Abstract
Aims
Chronic obstructive pulmonary disease is an independent predictor of mortality in patients with myocardial infarction (MI). However, the impact on mode of death and risk of atherosclerotic events is unknown.
Methods and results
We assessed the risk of death and major cardiovascular (CV) events associated with chronic obstructive pulmonary disease in 14 703 patients with acute MI enrolled in the Valsartan in Acute Myocardial Infarction (VALIANT) trial. Cox proportional hazards models were used to evaluate the relationship between chronic obstructive pulmonary disease and CV outcomes. A total of 1258 (8.6%) patients had chronic obstructive pulmonary disease. Over a median follow-up period of 24.7 months, all-cause mortality was 30% in patients with chronic obstructive pulmonary disease, compared with 19% in those without. The adjusted hazard ratio (HR) for mortality was 1.14 (95% confidence interval 1.02–1.28). This reflected increased incidence of both non-CV death [HR 1.86 (1.43–2.42)] and sudden death [HR 1.26 (1.03–1.53)]. The unadjusted risk of all pre-specified CV outcomes was increased. However, after multivariate adjustment, chronic obstructive pulmonary disease was not an independent predictor of atherosclerotic events [MI or stroke: HR 0.98 (0.77–1.23)]. Mortality was significantly lower in patients receiving beta-blockers, irrespective of airway disease.
Conclusion
In high-risk patients with acute MI, chronic obstructive pulmonary disease is associated with increased mortality and non-fatal clinical events (both CV and non-CV). However, patients with chronic obstructive pulmonary disease did not experience a higher rate of atherosclerotic events.
Keywords: Chronic obstructive pulmonary disease, Heart failure, Left ventricular systolic dysfunction, Myocardial infarction
Introduction
Chronic obstructive pulmonary disease is a global epidemic affecting 5–15% of all adults.1 Both prevalence and mortality are increasing and projected to escalate still further. Cardiovascular (CV) and pulmonary deaths are equally common, accounting for 3 million lives per year worldwide.2 The shared aetiology of tobacco smoking is partly responsible. However, airflow obstruction independently predicts CV mortality in population studies, even after adjusting for smoking history.3 Atherosclerotic consequences of chronic systemic inflammation in chronic obstructive pulmonary disease have been postulated.4–6 Whether these aggravate established coronary artery disease is uncertain.
Two contemporary studies have examined patients with myocardial infarction (MI) and concomitant chronic obstructive pulmonary disease.7,8 Both found chronic obstructive pulmonary disease to be an independent predictor of long-term mortality.7,8 Neither report investigated the relationship between chronic obstructive pulmonary disease and mode of death or risk of non-fatal CV events. Furthermore, the increased mortality was confined to patients without heart failure (HF) in one study.7 We used the Valsartan in Acute Myocardial Infarction (VALIANT) trial to characterize the impact of chronic obstructive pulmonary disease on treatment and clinical outcomes in patients with MI complicated by HF, left ventricular systolic dysfunction (LVSD), or both.
Methods
Trial design
VALIANT enrolled 14 703 patients with MI complicated by LVSD, HF, or both. The former was defined by ejection fraction ≤0.35 on echocardiography or contrast angiography and ≤0.40 on radionuclide ventriculography, whereas the latter by clinical signs of HF or radiological evidence of pulmonary venous congestion.9 The randomized, double-blind, active-controlled design compared treatment with valsartan, captopril, or both. The rationale, methods, inclusion and exclusion criteria, and main outcomes have been reported previously.9,10 The study was approved by local Ethics Committees in all participating centres, and all patients provided written informed consent.
Trial endpoints
The primary outcome was mortality from any cause within 3 years following the index MI. Secondary pre-specified endpoints included CV death and components (sudden cardiac death, fatal MI, and fatal worsening HF); non-fatal MI; hospital admission for worsening HF; and the composite of CV death, MI, or HF hospitalization. The presence of clinically recognized chronic obstructive pulmonary disease was recorded using a yes/no check box by individual site investigators at study entry according to their clinical judgement.
All pre-specified endpoints were adjudicated by an independent clinical endpoint committee. Definitions of the endpoints are published previously.10 Hospitalization for HF was defined as admission with symptoms or signs of HF requiring intravenous treatment with diuretic, inotropic, or vasodilator therapy. Members of the committee distinguished HF from chronic obstructive pulmonary disease using clinical judgement supported by hospital records and results of investigations.
Statistical analysis
Data analyses were performed independently by the Duke Clinical Research Institute. The chronic obstructive pulmonary disease status is defined as having a known history prior to the qualifying MI for the trial. Baseline characteristics of patients with and without chronic obstructive pulmonary disease are presented as means with standard deviations for continuous variables or by frequencies and per cents for categorical variables. Means were compared using the Wilcoxon rank sum test or Student's t-test, depending on the distribution of the data and proportions using the χ2 test. All analyses were performed on an intention-to-treat basis. Cumulative event rates were estimated using the Kaplan–Meier method and were compared using log-rank test. A two-tailed P-value of less than 0.05 was considered statistically significant.
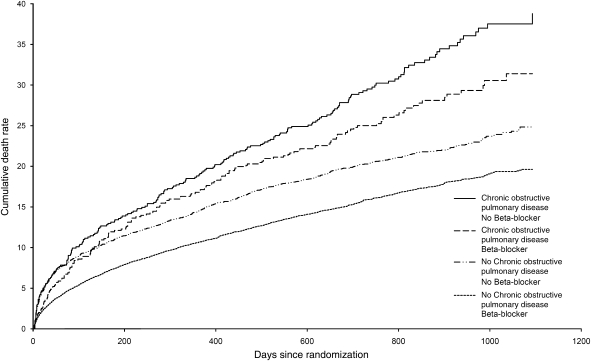
The prognostic significance of chronic obstructive pulmonary disease was evaluated for pre-defined outcomes, including the primary endpoint and other major CV events. The estimated hazard ratios (HRs) were adjusted for all important predictors of mortality and morbidity using Cox proportional hazards models. A separate model was built for each outcome of interest. Starting from over 70 candidate variables collected at randomization, both backward elimination and stepwise selection were used to identify independent factors. A P-value of 0.10 was required for a variable to enter and 0.05 to stay in the model. Bootstrap method with a re-sample of 200 was employed to validate the selection. Randomized treatments were added to the final model. The multivariable model for mortality included the following covariates: age, heart rate, systolic and diastolic blood pressures, weight, baseline creatinine, smoking status, diabetes, dyslipidaemia, history of hypertension, Killip classification, anterior MI, new left bundle branch block, thrombolytic therapy, primary percutaneous intervention, coronary artery bypass graft surgery after the qualifying MI, history of HF, atrial fibrillation, previous MI, angina or unstable angina, previous stroke, peripheral arterial disease, renal insufficiency, alcohol abuse, country of enrolment, beta-blocker use, and randomized treatment. The multivariate model for mortality stratified by chronic obstructive pulmonary disease status and baseline beta-blocker was employed to estimate the adjusted death rates for each of the four strata at different time points within the 3-year follow-up period. Event curves were created to display the cumulative adjusted mortality rates over time (Figure 1).
Figure 1.
Adjusted cumulative all-cause mortality rate by chronic obstructive pulmonary disease status and beta-blocker use.
An analysis of post-randomization periods (‘landmarks’) was employed to address potential survivor bias in analysis of the composite atherosclerotic outcome of MI or stroke. Patients with chronic obstructive pulmonary disease may die earlier than their counterparts, before developing arterial disease. The association between chronic obstructive pulmonary disease and atherosclerotic events may thus be underestimated due to unequal survivorship. To minimize this effect, the relationship between chronic obstructive pulmonary disease and atherosclerotic events was examined in four different periods: inpatient (1–16 days since randomization), post-discharge (17–45 days), early (46–198 days), and later (199–1096 days) follow-up. Only patients alive at the beginning of each period were included in each analysis. When the risk is similar across intervals, a combined HR was estimated by treating each interval as a cluster in the Cox model. All analyses were performed using SAS software version 8 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
VALIANT enrolled 14 703 patients, 1258 (8.6%) of which had a diagnosis of chronic obstructive pulmonary disease. The median duration of follow-up was 24.7 months. The baseline characteristics of patients with and without chronic obstructive pulmonary disease differed significantly (Table 1). Patients with chronic obstructive pulmonary disease were older with more CV risk factors including current or previous smoking, diabetes, hypertension, and dyslipidaemia. Comorbidity was likewise greater in patients with chronic obstructive pulmonary disease, particularly coronary (MI, percutaneous coronary intervention, and angina), peripheral, and cerebrovascular disease.
Table 1.
Baseline characteristics of patients with chronic obstructive pulmonary disease
| Characteristics | Chronic obstructive pulmonary disease (n = 1258, 8.6%) | No chronic obstructive pulmonary disease (n = 13 445, 91.4%) |
|---|---|---|
| Demographics | ||
| Age (years) | 68.1 ± 9.9* | 64.5 ± 11.9 |
| Female sex | 363 (28.9) | 4207 (31.3) |
| Body mass index (kg/m2) | 27.5 ± 5.5† | 27.9 ± 4.8 |
| Cardiovascular risk factors | ||
| Current smoker | 528 (42.0)* | 4136 (30.8) |
| Previous smoker | 518 (41.2)* | 4162 (31.0) |
| Diabetes mellitus | 323 (25.7)† | 3077 (22.9) |
| Hypertension | 731 (58.1)† | 7391 (55.0) |
| Dyslipidaemia | 453 (36.4)* | 3882 (29.3) |
| Previous comorbidity | ||
| Heart failure | 343 (27.3)* | 1831 (13.6) |
| Myocardial infarction | 502 (39.9)* | 3602 (26.8) |
| Angina | 580 (46.1)* | 5261 (39.1) |
| PCI | 138 (11.0)* | 929 (6.9) |
| Stroke | 103 (8.2)† | 792 (5.9) |
| Peripheral arterial disease | 220 (17.5)* | 1017 (7.6) |
| Atrial fibrillation | 120 (9.5)* | 840 (6.2) |
| Characteristics of qualifying MI | ||
| Heart rate | 78.2 ± 13.5* | 76.0 ± 12.8 |
| Systolic blood pressure (mmHg) | 123.9 ± 16.9† | 122.5 ± 17.0 |
| Diastolic blood pressure (mmHg) | 71.3 ± 11.3† | 72.4 ± 11.3 |
| Killip class III–IV | 369 (29.3)* | 3091 (23.0) |
| Radiological LV failure | 534 (42.4)† | 5208 (38.7) |
| ECG site—anterior | 646 (51.4)* | 8063 (60.0) |
| Q-wave MI | 632 (53.1)* | 8811 (67.8) |
| Non-Q-wave MI | 520 (44.3)* | 3938 (30.8) |
| Ejection fraction | 34.0 (10.2) | 35.4 (10.4) |
| Initial treatment of qualifying MI | ||
| Aspirin | 1065 (84.7)* | 12018 (89.4) |
| Beta-blocker | 526 (41.8)* | 8185 (60.9) |
| Thrombolysis | 346 (27.5)* | 4824 (35.9) |
| Catheterization | 352 (28.0) | 3769 (28.1) |
| Primary PCI | 133 (10.6)* | 2045 (15.2) |
| Medications at randomization | ||
| Aspirin | 1101 (87.5)* | 12317 (91.6) |
| Beta-blocker | 643 (51.1)* | 9707 (72.2) |
| Digoxin | 268 (21.3)* | 1588 (11.8) |
| Statin | 399 (31.7) | 4615 (34.3) |
| Calcium channel blocker | 214 (17.0)* | 1047 (7.8) |
*P<0.0001 compared with patients without chronic obstructive pulmonary disease.
†P<0.05 compared with patients without chronic obstructive pulmonary disease.
Values are means ± SD or n (%).
ECG, electrocardiogram; PCI, percutaneous coronary intervention; MI, myocardial infarction.
At randomization, patients with chronic obstructive pulmonary disease had a higher heart rate, Killip classification, and frequency of radiological pulmonary oedema. The qualifying electrocardiogram and ensuing treatment also varied. Patients with chronic obstructive pulmonary disease more frequently presented with non Q-wave MI (44.3 vs. 30.8%). Fewer patients with chronic obstructive pulmonary disease received primary percutaneous intervention (10.6 vs. 15.2%) or thrombolysis (27.5 vs. 35.9%), although a similar proportion underwent cardiac catheterization. Patients with chronic obstructive pulmonary disease were less likely to receive some risk-modifying CV medications, most notably beta-blockers (51.1 vs. 72.2% at randomization).
Mortality
Chronic obstructive pulmonary disease was independently associated with increased mortality. A total of 382 patients with chronic obstructive pulmonary disease (30.4%) died from any cause, compared with 2496 (18.6%) of those without disease (Table 2). After adjusting for additional predictors of mortality, the risk of death was increased by 14% in patients with chronic obstructive pulmonary disease [HR 1.14 (95% confidence interval 1.02–1.28)]. Mortality was greater in those with chronic obstructive pulmonary disease, regardless of beta-blocker prescription (Figure 1). Increased incidence of both non-CV death [6.0 vs. 2.4%, HR 1.86 (1.43–2.42)] and sudden death [10.0 vs. 5.9%, HR 1.26 (1.03–1.53)] contributed to the excess mortality in patients with chronic obstructive pulmonary disease (Table 2). However, the overall risk of CV death was not significantly elevated after correcting for baseline differences. The increased risk of sudden death was independent of age, beta-blocker use, ischaemic heart disease, diabetes, and other recognized predictors of sudden death. Of the 75 non-CV deaths in patients with chronic obstructive pulmonary disease, two-thirds were attributed to pulmonary disease (25%, n = 19), malignancy (33%, n = 25), or infection (9%, n = 7). The respective frequencies in patients without chronic obstructive pulmonary disease were 9% (n = 30), 43% (n = 137), and 13% (n = 43).
Table 2.
Risk of death and cardiovascular events in patients with chronic obstructive pulmonary disease
| Outcome | Chronic obstructive pulmonary disease present (n = 1258), % | Chronic obstructive pulmonary disease absent (n = 13 445), % | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| All-cause mortality | 382 (30.4) | 2496 (18.6) | 1.70 (1.53–1.90) | <0.0001 | 1.14 (1.02–1.28) | 0.021 |
| Non-cardiovascular death | 75 (6.0) | 319 (2.4) | 2.61 (2.02–3.36) | <0.0001 | 1.86 (1.43–2.42) | <0.0001 |
| Cardiovascular death | 307 (24.4) | 2177 (16.2) | 1.57 (1.39–1.77) | <0.0001 | 1.04 (0.92–1.19) | 0.506 |
| Sudden death | 126 (10.0) | 799 (5.9) | 1.77 (1.47–2.14) | <0.0001 | 1.26 (1.03–1.53) | 0.025 |
| HF hospitalization | 317 (25.2) | 2071 (15.4) | 1.77 (1.57–1.99) | <0.0001 | 1.19 (1.05–1.34) | 0.007 |
| MI or stroke | 190 (15.1) | 1570 (11.7) | 1.58 (1.27–1.94) | <0.0001 | 0.98 (0.77–1.23) | 0.871 |
| CV death, MI, HF hospitalization | 567 (45.1) | 4047 (30.1) | 1.64 (1.50–1.79) | <0.0001 | 1.14 (1.04–1.25) | 0.005 |
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction.
Cardiovascular morbidity and mortality
Chronic obstructive pulmonary disease was an independent predictor of hospitalization for HF [HR 1.19 (1.05–1.34)]. The combined endpoint of CV death, MI, or HF hospitalization occurred in 45% of the patients with chronic obstructive pulmonary disease, compared with 30% of those without. The adjusted risk for the combined endpoint remained significantly increased in patients with chronic obstructive pulmonary disease [1.14 (1.04–1.25)].
A composite atherosclerotic outcome was examined, incorporating fatal or non-fatal MI and stroke. This combined endpoint occurred in 190 (15.1%) as opposed to 1570 (11.7%) patients with and without chronic obstructive pulmonary disease, respectively. The adjusted risk of atherosclerotic events was not increased [0.98 (0.77–1.23), P = 0.871]. According to the analysis of landmarks, the adjusted HR of the relation between chronic obstructive pulmonary disease and atherosclerotic events was 0.94 [(0.70–1.25), P = 0.657], 1.36 [(0.96–1.93), P = 0.085], 0.91 [(0.71–1.17), P = 0.381], and 0.86 [(0.70–1.07), P = 0.648] for inpatient, post-discharge, early, and later follow-up, respectively. The higher hazard of atherosclerotic events during the post-discharge period was not statistically significant (P = 0.085). Combining results from all periods yielded a similar HR [0.94 (0.81–1.08), P = 0.348].
The impact of chronic obstructive pulmonary disease on atherosclerotic events was far outweighed by alternative CV risk factors (Table 3). The relative contribution of each factor to the variance of the outcome was determined by the χ2 statistic. Multivariate analysis revealed diabetes to be the strongest determinant of MI or stroke [HR 1.36 (1.24–1.50), P < 0.001]. Smoking, hypertension, obesity, and established coronary, peripheral and cerebrovascular disease were all independent predictors of atherosclerotic events.
Table 3.
Independent predictors of myocardial infarction or stroke
| Predictor | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Diabetes | 1.36 (1.24–1.50) | <0.001 |
| Age (per 10 years) | 1.17 (1.11–1.24) | <0.001 |
| Angina | 1.31 (1.19–1.44) | <0.001 |
| Previous MI | 1.28 (1.16–1.42) | <0.001 |
| Killip class 3 | 1.42 (1.22–1.66) | <0.001 |
| Killip class 4 | 1.49 (1.23–1.81) | <0.001 |
| Previous stroke | 1.28 (1.12–1.47) | <0.001 |
| Heart failure post-MI | 1.19 (1.08–1.31) | <0.001 |
| Heart rate (per 10 bpm) | 1.06 (1.03–1.10) | <0.001 |
| Current smoker | 1.21 (1.08–1.36) | 0.002 |
| Previous unstable angina | 1.17 (1.06–1.30) | 0.002 |
| Hypertension | 1.17 (1.06–1.30) | 0.002 |
| Angina post-MI | 1.16 (1.05–1.28) | 0.003 |
| Peripheral vascular disease | 1.19 (1.05–1.35) | 0.007 |
| Killip class 2 | 1.18 (1.03–1.34) | 0.014 |
| New diabetes | 1.28 (1.04–1.57) | 0.018 |
| Left bundle branch block | 1.23 (1.03–1.45) | 0.019 |
| Weight (per 10 kg) | 1.55 (1.07–2.22) | 0.019 |
| Previous CABG | 1.19 (1.03–1.37) | 0.020 |
| Previous heart failure | 1.13 (1.01–1.26) | 0.035 |
CABG, coronary artery bypass grafting; CI, confidence interval; MI, myocardial infarction.
Relationship between beta-blocker use and outcomes
Mortality was significantly lower in patients receiving beta-blockers, irrespective of airway disease (Figure 1). Overall, the adjusted HR for mortality comparing patients with and without beta-blockade was 0.74 (0.68–0.80) (P = 0.002). In patients with chronic obstructive pulmonary disease, 25.2% of those prescribed beta-blockers died from any cause, compared with 35.0% of those not prescribed beta-blockers. Results were similar in patients without chronic obstructive pulmonary disease (mortality 15.1 vs. 27.9%, respectively). Formal testing for interaction between chronic obstructive pulmonary disease and beta-blocker use with respect to mortality revealed no significant difference. Beta-blocker use was not associated adversely with any pre-specified outcome in patients with chronic obstructive pulmonary disease.
Discussion
Numerous studies have addressed the prognosis of patients with MI, HF, or both. Remarkably, few have described the impact of pulmonary comorbidity. Chronic obstructive pulmonary disease is known to independently reduce survival after MI.7,8 Our findings extend prior reports by defining the relative risk of CV and non-CV death, together with ischaemic and non-fatal events.
Chronic obstructive pulmonary disease was an independent predictor of mortality, largely due to increased non-CV and sudden death. The former is expected. Cigarette smoking and chronic obstructive pulmonary disease predispose to fatal outcomes from malignancy, pneumonia, and respiratory failure.11 The excess risk of sudden death corroborates findings of the recent TORCH (Towards a Revolution in COPD Health) trial.12 This was the first international trial of chronic obstructive pulmonary disease therapy to employ all-cause mortality as the primary endpoint and the first to adjudicate cause of death using a clinical endpoint committee. Sixteen per cent of deaths was classified as sudden and speculated to be the consequence of acute respiratory failure.11
Sudden death was explicitly defined in VALIANT as death that occurred suddenly and unexpectedly in a patient in otherwise stable conditions and included witnessed deaths.13 Some ‘out of hospital acute respiratory failure’ may be included in the category of sudden death. However, numerous substrates for ventricular arrhythmia exist in patients with chronic obstructive pulmonary disease: hypoxia, acidosis, hypercapnia, sympathetic activation, tachycardia, hypokalaemia, and QTc prolongation secondary to inhaled β2-agonists.14,15 Although safe in unselected populations, inhaled β2-agonists may precipitate CV events in susceptible patients.14–16 After MI, the risk of sudden death is greatest in the early months and among those with lowest ejection fraction.13 In high-risk patients with chronic obstructive pulmonary disease, early treatment of exacerbations and correction of arrhythmic substrates are therefore paramount.
In a recent cohort study of 2481 patients presenting with acute MI, rehospitalization rates were 22% higher among patients with chronic obstructive pulmonary disease.8 The reasons for admission were not defined. Our analysis reveals this disease to be an independent predictor of HF hospitalization after infarction. This mirrors findings in patients with chronic HF, in whom chronic obstructive pulmonary disease is a frequent comorbidity and infection a recognized precipitant of decompensation.17,18 Once hospitalized, concomitant pulmonary disease also prolongs inpatient stay and increases risk of re-admission.18,19
Chronic obstructive pulmonary disease is increasingly considered a systemic inflammatory disorder with putative atherosclerotic consequences.4–6 The hypothesis is founded on the epidemiological association between airflow obstruction and CV mortality.3 The key issue is whether chronic obstructive pulmonary disease contributes to atherosclerosis or merely serves as a marker of CV disease. This is the first analysis to evaluate chronic obstructive pulmonary disease as a modifier of CV events in subjects with pre-existing coronary disease. Previous studies have focused on overall survival following MI,7,8,20,21 percutaneous intervention,22,23 or surgical revascularization.24,25 Although we expected a strong association with atherosclerotic events, this was not found and merits careful consideration.
Many population studies adjusted only for age, gender, and smoking history.3 Residual confounding by established risk factors and unmeasured variables limits such reports. Numerous potential confounders exist: diabetes, hypertension, blood pressure, dyslipidaemia, low socio-economic class, occupation, poor diet, sedentary lifestyle, and obesity. In our high-risk cohort, all major CV risk factors occurred more frequently among patients with chronic obstructive pulmonary disease. The prevalence of existing coronary, peripheral, and cerebrovascular disease was likewise increased. Finally, patients with chronic obstructive pulmonary disease received fewer risk-modifying medications, notably beta-blockers. All these factors are established predictors of worse clinical outcomes. Comprehensive multivariate adjustment is thus crucial when considering prognosis. The 58% increased risk of atherosclerotic events was reduced by adjusting multivariate analyses [HR 0.98 (0.77–1.23)]. Exploring the relative contribution of covariates to the atherosclerotic endpoint confirmed our suspicions. The independent predictors of MI or stroke were all established CV risk factors or comorbidity. Our findings corroborate those of the TORCH study, in which just 3% of the 911 adjudicated deaths were attributed to MI.11
Population studies have further limitations. Survival estimates are potentially biased by loss to follow-up. Reliance on hospital coding and death certificates overestimates the burden of CV events in the community.26,27 Sudden death may be incorrectly attributed to coronary events. As discussed earlier, there are numerous other arrhythmic substrates.14,15 Unmeasured changes in baseline risk factors may influence survival during long follow-up periods. Differences in CV treatment are likewise unaccounted for. Beta-blockers are underutilized in patients with airflow obstruction and concomitant hypertension, HF, angina, or MI.28,29 The robust epidemiological association between airflow obstruction and CV mortality does not necessarily equate to chronic obstructive pulmonary disease causing atherosclerosis.
Two observational cohort studies from the Cooperative Cardiovascular Project suggested that beta-blockers are safe and effective for post-MI in patients with chronic obstructive pulmonary disease.21,29 Neither reported the outcomes of patients with HF or LVSD. Our analysis extends the prognostic benefits of beta-blockade to this important patient group. Furthermore, no adverse effects were observed for any pre-specified endpoint. In particular, non-CV mortality was not increased in patients with chronic obstructive pulmonary disease receiving beta-blockers. This observation should help alleviate historical concerns regarding safety. As with previous reports,21,29 interpretation is hindered by the lack of spirometry or stratification of chronic obstructive pulmonary disease severity. Recruitment bias and preferential prescribing habits confound applicability to patients with severe or reversible airflow obstruction.
Several limitations must be acknowledged, foremost being the investigator-derived diagnosis of chronic obstructive pulmonary disease. This was obtained from hospital records, pulmonary function if available, and questioning the patient. No pre-specified criteria were defined in the investigators brochure. Misdiagnosis is unavoidable and inherent to all clinical trials lacking spirometry.7,20,30 The prevalence of chronic obstructive pulmonary disease in VALIANT (8.6%) was akin to these trials and also the general population.7,20,30,31 No study has assessed the validity of self-reported chronic obstructive pulmonary disease in patients with MI. Only one has examined those admitted with HF, confirming airflow obstruction in 67%.32 However, the proportion of that cohort with HF and chronic obstructive pulmonary disease was higher than in VALIANT (HF 100 vs. 15% and COPD 22 vs. 9%), providing far greater scope for misdiagnosis. Furthermore, the VALIANT chronic obstructive pulmonary disease group is characterized by its three major predictors: male gender (71%), advanced age, and smoking history (83%).33,34 Recruitment bias will exclude many individuals with severe pulmonary disease. However, the generalizability of results is reasonable as severe airflow obstruction is also uncommon in the wider population.4
In summary, chronic obstructive pulmonary disease is an independent predictor of mortality in patients with MI, specifically of non-CV and sudden death. No excess risk of atherosclerotic events was observed after adjusting for baseline risk factors and comorbidity. The proposed atherosclerotic effects of chronic obstructive pulmonary disease are of limited clinical significance, at least during intermediate follow-up. There is a simple message. We must optimize both pulmonary and CV therapies in patients with chronic obstructive pulmonary disease. Greater collaboration is required between the specialties to achieve this. Intensive treatment of established CV risk factors and disease is essential to improve outcomes in this high-risk group.
Funding
The VALIANT study was supported by Novartis Pharmaceuticals.
Acknowledgements
Z.H. and K.S.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: N.M.H., A.P.M., J.J.V.M., M.A.P., K.S., S.D.S., E.J.V., and L.K. have received research grants, honoraria for lectures, and/or consulting fees from a number of pharmaceutical companies manufacturing and selling inhibitors of the renin–angiotensin–aldosterone system, including Novartis, AstraZeneca, Bristol-Myers Squibb, Sanofi-Aventis, Merck, and Pfizer.
References
- 1.GOLD Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Workshop Report. Updated 2006, http://www.goldcopd.com . [Google Scholar]
- 2.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370:797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 6.Maclay JD, McAllister DA, MacNee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12:634–641. doi: 10.1111/j.1440-1843.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 7.Kjoller E, Kober L, Iversen K, Torp-Pedersen C. Importance of chronic obstructive pulmonary disease for prognosis and diagnosis of congestive heart failure in patients with acute myocardial infarction. Eur J Heart Fail. 2004;6:71–77. doi: 10.1016/j.ejheart.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Salisbury AC, Reid KJ, Spertus JA. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am J Cardiol. 2007;99:636–641. doi: 10.1016/j.amjcard.2006.09.112. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer MA, McMurray J, Leizorovicz A, Maggioni AP, Rouleau JL, Van de WF, Henis M, Neuhart E, Gallo P, Edwards S, Sellers MA, Velazquez E, Califf R. Valsartan in Acute Myocardial Infarction Trial (VALIANT): rationale and design. Am Heart J. 2000;140:727–750. doi: 10.1067/mhj.2000.108832. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de WF, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 11.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de WF, Pieper K, Califf RM, Pfeffer MA. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M, Matera MG, Donner CF. Inhaled beta2-adrenoceptor agonists: cardiovascular safety in patients with obstructive lung disease. Drugs. 2005;65:1595–1610. doi: 10.2165/00003495-200565120-00001. [DOI] [PubMed] [Google Scholar]
- 15.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 16.Au DH, Udris EM, Fan VS, Curtis JR, McDonell MB, Fihn SD. Risk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunction. Chest. 2003;123:1964–1969. doi: 10.1378/chest.123.6.1964. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 18.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 19.Wright SP, Verouhis D, Gamble G, Swedberg K, Sharpe N, Doughty RN. Factors influencing the length of hospital stay of patients with heart failure. Eur J Heart Fail. 2003;5:201–209. doi: 10.1016/s1388-9842(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 20.Behar S, Panosh A, Reicher-Reiss H, Zion M, Schlesinger Z, Goldbourt U. Prevalence and prognosis of chronic obstructive pulmonary disease among 5,839 consecutive patients with acute myocardial infarction. SPRINT Study Group. Am J Med. 1992;93:637–641. doi: 10.1016/0002-9343(92)90196-i. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339:489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 22.Berger JS, Sanborn TA, Sherman W, Brown DL. Effect of chronic obstructive pulmonary disease on survival of patients with coronary heart disease having percutaneous coronary intervention. Am J Cardiol. 2004;94:649–651. doi: 10.1016/j.amjcard.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj CL, Gurm HS, Gupta R, Ellis SG, Bhatt DL. Chronic obstructive pulmonary disease as a predictor of mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2005;96:756–759. doi: 10.1016/j.amjcard.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Gardner SC, Grunwald GK, Rumsfeld JS, Mackenzie T, Gao D, Perlin JB, McDonald G, Shroyer AL. Risk factors for intermediate-term survival after coronary artery bypass grafting. Ann Thorac Surg. 2001;72:2033–2037. doi: 10.1016/s0003-4975(01)03217-9. [DOI] [PubMed] [Google Scholar]
- 25.Gao D, Grunwald GK, Rumsfeld JS, Mackenzie T, Grover FL, Perlin JB, McDonald GO, Shroyer AL. Variation in mortality risk factors with time after coronary artery bypass graft operation. Ann Thorac Surg. 2003;75:74–81. doi: 10.1016/s0003-4975(02)04163-2. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Epidemiol. 2001;54:40–50. doi: 10.1016/s0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 28.Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, Dietz R, Gavazzi A, van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24:464–474. doi: 10.1016/s0195-668x(02)00700-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol. 2001;37:1950–1956. doi: 10.1016/s0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- 30.Staszewsky L, Wong M, Masson S, Barlera S, Carretta E, Maggioni AP, Anand IS, Cohn JN, Tognoni G, Latini R. Clinical, neurohormonal, and inflammatory markers and overall prognostic role of chronic obstructive pulmonary disease in patients with heart failure: data from the Val-HeFT heart failure trial. J Card Fail. 2007;13:797–804. doi: 10.1016/j.cardfail.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 32.Iversen KK, Kjaergaard J, Akkan D, Kober L, Torp-Pedersen C, Hassager C, Vestbo J, Kjoller E. Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med. 2008;264:361–369. doi: 10.1111/j.1365-2796.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 33.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 34.Pena VS, Miravitlles M, Gabriel R, Jimenez-Ruiz CA, Villasante C, Masa JF, Viejo JL, Fernandez-Fau L. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118:981–989. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]