.3 ± 4.4%, P < 0.001) (Figure 4G).
Effect of treatment with recombinant S100A8/A9 on mRNA expression of proinflammatory cytokines
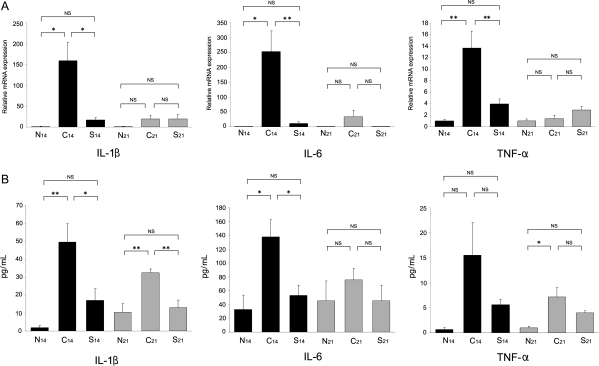
Real-time RT–PCR analysis was performed to assess the expression of IL-1β, IL-6, and TNF-α. The mRNA expression of these proinflammatory cytokines in the myocardium was significantly depressed in Group S14 compared with Group C14. However, between Groups S21 and C21, there were no significant differences in the mRNA expression of IL-1β, IL-6, and TNF-α (Figure 5A).
Figure 5.
Effect of recombinant S100A8/A9 on proinflammatory cytokines: mRNA expression in EAM heart and serum concentrations. (A) Total RNA was extracted from the heart and real-time RT–PCR analysis was performed. Bar graphs show relative mRNA expression of IL-1β, IL-6, and TNF-α. *P < 0.05, **P < 0.01. (B) Serum IL-1β, IL-6, and TNF-α concentrations were determined using an ELISA method. The Y-axis represents the concentrations of each proinflammatory cytokine. *P < 0.05; **P < 0.01.
Effect of treatment with recombinant S100A8/A9 on serum proinflammatory cytokine concentrations
The serum IL-1β and IL-6 concentrations in Group S14 markedly decreased compared with those in group C14 (P = 0.008 and P = 0.019, respectively). The serum TNF-α concentration in Group S14 tended to decrease compared with that in Group C14. On Day 21, there was a significant difference only in the serum concentration of IL-1β (P = 0.002) between Groups S21 and C21 (Figure 5B).
Suppression of NF-κB expression in the heart of S100A8/A9-treated rats
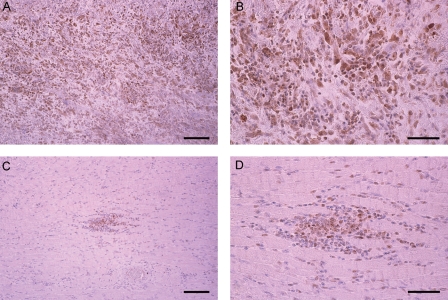
To evaluate NF-κB activity, we immunohistochemically examined the expression of the nuclear p65 protein in the myocardium. This revealed that the expression of p65 was enhanced in Group C14, whereas in Group S14, it was significantly suppressed (Figure 6). There was no detectable expression of p65 in naive rat hearts in Group N14.
Figure 6.
Representative results of immunohistochemical detection of NF-κB. Enhanced expression of NF-κB (p65) was observed in non-treated EAM heart (Group C14) (A and B), while recombinant S100A8/A9 (Group S14) suppressed expression (C and D). Bar indicates 100 µm in (A and C) and 50 µm in (B and D).
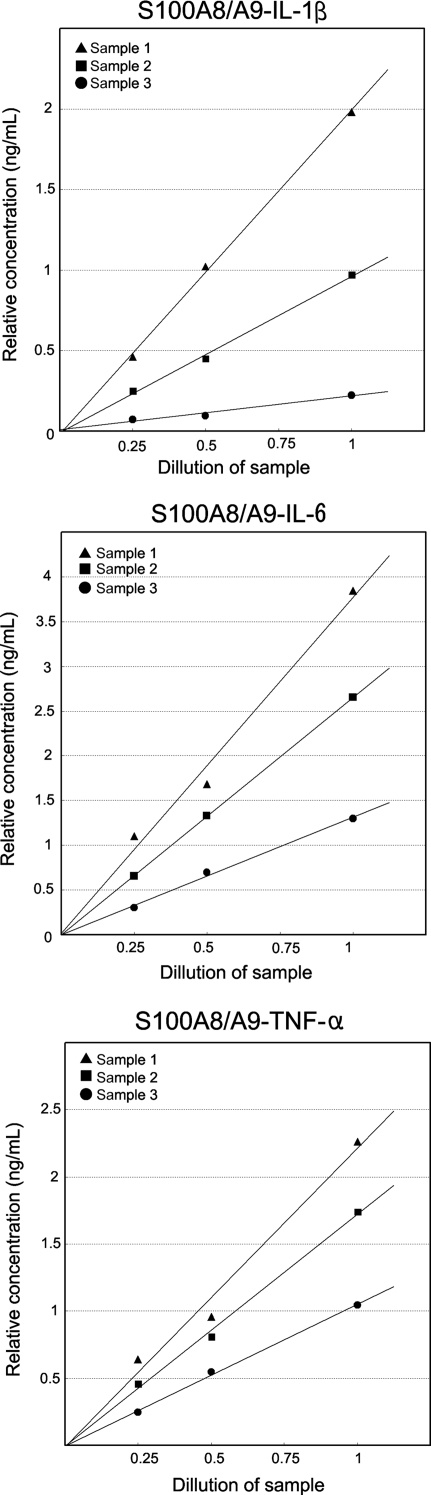
In vivo binding between S100A8/A9 and proinflammatory cytokines
To confirm the in vivo binding of S100A8/A9 with IL-1β, IL-6, and TNF-α, we used the ELISA test for S100A8/A9 to the supernatant of the homogenized heart tissue from EAM rats treated with recombinant S100A8/A9 (Group S14). We demonstrated three kinds of S100A8/A9–proinflammatory cytokine complexes in heart tissue. As determined by ELISA, these complexes were quantitatively measured (Figure 7).
Figure 7.
Evidence of binding of S100A8/A9 with proinflammatory cytokines. For detection of S100A8/A9–proinflammatory cytokine complexes, we used the ELISA plate coated with anti-S100A8/A9 monoclonal IgG (Mo2B9). Biotinylated anti-rat IL-1β, IL-6, and TNF-α IgGs were used as the second antibody. Finally, colour development was achieved by measuring horseradish peroxidase activity after incubation of streptavidin–horseradish peroxidase conjugate for 30 min. The reaction was significantly positive and quantitative, indicating the existence of S100A8/A9–cytokine complexes. This is a representative result of three samples.
Discussion
We first examined the localization of endogenous S100A8/A9 in the inflamed myocardium of EAM rats. Fluorescent immunohistochemistry revealed that mononuclear cells both positive for CD68 and S100A8/A9 infiltrated into the myocardium to a great extent. This suggested that S100A8/A9 might play an important role in the pathogenesis of acute inflammation in the EAM model. However, it remains unclear how endogenous S100A8/A9 acts in this myocarditis model.
The pathogenic mechanism of the EAM model involves three sequential processes: (i) autoreactive macrophages and T lymphocytes are activated and expanded by a fragment of cardiac myosin; (ii) activated macrophages and T lymphocytes are recruited to the target organ; and (iii) effector–target interaction occurs.14 Thus, inflammation of the EAM model consists mainly of macrophages and T lymphocytes. During the inflammatory phase, proinflammatory and Th1-type cytokines (e.g. IL-1β, IL-6, TNF-α, IFN-γ, and IL-2) are produced, which induce inflammation.15,16 Pilot studies of the EAM model have shown that the expression of IL-1β, IL-6, and TNF-α in heart tissue had already increased on Day 14 when histological myocarditis did not reach the severest phase. Therefore, to verify our hypothesis that S100A8/A9 suppresses the inflammation of EAM by neutralizing the activity of proinflammatory cytokines, we intraperitoneally injected the recombinant S100A8/A9 into immunized EAM rats from Days 8 to 13 and sacrificed them on Day 14 or 21. On Day 14, data such as echocardiographic parameters, heart weight/body weight ratio, and histological assessment revealed that acute inflammation in recombinant S100A8/A9-treated EAM rats (Group S14) was significantly suppressed compared with the vehicle group that had not received S100A8/A9 (Group C14). Additionally, on Day 21, when histologically severe myocarditis was reported to be observed in EAM rats,15 the area affected with myocarditis in the S100A8/A9-treated EAM rats (Group S21) was significantly smaller than that in the vehicle group (Group C21).
These data indicate that treatment with S100A8/A9 inhibits the development of acute inflammation in EAM, and are in agreement with the results of our previous study that intraperitoneal injection of S100A8/A9 suppresses liver injury induced by lipopolysaccharides in rats.8 In the present study, the mRNA expression of IL-1β, IL-6, and TNF-α in the myocardium was dramatically suppressed in Group S14 compared with Group C14, whereas there was no significant difference in the mRNA expression of these cytokines between Groups S21 and C21. It has been reported that the mRNA expression of proinflammatory cytokines in the EAM model has only one peak during the inflammatory phase.15 Thus, it may be said that mRNA levels of proinflammatory cytokines in our EAM model peaked around Day 14, and that recombinant S100A8/A9 ameliorated the peak expression of proinflammatory cytokines. On Day 21, there was no significant difference in mRNA levels of proinflammatory cytokines between Groups S21 and C21, which may be due to the natural decrease in the mRNA expression of these cytokines in Group C21.15
NF-κB is a rapid-response transcription factor that regulates the expression of the genes encoding cytokines, chemokines, and adhesion molecules. NF-κB exists in the cytoplasm as a heterodimer of 50-kDa (p50) and 65-kDa (p65) subunits associated with an inhibitory protein of the IκB family. When cells are stimulated, the IκB inhibitory protein is phosphorylated and dissociates from the NF-κB heterodimer, followed by translocation of free NF-κB into the nucleus.17 In the cytokine network, in which the actions of certain cytokines are regulated by the activity of others, IL-1β and TNF-α both activate and are activated by NF-κB.17–20 In addition, NF-κB-binding sequences have been found in the promoter regions of cytokine genes associated with inflammatory responses, including IL-6, TNF-α, IL-2, and the IL-2 receptor.20,21 In our EAM model, immunohistochemical findings demonstrated that S100A8/A9 suppressed the activation of NF-κB as reflected by p65. Treatment with S100A8/A9 ameliorated the expression of proinflammatory cytokines and the activity of NF-κB; this suggests that S100A8/A9 has a suppressive function in the cytokine network.
In our previous study, affinity chromatography was performed using the purified S100A8/A9–Sepharose 4B column to confirm the binding of S100A8/A9 with proinflammatory cytokines.8 A significant amount of IL-1β, IL-6, and TNF-α was eluted from the column, but anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β were not, indicating that S100A8/A9 binds with these proinflammatory cytokines in vitro.8 However, the presence of the S100A8/A9–proinflammatory cytokine complexes was not documented in vivo in that study. Therefore, in the present study, effort was made to clarify the presence of these complexes in vivo using an antibody specific for S100A8/A9 in the ELISA system. It was established that in the acute phase of EAM, S100A8/A9 binds to at least three kinds of proinflammatory cytokines, IL-1β, IL-6, and TNF-α, in vivo. On the other hand, using anti-inflammatory cytokines IgGs as the second antibody, biotinylated anti-rat IL-4 and TGF-β IgGs, the reaction was not detected in the ELISA system (data not shown). It has been reported that extracellular S100A8/A9 interacts with binding sites on specific surface molecules, such as heparan sulfate, carboxylated glycans, and arachidonic acid.22–24 Thus, S100A8/A9 may be a protein with multiple binding sites for many substances and may often bind to proinflammatory cytokines. The present study revealed that mRNA expression in the myocardium and also serum concentrations of proinflammatory cytokines were significantly decreased by the S100A8/A9 treatment. Possible causes of this effect may be the binding of the proinflammatory cytokines with S100A8/A9, as well as a decrease in cytokine production via the suppression of mRNA. Treatment with S100A8/A9 presents a new mechanistic approach for mitigating inflammation in the EAM model by trapping proinflammatory cytokines and altering the cytokine network.
Although the biological function of S100A8/A9 is yet to be described in detail, it has been proposed that S100A8/A9 has several functions, including antimicrobial activity, enhancement of transendothelial leucocyte migration, and induction of apoptosis.25–27 While several studies to date have asserted that S100A8/A9 has a proinflammatory function,28,29 few have revealed its anti-inflammatory function. The present study may shed some light on the novel anti-inflammatory function of S100A8/A9, which occurs by its binding to proinflammatory cytokines and modulating the cytokine network. Thus, treatment with S100A8/A9 is capable of neutralizing several kinds of proinflammatory cytokines, which may be unique because so-called anti-cytokine therapy generally targets a certain cytokine.
To summarize, we found that treatment with recombinant S100A8/A9 attenuated acute myocarditis in rats with EAM. At least three kinds of S100A8/A9 complexes with IL-1β, IL-6, and TNF-α were found in the inflamed organ tissues, which might have contributed to the reduction in acute inflammatory responses.
Study limitations
There are some limitations to the present study. First, the dosage of S100A8/A9 (1 mg/day) was chosen based on our previous study.8 Myocarditis was not suppressed significantly when one-tenth of the dosage (0.1 mg/day) was given to EAM rats in the preliminary experiment. We did not attempt to use any other doses of S100A8/A9, which might have led to different results. Since only a single timing framework for S100A8/A9 administration was used in this study, the time dependency of the observed effects could not be confirmed. Further investigations on the efficacy of S100A8/A9 at different dosages and timings are therefore needed. Second, Th1/Th2 balance has been reported to play an important role in the pathogenesis of the inflammatory process in the EAM model.13 As a counter-regulator of inflammatory cytokines, the suppressor of cytokine signalling (SOCS) family has also attracted attention.30 However, we could not evaluate the involvement of Th1/Th2 balance and SOCS in the present study. Further studies on these factors are necessary.
Conflict of interest: none declared.
Funding
This study was supported in part by a research grant for intractable diseases from the Ministry of Health, Labour and Welfare of Japan (YK), a grants-in-aid for scientific research from the Ministry of Health, Labour and Welfare of Japan (FT), and a grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 20590567) (MI).
References
- 1.Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448:200–211. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- 2.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 3.Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 4.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991;266:13462–13467. [PubMed] [Google Scholar]
- 5.Frosch M, Vogl T, Seeliger S, Wulffraat N, Kuis W, Viemann D, Foell D, Sorg C, Sunderkötter C, Roth J. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2003;48:2622–2626. doi: 10.1002/art.11177. [DOI] [PubMed] [Google Scholar]
- 6.Ikemoto M, Tanaka T, Takai Y, Murayama H, Tanaka K, Fujita M. New ELISA system for myeloid-related protein complex (MRP8/14) and its clinical significance as a sensitive marker for inflammatory responses associated with transplant rejection. Clin Chem. 2003;49:594–600. doi: 10.1373/49.4.594. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki F, Fujita M, Shimomura H, Tsukada B, Otsuka K, Otsuka K, Katashima T, Ikemoto M, Kitaura Y. Enhanced expression of myeloid related protein complex (MRP8/14) in macrophages and multinucleated giant cells in granulomas of patients with active cardiac sarcoidosis. Circ J. 2007;71:1545–1550. doi: 10.1253/circj.71.1545. [DOI] [PubMed] [Google Scholar]
- 8.Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007;376:197–204. doi: 10.1016/j.cca.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization, with cardiac myosin. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 10.Kamiyoshi Y, Takahashi M, Yokoseki O, Yazaki Y, Hirose S, Morimoto H, Watanabe N, Kinoshita O, Hongo M, Ikeda U. Mycophenolate mofetil prevents the development of experimental autoimmune myocarditis. J Mol Cell Cardiol. 2005;39:467–477. doi: 10.1016/j.yjmcc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Qing G, Ma LC, Khorchid A, Swapna GV, Mal TK, Takayama MM, Xia B, Phadtare S, Ke H, Acton T, Montelione GT, Ikura M, Inouye M. Cold-shock induced high-yield protein production in Escherichia coli. Nat Biotechnol. 2004;22:877–882. doi: 10.1038/nbt984. [DOI] [PubMed] [Google Scholar]
- 12.Matsui Y, Okamoto H, Jia N, Akino M, Uede T, Kitabatake A, Nishihira J. Blockade of macrophage migration inhibitory factor ameliorates experimental autoimmune myocarditis. J Mol Cell Cardiol. 2004;37:557–566. doi: 10.1016/j.yjmcc.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail. 2007:152–159. doi: 10.1016/j.ejheart.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kodama M, Hanawa H, Zhang S, Saeki M, Koyama S, Hosono H, Miyakita Y, Katoh K, Inomata T, Izumi T, Shibata A. FK506 therapy of experimental autoimmune myocarditis after onset of the disease. Am Heart J. 1993;126:1385–1392. doi: 10.1016/0002-8703(93)90538-k. [DOI] [PubMed] [Google Scholar]
- 15.Okura Y, Yamamoto T, Goto S, Inomata T, Hirono S, Hanawa H, Feng L, Wilson CB, Kihara I, Izumi T, Shibata A, Aizawa Y, Seki S, Abo T. Characterization of cytokine and iNOS mRNA expression in situ during the course of experimental autoimmune myocarditis in rats. J Mol Cell Cardiol. 1997;29:491–502. doi: 10.1006/jmcc.1996.0293. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Karin M. Nuclear factor-kappa B: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim JJ, Feldmann M. Introduction of the role of cytokines in innate host defense and adaptive immunity. In: Oppenheim JJ, Feldmann M, Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine Reference. San Diego: Academic Press; 2001. pp. 3–20. [Google Scholar]
- 19.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 20.Van Miert AS. Present concepts on the inflammatory modulators with special reference to cytokines. Vet Res Commun. 2002;26:111–126. doi: 10.1023/a:1014043601287. [DOI] [PubMed] [Google Scholar]
- 21.Ginn-Pease ME, Whisler RL. Redox signals and NF-kappa B activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 22.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277:3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- 23.Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166:4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 24.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 25.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 26.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 27.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–760. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]
- 28.Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37–51. doi: 10.1016/j.cccn.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Metcalf D, Di Rago L, Mifsud S, Hartley L, Alexander WS. The development of fatal myocarditis and polymyositis in mice heterozygous for IFN-gamma and lacking the SOCS-1 gene. Proc Natl Acad Sci USA. 2000;97:9174–9179. doi: 10.1073/pnas.160255197. [DOI] [PMC free article] [PubMed] [Google Scholar]