Abstract
Aims
A standard metric to estimate absolute treatment effects is numbers-needed-to-treat (NNT), which implicitly assumes that all benefits reverse at trial-end. However, in-trial survival benefits typically do not reverse until long after trial-end, so that NNT will substantially underestimate lifetime benefits.
Methods and results
We developed a new concept, years-needed-to-treat (YNT) to add 1 year of life, that quantifies the expected average life expectancy for two treatments including the estimated years of life remaining post-trial. Numbers-needed-to-treat and YNT were calculated in the COMET trial, in which carvedilol vs. metoprolol tartrate resulted in 17% lower mortality over 4.8 years. A multivariate Cox model was used to predict survival. Remaining years of life were estimated using the mortality-life-table method. At trial-end, survival was 9% higher in the carvedilol arm. Assuming that patients remained on the same therapy post-trial, the average total years of life for carvedilol vs. metoprolol were 10.63 ± 0.19 vs. 9.48 ± 0.18 (P < 0.0001) or 1.15 (95% confidence interval 0.64–1.66) additional years of life. The YNT was 9.2, indicating that 9.2 person-years of treatment added 1 person-year of life, compared with NNT of 59.
Conclusion
Compared with NNT, the YNT method more accurately accounts for potential long-term benefits of interventions in randomized trials.
Keywords: Heart failure, Prognostication, Outcomes, Epidemiology, Gompertz, Beta-blocker, Number-needed-to-treat, Years-needed-to-treat
Introduction
Use of effective therapies in heart failure and cardiovascular disease is an area of particular interest.1 A common metric to estimate effectiveness is the numbers-needed-to-treat (NNT),2,3 which limits the entire potential benefit to the period within the trial. In other words, the implicit unstated assumption of NNT is that all survival benefits that accrue during the trial are completely and immediately reversed at the end of the trial, i.e. any additional individuals surviving in the treatment arm immediately die at end-trial. Because this is very unlikely to be the case, this metric of effectiveness can substantially underestimate the true benefit of an intervention over the remaining lifetime of a patient, for example, as seen with long-term follow-up of enalapril in SOLVD4 and CONSENSUS trials,5 simvastatin in the 4S trial,6 and streptokinase in the ISIS-2 trial.7 This is best illustrated by streptokinase therapy in ISIS-2, in which the life-years saved per patient treated was 0.0029 during the 35 days post-myocardial infarction,7 but increased to ∼0.23 years (an 80-fold increase) over the subsequent 10 years of follow-up.7 Similarly, in GUSTO, patients receiving tissue plasminogen activator vs. streptokinase for an acute myocardial infarction gained 0.011 years at 1 year, but gained an estimated 0.14 years over a lifetime.8 In SOLVD, the benefit of enalapril during the trial was 0.16 years,4 whereas the benefit at 12 years was ∼0.72 years.4
Challenges with estimation of long-term benefits include estimating the benefit following the duration of observation (e.g. does the in-trial accrued benefit remain, increase, or decrease) and prediction of long-term survival. These issues are particularly relevant for medical therapy of heart failure, which may double lifetime survival9 [for example, therapy with an angiotensin-converting enzyme inhibitor (ACEI)10,11 and a β-blocker12–14]. Multivariate risk models have been developed for hospitalized or ambulatory heart failure patients. Most of these predict short (30 day)- or intermediate-term survival (1 or 2 years).15–17 A recent validated model, the Seattle Heart Failure Model, includes estimates of 1–5-year survival and average remaining years of life.9 This model allows users to estimate changes in short- and long-term survival due to alterations in heart failure medications and devices. Palm, PocketPC, PC, Mac, and web versions are available at SeattleHeartFailureModel.org. We applied a variation of this model to the Carvedilol or Metoprolol European Trial (COMET) in moderate heart failure patients18 to estimate the survival of patients after the end of the COMET trial. We developed a new concept of years-needed-to-treat (YNT), a metric that includes the estimated years of life remaining post-trial, to quantify the number of years of treatment required to add one person-year of life.
Methods
Participants
COMET was a randomized trial of carvedilol vs. metoprolol tartrate in 3029 patients with moderate heart failure. During a mean 4.8 years of follow-up, carvedilol resulted in a 17% [95% confidence interval (CI) 7–26%, P = 0.0017] relative reduction in mortality compared with metoprolol.18 At trial-end, 9% more patients were alive in the carvedilol arm.
Development of the model
Using a participant-level de-identified database and methods previously established for the Seattle Heart Failure Model,9 a multivariate Cox model was derived that included age, gender, ischaemic aetiology, New York Heart Association (NYHA) functional class, ejection fraction (EF), systolic blood pressure, heart rate, cancer, diabetes, serum sodium, haemoglobin, creatinine, anti-arrhythmic use, digoxin use, NSAID use, and aldosterone blocker use. Doses of ACEI, angiotensin receptor blockers, statins, carvedilol, metoprolol, open label β-blockers, loop, and thiazide diuretics were also entered in the model. Baseline data for EF, cancer, and diabetes were used; other available time-dependent variables were updated at 4 months, 8 months, and then yearly thereafter. Missing variables were imputed with the mean if missing at baseline, interpolated if early and later values were available, or the last value carried forward if no later value was available. Statistically significant univariate variables were visually inspected for linearity using a plot of the variable by deciles vs. the natural log of the hazard ratio. If the response was non-linear, simple transformations were used.9 A stepwise multivariate Cox model used a P-value of ≤0.05 for inclusion and >0.05 for exclusion.
The predicted 5-year survival by deciles of the model was plotted against the actual 5-year (Kaplan–Meier) survival for each decile. A correlation coefficient and standard error of the estimate were calculated. Accuracy of the model across deciles was determined by comparing the 5-year predicted with actual survival. Model discriminant ability was determined by the 1-year receiver-operating characteristic area under the curve (ROC AUC).
Estimation of long-term survival
For patients censored alive at trial-end, the most recent predicted survival from the modified risk model was used to estimate mean life years remaining as previously described, referred hereafter as the Levy method.9 We also used alternative methods of estimating individual patient post-trial survival, including the Gompertz19 and Deale20 methods. For the Deale method, estimated survival was based on age and gender mortality life tables.21 Total years of survival was calculated as survival during the trial (in-trial) plus the estimated years of survival following the conclusion of the trial (estimated post-trial). The additional years of life gained for each year of therapy was calculated [(total survival with therapy−total survival without therapy)/total survival with therapy]. The inverse of this value defined the YNT. Years-needed-to-treat was calculated on the basis of the scenario that all patients continue randomized treatment post-trial. In sensitivity analyses, YNT was also calculated on the basis of the scenarios that (i) all patients stop all therapy post-trial, (ii) all patients switch from metoprolol tartate to carvedilol therapy post-trial, or (iii) all patients live an additional 5 years post-trial regardless of therapy (the latter scenario being unrealistic but nevertheless a less drastic extension of the NNT assumptions). Numbers-needed to-treat was calculated as the 1/average annual difference in survival during the trial.
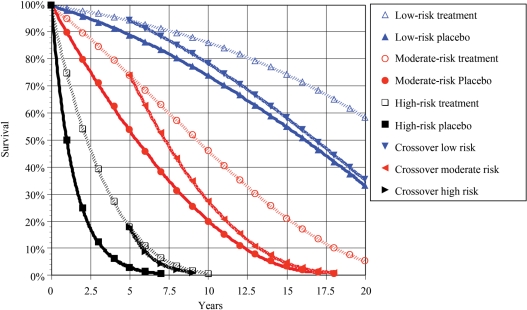
An alternative method to estimate the YNT is to draw a horizontal line from the treatment curve to the placebo curve at the end of the trial. This assumes that both arms have parallel survival after the end of the trial, i.e. lack of either continued benefit or reversal of benefit. Thus, the YNT is determined by using the horizontal distance between the two arms of the trial, whereas the NNT uses the vertical distance. This simplified method of estimating YNT tends to overestimate the benefit in lower risk populations, as patients die at an increasing rate as they age, but is compatible with restricting the analysis to the in-trial time like NNT (Figure 1).
Figure 1.
The methodological approach to determining years-needed-to-treat (YNT) is illustrated. Three groups of patients are randomized to treatment for 5 years with placebo (P) vs. a hypothetical therapy (T) that reduces mortality by 50%. Evaluating only the in-trial effect through 5 years, in different subgroups of patients with baseline annual mortality of 2% (low risk), 10% (moderate risk), or 50% (high risk), the numbers-needed-to-treat (NNT) for 1 year to add 1 year of life would be 100, 20, or 4, or a 25-fold difference in NNT between low- and high-risk groups. Using the YNT method and based on the scenario of continued randomized therapy, 6.3, 3.6, and 1.6 estimated years of life would be added over a lifetime for each patient treated in the low-, moderate-, and high-risk groups, corresponding to 0.27, 0.34, and 0.48 additional years of life per patient-year of treatment, respectively. The corresponding YNT to add 1 year of life is 3.7, 2.9, and 2.1, a 1.8-fold difference between low- and high-risk groups. Alternative scenarios would include stopping randomized therapy at the end of the trial (illustrated as a crossover to placebo) or starting the therapy in the placebo group at the end of the trial. An alternative method to estimate the YNT is to draw a horizontal line from the treatment curve to the placebo curve at the end of the trial. In all three groups, the length of that line is ∼2.5 years. Thus, it is required to treat for 2 years to add 1 year of life (5/2.5). This simplified method tends to overstate the benefit in lower risk populations.
Years-needed-to-treat was also extrapolated for other published cardiovascular trials based on the average annual mortality for each arm in the trial and assuming that patients remained on the assigned therapy post-trial. The methodological approach in this analysis is illustrated in Figure 1. Statistics were by Statview 5 (SAS Institute, NC, USA), with ROC AUC determined using SPSS 11 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P ≤ 0.05 (two-tailed).
Results
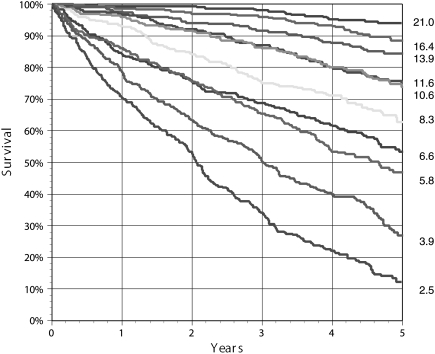
The multivariate risk model developed in the COMET database had a 1-year ROC of 0.78 (95% CI 0.76–0.79). Predicted vs. actual survival at 5 years was compared in deciles of risk, with excellent correspondence (R2 0.99, standard error of the 5-year estimate ±3%). The deciles of 5-year survival varied from 12 to 94%, with estimated total years of life ranging from a mean of 2.5 to 21 years (Figure 2).
Figure 2.
Actual Kaplan–Meier survival curve according to deciles of predicted risk in COMET varied from 12 to 94% 5-year survival. The estimated mean total life years for each decile is shown on the right.
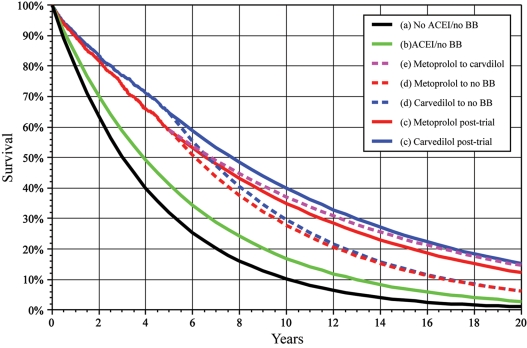
Carvedilol vs. metoprolol tartrate therapy resulted in 0.136 (95% CI 0.02–0.25) additional years of life during the trial period (mean absolute survival of 3.902 vs. 3.767 years, respectively). At trial-end, 9% more patients (n = 999 vs. n = 918) were alive in the carvedilol arm. The estimated average post-trial survival of all patients alive at trial-end was similar using the Levy, Gompertz, or Deale methods: 9.82 ± 0.11, 9.74 ± 0.12, and 10.53 ± 0.13 years, respectively. Under the scenario that each patient remained on his/her end-trial therapy, the total years of life (in-trial plus estimated post-trial) were 10.63 ± 0.19 vs. 9.48 ± 0.18 for the carvedilol vs. metoprolol arm, respectively (Levy method), corresponding to 1.15 (95% CI 0.64–1.66) additional years of life for patients on carvedilol vs. metoprolol tartrate (Figure 3). The estimated additional years of life for patients on carvedilol vs. metoprolol were very similar using the Gompertz method (1.18 years, 95% CI 0.66–1.69) or Deale method (1.24 years, 95% CI 0.23–1.29) to estimate post-trial survival. By all three methods, for each year of treatment with carvedilol vs. metoprolol, 0.11 (95% CI 0.06–0.16) years of life would be added (the minor variation in the estimation of post-trial survival between each method is similar for both arms of the trial, so that the difference is similar using any of the three methods). The inverse of the years of life added (1/0.11 = 9.2) defines the YNT: one patient treated by carvedilol vs. metoprolol tartrate for 9.2 years adds 1 year of life.
Figure 3.
Actual survival curves in COMET for the first 5 years. The predicted survival curves are illustrated with solid lines for (a) no angiotensin-converting enzyme inhibitor (ACEI) or β-blocker, (b) ACEI but no β-blocker, and (c) continuation of metoprolol tartrate or carvedilol at end-trial. The dashed lines represent predicted post-trial survival for (d) discontinuation of the randomized β blocker or (e) all patients in placebo switching from metoprolol tartrate to carvedilol. The total life years is the area under the appropriate curve, and life years saved under each scenario is the area between the corresponding curves. The remaining life years are estimated from patient-level data and used to construct this illustration of the concepts presented in the manuscript estimated from patient-level data. The patient-level data were used to construct this illustration of the concepts presented in the manuscript.
The estimated mean years of life for various patient subgroups are presented in Table 1, including values for patients divided into quartiles of overall mortality risk by the multivariate risk model. The YNT to add 1 year of life ranged from 14.7 in the lowest risk quartile to 4.7 in the highest risk quartile, indicating approximately one-third of the benefit in the lower vs. higher risk patient subgroup. In comparison, the NNT for 1 year ranged from 318 in the lowest risk quartile to 22 in the highest risk quartile or approximately only 7% of the benefit in the lower vs. higher risk patient subgroup. Thus, the NNT underestimated survival benefits in all patients and particularly among lower risk patients in whom long-term survival is greatest.
Table 1.
Estimated mean years of life for various COMET subgroups
| Metoprolol tartrate (years) | Carvedilol (years) | Numbers-needed-to-treat (NNT) | Life years saved (LYS) (95% CI) | LYS/year of therapy (95% CI) | Years-needed-to-treat (YNT) (95% CI) | |
|---|---|---|---|---|---|---|
| Overall | 9.48 | 10.63 | 59 | 1.15 (0.64–1.66) | 0.11 (0.06–0.16) | 9.2 (6.4–16.6) |
| EF | ||||||
| >25% | 10.26 | 11.38 | 62 | 1.12 (0.43–1.82) | 0.10 (0.04–0.16) | 10.2 (6.3–26.5) |
| <25% | 8.37 | 9.76 | 46 | 1.39 (0.66–2.13) | 0.14 (0.07–0.22) | 7.0 (4.6–14.8) |
| NYHA | ||||||
| 2 | 11.10 | 12.61 | 50 | 1.50 (0.78–2.23) | 0.12 (0.06–0.18) | 8.4 (5.7–16.2) |
| 3/4 | 7.94 | 8.77 | 75 | 0.83 (0.16–1.50) | 0.09 (0.02–0.17) | 10.6 (5.8–54.8) |
| Diabetes | ||||||
| No | 10.09 | 11.24 | 65 | 1.15 (0.56–1.74) | 0.10 (0.05–0.15) | 9.8 (6.5–20.1) |
| Yes | 7.61 | 8.67 | 49 | 1.06 (0.14–1.99) | 0.12 (0.02–0.23) | 8.2 (4.4–61.9) |
| Age | ||||||
| <63 | 12.07 | 13.20 | 97 | 1.13 (0.36–1.90) | 0.09 (0.03–0.14) | 11.7 (6.9–36.7) |
| >63 | 7.29 | 8.16 | 49 | 0.87 (0.28–1.46) | 0.11 (0.03–0.18) | 9.4 (5.6–29.1) |
| Quartile 1 | 15.40 | 16.52 | 318 | 1.12 (0.17–2.07) | 0.07 (0.01–0.13) | 14.7 (8.0–97.2) |
| Quartile 2 | 10.85 | 11.91 | 73 | 1.06 (0.17–1.95) | 0.09 (0.01–0.16) | 11.2 (6.1–70.0) |
| Quartile 3 | 5.57 | 8.92 | 40 | 1.34 (0.54–2.15) | 0.15 (0.06–0.24) | 6.6 (4.1–16.5) |
| Quartile 4 | 4.06 | 5.14 | 22 | 1.08 (0.47–1.70) | 0.21 (0.09–0.33) | 4.7 (3.0–10.9) |
Sensitivity analysis
Under the scenario that all patients stop β-blockers at trial-end, the estimated post-trial survival for the average individual patient would be similar in the two arms, but the total post-trial life-years gained would still be higher in the carvedilol vs. metoprolol arm due to the accrued survival benefit (9% more patients alive) at end-trial. Under this scenario, the estimated post-trial additional survival would be 0.23 years, resulting in 0.14 (in-trial) + 0.23 (estimated post-trial) = 0.37 total additional years of life (95% CI 0.01–0.74, Figure 3) due to the 3.9 years of treatment with carvedilol vs. metoprolol. This corresponds to a YNT of 10.5 (3.9 years of therapy/0.37 additional life years).
Under the scenario that all patients on metoprolol tartrate were switched to carvedilol at trial-end, the estimated post-trial survival for the average individual patient would again be similar in the two arms (as all patients are now taking carvedilol), but the total post-trial life-years gained comparing the carvedilol with metoprolol arm would be a bit higher than seen previously due to the similar accrued end-trial survival benefit (9% more patients alive) plus somewhat greater average post-trial survival due to benefits of carvedilol. Under this scenario, the estimated post-trial additional survival would be 0.37 years, resulting in 0.14 (in-trial) + 0.37 (estimated post-trial) = 0.51 total additional years of life (95% CI 0.03–1.06) from the 3.9 years of treatment with carvedilol vs. metoprolol. Note that the 3.9 years of treatment remains the same, as the total additional years of life are solely due to this period of treatment. This corresponds to a YNT of 7.6 (3.9 years of therapy/0.51 additional life years).
Under the scenario that all patients in both arms who are alive at the trial-end live an average 5 years after the trial, 0.42 (95% CI 0.15–0.69) total additional years of life (in-trial plus estimated post-trial) would be saved due to the 3.9 years of treatment with carvedilol vs. metoprolol, corresponding to a YNT of 9.3 (3.9 years of therapy/0.42 additional life years).
Estimate of benefit of angiotensin-converting enzyme inhibitor and beta-blockers vs. no treatment
Based on age/gender mortality life tables (i.e. no heart failure), the average years of life remaining in the COMET population was 17.3 years. Given the diagnosis of heart failure, the average years of life remaining in this cohort if untreated with ACEIs and β-blockers is 4.63 years (95% CI 4.55–4.71, Figure 3). Treating only with an ACEI would increase this to 5.88 years (95% CI 5.78–5.98, Figure 3) or an additional 1.25 years (YNT = 4.7). In this same cohort of patients, adding metoprolol tartrate to an ACEI would increase the lifespan by 3.6 years (9.48 vs. 5.88 years, YNT = 2.7), whereas adding carvedilol would add 4.75 years (10.63 vs. 5.88, YNT = 2.2).
Application of this method to published trials
The YNT method can be extended to published trials, based on the annual in-trial mortality in each arm. Estimates can be made for the scenario that patients remain on assigned therapy at trial-end (i.e. survival benefits continue to accrue) or, alternatively, that patients cross over to the treatment arm at trial-end (i.e. the average patient life expectancy post-trial is similar in both arms). The application of the first approach to selected published cardiovascular trials is shown in Table 2, including estimates of both YNT and NNT under the both scenarios. For these placebo-controlled trials that each demonstrated a statistically significant reduction in mortality with treatment, the YNT varies from ∼2 to 20, whereas NNT ranges from 6 to 625. For COMET using this approach with the published data (vs. the patient-level data in this analysis), the YNT was 8.9, similar to the estimate in the present analysis of 9.2 using the patient-level data. In the CONSENSUS trial, treatment with enalapril added 0.71 years of life at 10 years5 (2.14 vs. 1.43 years, YNT = 1.7). This compares with an estimated YNT of 2.1 years using the methodology in this paper. The 12-year follow-up for SOLVD4 suggests that treatment with an ACEI adds ∼0.72 years of life, compared with an estimate of 0.77 years using the methodology in this paper.
Table 2.
Application of years-needed-to-treat to published clinical trials
| Trial | Mortality reduction (%) | Numbers-needed-to-treat (NNT) | Life years saved per year of treatment | Years-needed-to-treat (YNT) |
|---|---|---|---|---|
| Theoretical | ||||
| 50% annual mortality | 50 | 4 | 0.48 | 2.1 |
| 10% annual mortality | 50 | 20 | 0.34 | 2.9 |
| 2% annual mortality | 50 | 100 | 0.27 | 3.7 |
| Heart failure trials | ||||
| CONSENSUS—enalapril10 | 40 | 6 | 0.47 | 2.1 |
| MERIT-HF—metoprolol succinate13 | 34 | 27 | 0.23 | 4.3 |
| CIBIS II—bisoprolol12 | 34 | 22 | 0.24 | 4.1 |
| COPERNICUS—carvedilol14 | 35 | 15 | 0.29 | 3.5 |
| COMET—carvedilol vs. metoprolol tartrate18 | 17 | 59 | 0.11 | 8.9 |
| SOLVD—enalapril11 | 16 | 54 | 0.11 | 9.1 |
| CARE-HF—CRT25 | 36 | 23 | 0.25 | 4.0 |
| CHARM—candesartan26 | 9 | 143 | 0.05 | 19.8 |
| COMPANION—CRT-D27 | 36 | 15 | 0.29 | 3.4 |
| MADIT II—ICD28 | 31 | 30 | 0.20 | 5.1 |
| SCD-HeFT—ICD29 | 23 | 60 | 0.14 | 7.0 |
| Other cardiovascular trials | ||||
| SHEP—chlorthalidone30 | 13 | 375 | 0.06 | 16.7 |
| HOPE—ramipril31 | 16 | 256 | 0.08 | 12.0 |
| ASCOT—BPLA32 | 11 | 625 | 0.05 | 20.5 |
| 4S—simvastatin24 | 30 | 145 | 0.16 | 6.3 |
| Heart Protection Study—simvastatin23 | 12 | 278 | 0.07 | 15.3 |
CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; MERIT-HF, Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; CIBIS II, Cardiac Insufficiency Bisoprolol Study II; COMET, Carvedilol Or Metoprolol European Trial; SOLVD, Study of Left Ventricular Dysfunction; CARE-HF, Cardiac Resynchronization in Heart Failure; CHARM, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure; MADIT II, Multicenter Automated Defibrillator Implantation Trial II; SCD-HeFT, Sudden Cardiac Death Heart Failure Trial; SHEP, Systolic Hypertension in the Elderly Program; HOPE, Heart Outcome Prevention Evaluation; ASCOT, Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm; 4S, Scandinavian Simvastatin Survival Study.
Discussion
We developed a new metric, YNT, to estimate the effectiveness of various treatments. Years-needed-to-treat quantitatively accounts for the accrued patient survival during the trial, acknowledging that any increased numbers of patients surviving at end-trial in the treatment arm will continue to live for some remaining years. In contrast, the traditional metric NNT implicitly assumes that any additional patients surviving in the treatment arm will immediately die at end-trial. Using the COMET trial as an example, we demonstrated a YNT of 9.2 for carvedilol vs. metoprolol, indicating that 9.2 person-years of treatment would add 1 person-year of life, compared with an NNT of 59. Thus, by acknowledging the continued post-trial survival, rather than limiting the estimate to the in-trial period, the YNT one patient to add 1 year of life is decreased by 84% (9.2 YNT vs. 59 NNT).
Notably, this difference is not dependent on the assumption of continued post-trial benefits in the treatment arm vs. the control arm. Rather, it largely stems from the continued life-years of all patients surviving during the trial. Thus, even assuming that all treatment is stopped at trial-end or that all patients in the control arm are switched to the treatment arm, the YNT would be 7.6 and 10.5, respectively. Each of these estimates are still considerably lower than the NNT.
Extending these methods to other published clinical trials, the marked difference in estimated effectiveness for using YNT vs. NNT was also clearly evident for other treatments. For example, compared with placebo, treatment with either an ACEI or β-blocker was very effective, with YNTs ranging from ∼2 to 9. Device-based heart failure therapy [cardiac resynchronization therapy (CRT), implantable cardioverter defibrillator, or CRT-D] was similarly effective, with YNTs ranging from 4 to 7. These estimates of clinical effectiveness can be used directly to assess cost-effectiveness; for example, devices are more expensive than treatment with an ACEI or a β-blocker. In contrast, device therapy is typically added, not used in place of medical therapy, so across-treatment comparisons may be less relevant than within-treatment comparisons.
In addition to providing more accurate estimates of overall effectiveness, the YNT method also corrects for bias against effectiveness of treatment in lower risk patients. For example, in the present analysis, treatment of lower risk patients (e.g. younger, lower NYHA, non-diabetic, or higher EF) yielded YNTs ranging from ∼5 to 15, i.e. not much greater than for higher risk patients. This is in direct contrast to other methods, such as NNT, that are biased towards greater benefits in higher risk patients by ignoring the greater post-trial lifetime of lower risk patients. For example, in the SAVE trial, treatment with captopril in a 50- vs. 80-year-old patient was estimated to be ∼17-fold more expensive using an NNT approach22 vs. a 4.4-fold difference using the YNT approach. Because the NNT neglects to incorporate the longer post-trial lifespan of the lower vs. higher risk patient, this could lead to erroneous conclusions to avoid or delay treatment in the lower risk patient. However, the YNT approach indicates that delaying therapy until a patient is at higher risk merely limits the total benefit for the patient.
Limitations
The YNT metric accounts for total survival and does not estimate quality-adjusted life-years. Nevertheless, the YNT method has the advantage of estimating the full lifetime effect of a treatment, rather than arbitrarily reversing benefits at the end of trial follow-up. The calculation of YNT for the scenario of continued end-trial treatment would overestimate effectiveness if benefits diminish over time, as may be seen, for example, with some surgical or device interventions. In these instances, the most conservative approach would be the YNT calculation for the scenario that all treatment ceases end-trial, as this scenario assumes no additional post-trial benefit of treatment for individual patients in either the placebo or treatment arm. Conversely, all YNT approaches would underestimate benefits if survival advantages continued to accrue over time. For example, in the HPS23 and 4S24 statin trials, benefits of statins increased during trial follow-up; if this were to continue, YNT calculations would underestimate long-term benefits. The actual benefit for compliant patients will be greater than the estimates using this approach, which uses only intention-to-treat benefits.
Conclusions
Compared with traditional metrics such as NNT, the YNT approach more accurately estimates effectiveness of interventions by accounting for post-trial survival, largely independent of assumptions about continued post-trial accrual of benefits of treatment. Extension of this approach to published trials suggests that, for treatment of heart failure, an ACEI, spironolactone, bisoprolol, metoprolol succinate, or carvedilol are all very effective in comparison with placebo (Table 2). The effectiveness of treatments is also seen to be much more similar for low-risk vs. high-risk patients using YNT, compared with metrics such as NNT that underestimate benefits in lower risk patients.
Funding
The manuscript was supported by GlaxoSmithKline as an investigator initiated proposal.
Conflict of interest: Most authors participated as steering committee members for COMET or have received research funding or honoraria from GlaxoSmithKline and/or Roche, sponsors of the COMET trial.
Acknowledgements
The authors would like to thank all the patients, study coordinators, and investigators who participated in the trial. The opinions expressed in the manuscript are those of the authors and do not necessarily reflect the opinions of GlaxoSmithKline or the COMET steering committee.
References
- 1.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:1516–1522. doi: 10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 3.Christensen PM, Kristiansen IS. Number-needed-to-treat (NNT)—needs treatment with care. Basic Clin Pharmacol Toxicol. 2006;99:12–16. doi: 10.1111/j.1742-7843.2006.pto_412.x. [DOI] [PubMed] [Google Scholar]
- 4.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 5.Swedberg K, Kjekshus J, Snapinn S. Long-term survival in severe heart failure in patients treated with enalapril. Ten year follow-up of CONSENSUS I. Eur Heart J. 1999;20:136–139. doi: 10.1053/euhj.1998.1098. [DOI] [PubMed] [Google Scholar]
- 6.Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, Pedersen TR, Kjekshus J. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ. 1998;316:1337–1343. doi: 10.1136/bmj.316.7141.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark DB, Hlatky MA, Califf RM, Naylor CD, Lee KL, Armstrong PW, Barbash G, White H, Simoons ML, Nelson CL. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med. 1995;332:1418–1424. doi: 10.1056/NEJM199505253322106. [DOI] [PubMed] [Google Scholar]
- 9.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 10.Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) Am J Cardiol. 1988;62:60A–66A. doi: 10.1016/s0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- 11.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 12.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 13.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 18.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 19.Haybittle JL. The use of the Gompertz function to relate changes in life expectancy to the standardized mortality ratio. Int J Epidemiol. 1998;27:885–889. doi: 10.1093/ije/27.5.885. [DOI] [PubMed] [Google Scholar]
- 20.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the ‘DEALE’). I. Validation of the method. Am J Med. 1982;73:883–888. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 21.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsevat J, Duke D, Goldman L, Pfeffer MA, Lamas GA, Soukup JR, Kuntz KM, Lee TH. Cost-effectiveness of captopril therapy after myocardial infarction. J Am Coll Cardiol. 1995;26:914–919. doi: 10.1016/0735-1097(95)00284-1. [DOI] [PubMed] [Google Scholar]
- 23.Heart protection study collaborative group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 24.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 25.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;15:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 27.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;21:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 28.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 29.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 30.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 31.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 32.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]