Abstract
Aims
Few prognostic models in heart failure have been developed in typically elderly patients treated with modern pharmacological therapy and even fewer included simple biochemical tests (such as creatinine), new biomarkers (such as natriuretic peptides), or, especially, both. In addition, most models have been developed for the single outcome of all-cause mortality.
Methods and results
We built a series of models for nine different fatal and non-fatal outcomes. For each outcome, a model was first built using demographic and clinical variables (Step 1), then with the addition of biochemical measures (serum creatinine, alanine aminotransferase, creatine kinase, thyrotrophin, apolipoproteins A-1 and B, and triglycerides) (Step 2) and finally with the incorporation of high-sensitivity C-reactive protein (hsCRP) and N-terminal pro B-type natriuretic peptide (NT-proBNP). Ranked according to the Wald χ2 value, age (56), ejection fraction (44), and body mass index (42) were most predictive of all-cause mortality in Step 1 (total model χ2 343). Creatinine was the most powerful predictor at Step 2 (48) and ApoA-1 ranked fifth (25), with the overall χ2 increasing to 440. Log NT-proBNP (167) was the most powerful of the 14 independently predictive variables identified at Step 3 and the overall χ2 increased to 600. NT-proBNP was the most powerful predictor of each other outcome. hsCRP was not a predictor of all-cause mortality but did predict the composite atherothrombotic outcome.
Conclusion
Of the two new biomarkers studied in prognostic models in heart failure, NT-proBNP, but not hsCRP, added substantial and independent predictive information, for a range of clinical outcomes, to that provided by simple demographic, clinical, and biochemical measures. ApoA-1 was more predictive than LDL or HDL.
Keywords: Chronic heart failure
Introduction
Chronic heart failure remains a common, disabling and deadly problem despite recent treatment advances.1,2 Models that predict outcome in patients with heart failure are important for counselling patients and their families about prognosis and informing decisions about treatment choices.3,4 Such models help in deciding about intensity of monitoring, in targeting expensive treatments (e.g. devices and transplantation) and in determining when palliative rather than life-prolonging therapy is most appropriate. Prognostic models also aid understanding of the patho-physiology of disease progression in heart failure and may identify new targets for therapeutic intervention.
Several such models exist, the best validated of which is the Seattle model.4 Because the Seattle model was tested in historical patient populations, however, it suffers from some limitations. Beta-blockers were not used in the derivation cohort and only in a minority of patients in the validation cohorts.4 The Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) model is a more contemporary one in which over half the patients were treated with a beta-blocker.5 However, neither the Seattle nor the CHARM model included biomarkers thought to be of prognostic importance in heart failure. High-sensitivity C-reactive protein (hsCRP), a biomarker indicative of inflammation (which is also thought to play a patho-physiological role in heart failure), was not measured in either study.6 Natriuretic peptides were not measured in CHARM patients or in the Seattle derivation cohort (and in only one of the five Seattle validation cohorts).7
In order to investigate the role of both established factors and these newer biomarkers in predicting fatal and non-fatal outcomes, we examined the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) population, a contemporary cohort of patients with low ejection fraction (EF) heart failure receiving modern pharmacological therapy.8,9 Our primary aim was to determine whether hsCRP and N-terminal pro B-type natriuretic peptide (NT-proBNP) provided additional, independent, prognostic information in these.10 A further objective was to examine, in more detail than previously, the prognostic importance of lipoprotein measurements in heart failure. Low total cholesterol is known to predict a high mortality in patients with heart failure but the predictive value of other lipoprotein measurements is uncertain.11
Methods
Patients
The design and principal findings of CORONA have been reported in detail.8,9 Patients ≥60 years with chronic New York Heart Association (NYHA) class II–IV heart failure of investigator-reported ischaemic aetiology and an EF of ≤40% (≤35% if NYHA class II) were eligible, provided that the investigator felt they did not need treatment with a cholesterol-lowering drug.
Exclusion criteria included: recent cardiovascular events, procedures or operations (or planned procedures or operations), acute or chronic liver disease or alanine aminotransferase (ALAT) ≥2 upper limit of normal (ULN); serum creatinine (s/creatinine) >220 µmol/L (2.5 mg/dL); chronic muscle disease or unexplained creatine kinase (CK) >2.5 ULN; thyroid-stimulating hormone (TSH) >2ULN; any other condition substantially reducing life expectancy.
Study procedures
The trial was approved by the Ethics Committees of the participating hospitals and patients provided written informed consent. Patients were allocated, equally, to 10 mg of rosuvastatin or matching placebo, once-daily. The first patient was randomized on 15 September 2003.
We measured s/creatinine, CK, TSH, ALAT, hsCRP, and lipid/lipoproteins [total, LDL and HDL cholesterol, triglycerides (TGs), and apolipoproteins A-1 (ApoA-1) and B (ApoB)] at baseline in all patients. After the study started, the protocol was amended to include measurement of NT-proBNP which, therefore, was not available in all patients. Complete sets of measurements were recorded in 3442 patients. These constitute the focus of this report.
All blood samples were non-fasting and analysed on fresh samples at a central laboratory [Medical Research Laboratories (MRL), Zaventem, Belgium]. NT-proBNP was analysed using a commercially available assay (Roche). An immunonephelometric method was used to measure hs-CRP (Dade Behring BNII instrument using CardioPhase hsCRP reagent from Dade Behring; assay sensitivity 0.040 mg/L and reference-range 0.0–8.4 mg/L).
Study outcomes and definitions
The primary outcome was the composite of cardiovascular mortality, non-fatal myocardial infarction (MI) or non-fatal stroke, analysed as time to the first event. The secondary outcomes were (in listed order): all-cause mortality, any coronary event [defined as sudden death, fatal or non-fatal MI, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), ventricular defibrillation by an ICD, resuscitation from cardiac arrest, or hospitalization for unstable angina], cardiovascular mortality (cause-specific cardiovascular death was also analysed), and total number of hospitalizations (for cardiovascular causes, unstable angina, and worsening heart failure). In the present analyses, we examined the predictors of these outcomes and their important components (e.g. sudden death, death from worsening heart failure and hospitalization for heart failure). We also included two additional ‘post hoc’ composite outcomes: death from any cause or hospitalization for worsening heart failure which is commonly reported in heart failure trials and an ‘atherothrombotic endpoint’ (fatal or non-fatal MI or fatal or non-fatal non-haemorrhagic stroke) comprising events likely to be related to atherosclerosis rather than myocardial disease. The definition and adjudication of these outcomes has been described in detail previously.
Statistical analyses: multi-variable analyses
Patients included in the present multi-variable analyses (n = 3342; 67% of all randomized) were those with no missing data for the variables included in the model (see below).
Multi-variable Cox regression models were applied to identify variables associated with each of the endpoints defined above. The multi-variable analysis was performed in three steps.
In Step 1, the following variables were included: age, sex, EF, NYHA class, systolic blood pressure (SBP), heart rate, body mass index (BMI), history of MI, angina pectoris, diabetes mellitus (DM), hypertension, stroke, intermittent claudication, aortic aneurysm, PTCA/PCI, CABG, atrial fibrillation, implanted pacemaker, implanted cardiac defibrillator, and smoking status.
In Step 2, the following laboratory variables were added: s/creatinine, ALAT, CK, TSH, and TGs. Furthermore, it was decided to add one of two alternatives. Alternative 1 focused on LDL and HDL and their ratio (both or the LDL/HDL ratio). Alternative 2 focused on ApoA-1 and ApoB and their ratio.
In Step 3, hsCRP and NT-proBNP were added.
SAS statistical package version 9.1 was used for most analyses. The C-statistics, which are the same as the area under the ROC curve, were calculated by Harrell's method using the STATA statistical package.
Results
Baseline characteristics
Patient characteristics are shown in Table 1. The mean age was 72 years and 40% were at least 75 years old. There was a high prevalence of prior or current hypertension, atrial fibrillation/flutter, DM, and chronic kidney disease (55% had an estimated glomerular filtration rate value <60 mL/min/1.73 m2). The mean (SD) total cholesterol was 5.36 (1.09) mmol/L; other lipid/lipoprotein measurements are shown in Table 1. The median (IQR) hsCRP was 3.4 (1.6–7.3) mg/L and the median NT-proBNP concentration was 166 pmol/L (70–358) corresponding to 1404 (592–3028) pg/mL. The patients were well treated for systolic heart failure and most were also treated with an anti-platelet or anti-coagulant drug.
Table 1.
Baseline characteristics for patients included in the prognostic model
| Variables | Survivors, n = 2408 | Non-survivors, n = 934 | All included, n = 3342 |
|---|---|---|---|
| Demographics | |||
| Age, years | 71.8 (6.9) | 74.3 (7.5) | 72.5 (7.1) |
| ≥75 years, n (%) | 870 (36) | 457 (49) | 1327 (40) |
| Female sex, n (%) | 658 (27) | 184 (20) | 842 (25) |
| NYHA class | |||
| II, n (%) | 920 (38) | 291 (31) | 1211 (36) |
| III, n (%) | 1462 (61) | 621 (67) | 2083 (62) |
| IV, n (%) | 26 (1.1) | 22 (2.4) | 48 (1.4) |
| Ejection fraction | 0.316 (0.063) | 0.296 (0.069) | 0.310 (0.065) |
| BMI, kg/m2 | 27.6 (4.6) | 26.2 (4.4) | 27.2 (4.6) |
| Systolic blood pressure, mmHg | 131 (16) | 127 (17) | 130 (16) |
| Diastolic blood pressure, mmHg | 77 (8.8) | 75 (9.2) | 76 (8.9) |
| Heart rate, b.p.m. | 71 (11) | 73 (12) | 72 (11) |
| Current smoker, n (%) | 200 (8.3) | 85 (9.1) | 285 (8.5) |
| Medical history | |||
| Myocardial infarction, n (%) | 1347 (56) | 580 (62) | 1927 (58) |
| Angina pectoris, n (%)a | 1751 (73) | 675 (72) | 2426 (73) |
| PTCA/PCI, n (%) | 297 (12) | 104 (11) | 401 (12) |
| CABG, n (%) | 382 (16) | 134 (14) | 516 (15) |
| CABG or PTCA/PCI, n (%) | 616 (26) | 214 (23) | 830 (25) |
| Hypertension, n (%) | 1589 (66) | 575 (62) | 2164 (65) |
| Diabetes mellitus, n (%) | 670 (28) | 311 (33) | 981 (29) |
| Atrial fibrillation or flutter (ECG), n (%)b | 598 (25) | 266 (29) | 864 (26) |
| Stroke, n (%) | 270 (11) | 140 (15) | 410 (12) |
| Intermittent claudication, n (%) | 267 (11) | 167 (18) | 434 (13) |
| Aortic aneurysm, n (%) | 57 (2.4) | 43 (4.6) | 100 (3.0) |
| Pacemaker, n (%) | 119 (4.9) | 62 (6.6) | 181 (5.4) |
| Implanted cardioverter defibrillator, n (%) | 57 (2.4) | 20 (2.1) | 77 (2.3) |
| Laboratory measurements | |||
| Total cholesterol, mmol/Lc | 5.43 (1.08) | 5.18 (1.09) | 5.36 (1.09) |
| LDL cholesterol, mmol/Lc | 3.63 (0.94) | 3.43 (0.94) | 3.57 (0.94) |
| HDL cholesterol, mmol/Lc | 1.24 (0.35) | 1.22 (0.37) | 1.24 (0.35) |
| ApoA-1, g/L | 1.52 (0.28) | 1.45 (0.29) | 1.50 (0.28) |
| ApoB, g/L | 1.28 (0.30) | 1.23 (0.30) | 1.27 (0.30) |
| ApoB/ApoA-1 | 0.86 (0.24) | 0.88 (0.26) | 0.87 (0.25) |
| Triglycerides, mmol/Ld | 2.02 (1.34) | 1.83 (1.08) | 1.96 (1.28) |
| Serum creatinine, μmol/Le | 111 (25) | 122 (30) | 114 (27) |
| Serum creatinine >130 µmol/L,en (%) | 409 (17) | 296 (32) | 705 (21) |
| eGFRMDRD mL/min/1.73 m2 | 59 (14) | 55 (15) | 58 (14) |
| eGFRMDRD<60 mL/min/1.73 m2, n (%) | 1243 (52) | 606 (65) | 1849 (55) |
| NT-proBNP pmol/L median (IQ range)f | 132 (54–271) | 313 (147–593) | 166 (70–358) |
| hsCRP mg/L median (IQ range) | 3.0 (1.5–6.4) | 4.6 (1.9–9.9) | 3.4 (1.6–7.3) |
| Medication | |||
| Loop diuretic, n (%) | 1723 (72) | 793 (85) | 2516 (75) |
| Loop or thiazide diuretic, n (%)g | 2077 (86) | 869 (93) | 2946 (88) |
| Aldosterone antagonist, n (%) | 887 (37) | 422 (45) | 1309 (39) |
| ACE inhibitor, n (%) | 1969 (82) | 754 (81) | 2723 (82) |
| ACE inhibitor or ARBS, n (%) | 2235 (93) | 848 (91) | 3083 (92) |
| Beta-blocker, n (%) | 1889 (78) | 669 (72) | 2558 (77) |
| Digitalis glycoside, n (%) | 710 (30) | 375 (40) | 1085 (33) |
| Anti-arrhythmic therapy, n (%) | 240 (10) | 134 (14) | 374 (11) |
| Anti-platelet, n (%) | 1470 (61) | 554 (59) | 2024 (61) |
| Anti-coagulant, n (%) | 812 (34) | 333 (36) | 1145 (34) |
| Anti-platelet or anti-coagulant, n (%) | 2172 (90) | 840 (90) | 3012 (90) |
All values are given as mean (standard deviation) unless stated otherwise.
NYHA, New York Heart Association; BMI, body mass index; b.p.m., beats per minute; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; ECG, electrocardiogram; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Apo, apolipoprotein; eGFR, estimated glomerular filtration rate; MDRD, modified diet in renal disease; NT-proBNP, N-terminal pro B-type natriuretic peptide; IQ, inter-quartile; hsCRP, high-sensitivity C-reactive protein; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
aPast or current.
bCurrent on ECG.
cTo convert to mg/dL multiply by 38.6.
dTo convert to mg/dL divide by 88.5.
eTo convert to mg/dL multiply by 0.0113.
fTo convert to pg/ml multiply by 8.457. NT-proBNP was measured in 1820 placebo and 1844 rosuvastatin treated patients.
gIncludes thiazide-like diuretics.
Multi-variable analyses
Step 1
Table 2 shows the prognostic model for total mortality based on demographics and medical history. Age, EF, and BMI were the top three variables, ordered by the Wald χ2 value. Results of the Step 1 models for all other endpoints are available on request (from the corresponding author).
Table 2.
Prognostic model for total mortality based on demographics and medical history (Step 1; all variables included in Step 1 are listed here)
| Variables | β-coeff. | SE | HR | 95% CI | Wald | P-value |
|---|---|---|---|---|---|---|
| Age, years/10 | 0.3640 | 0.0484 | 1.44 | 1.31–1.58 | 56.4 | <0.0001 |
| EF × 100 | −0.0329 | 0.0050 | 0.97 | 0.96–0.98 | 43.6 | <0.0001 |
| BMI, kg/m2 | −0.0547 | 0.0085 | 0.95 | 0.93–0.96 | 41.7 | <0.0001 |
| Diabetes | 0.3625 | 0.0720 | 1.44 | 1.25–1.66 | 25.4 | <0.0001 |
| Female sex | −0.3959 | 0.0848 | 0.67 | 0.57–0.80 | 21.8 | <0.0001 |
| NYHA class | 0.3328 | 0.0748 | 1.40 | 1.21–1.62 | 19.8 | <0.0001 |
| Intermittent claudication | 0.3390 | 0.0894 | 1.40 | 1.18–1.67 | 14.4 | 0.0002 |
| Heart rate, b.p.m./10 | 0.1018 | 0.0293 | 1.11 | 1.05–1.17 | 12.1 | 0.0005 |
| SBP, mmHg/10 | −0.0665 | 0.0216 | 0.94 | 0.90–0.98 | 9.5 | 0.0020 |
| CABG | −0.2557 | 0.0966 | 0.77 | 0.64–0.94 | 7.0 | 0.0081 |
| Myocardial infarction | 0.1796 | 0.0708 | 1.20 | 1.04–1.38 | 6.4 | 0.0112 |
| Stroke | 0.1789 | 0.0926 | 1.20 | 1.00–1.43 | 3.7 | 0.0535 |
| PTCA/PCI | −0.1324 | 0.1059 | 0.88 | 0.71–1.08 | 1.6 | >0.2 |
| Aortic aneurysm | 0.1703 | 0.1621 | 1.19 | 0.86–1.63 | 1.1 | >0.2 |
| Imp. cardioversion defib. | −01763 | 0.2308 | 0.84 | 0.53–1.32 | 0.6 | >0.2 |
| Hypertension | 0.0512 | 0.0728 | 1.05 | 0.91–1.21 | 0.5 | >0.2 |
| Angina pectoris | −0.0421 | 0.0760 | 0.96 | 0.83–1.11 | 0.3 | >0.2 |
| Atrial fibrillation | −0.0248 | 0.0774 | 0.98 | 0.84–1.14 | 0.1 | >0.2 |
| Pacemaker | 0.0112 | 0.1343 | 1.01 | 0.78–1.32 | 0.0 | >0.2 |
| Smoking | 0.0055 | 0.1096 | 1.01 | 0.81–1.25 | 0.0 | >0.2 |
| All variables total | 343 | <0.0001 |
Variables are ordered after value for Wald χ2. The model for total mortality is based on n=934 deaths.
Coeff., coefficient; SE, standard error; HR, hazard ratio; CI, confidence interval; EF, ejection fraction; BMI, body mass index; NYHA, New York Heart Association; b.p.m., beats per minute; SBP, systolic blood pressure; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; Imp., implanted; defib., defibrillator.
Choosing which lipid/lipoprotein variables to include in Step 2
Table 3 shows the results obtained from testing eight different lipid variables, individually, in separate steps. These variables were added to the model shown in Table 2 (i.e. the model based only on demographics and medical history). The lipid variables were tested for three different endpoints: total mortality (n = 934 deaths), the primary endpoint (cardiovascular death or non-fatal MI or non-fatal stroke; time to first event, n = 883 events), and the atherothrombotic endpoint (fatal or non-fatal MI or fatal or non-fatal non-haemorrhagic stroke; time to first event, n = 284 events). Higher baseline LDL, HDL, ApoA-1, ApoB, and TG were associated with a better prognosis. Table 3 shows the total Wald χ2 value of the resulting overall models (in descending order), after adding each lipid/lipoprotein variable. Note that the total Wald χ2 value of the basic model for all-cause mortality in Table 2 was 343 and this increased to between 410 and 440 after addition of the lipid/lipoprotein variables. On the basis of these analyses, we chose to use ApoA-1 and ApoB, added simultaneously, in subsequent Step 2 models.
Table 3.
Prognostic model for testing eight different lipid variables, one at a time, in eight separate different steps
| Endpoint/variables | Variable Wald | P-value | Total Wald |
|---|---|---|---|
| Total mortality | |||
| ApoB and ApoA-1 simultaneously | 1.0/25.3 | >0.2/<0.0001 | 440 |
| ApoA-1 only | 26.7 | <0.001 | 439 |
| LDL and HDL simultaneously | 7.2/7.1 | 0.0074/0.0076 | 429 |
| LDL only | 8.9 | 0.0029 | 422 |
| HDL only | 8.8 | 0.0030 | 420 |
| ApoB/ApoA-1 ratio | 6.7 | 0.0098 | 416 |
| ApoB only | 2.5 | 0.12 | 414 |
| LDL/HDL ratio | 0.3 | >0.2 | 410 |
| Primary endpoint | |||
| ApoB and ApoA-1 simultaneously | 0.12/22.4 | >0.2/<0.0001 | 315 |
| ApoA-1 only | 23.0 | <0.0001 | 315 |
| LDL and HDL simultaneously | 3.8/9.6 | 0.053/0.0019 | 307 |
| HDL only | 11.0 | 0.0009 | 303 |
| ApoB/ApoA-1 ratio | 7.4 | 0.0066 | 298 |
| LDL only | 5.2 | 0.023 | 297 |
| ApoB only | 0.7 | >0.2 | 292 |
| LDL/HDL ratio | 1.0 | >0.2 | 291 |
| Atherothrombotic endpoint | |||
| ApoB and ApoA-1 simultaneously | 0.13/6.7 | >0.2/0.0097 | 74 |
| ApoA-1 only | 6.6 | 0.010 | 74 |
| LDL and HDL simultaneously | 0.3/4.8 | >0.2/0.029 | 73 |
| HDL only | 5.1 | 0.024 | 72 |
| ApoB/ApoA-1 ratio | 2.3 | 0.13 | 69 |
| LDL only | 0.6 | >0.2 | 68 |
| LDL/HDL ratio | 0.3 | >0.2 | 68 |
| ApoB only | 0.0 | >0.2 | 67 |
The lipid variables have been added to the model based on demographics and medical history (see Table 2). Testing has been done for three different endpoints: total mortality (n = 934 deaths), the primary endpoint (cardiovascular death or non-fatal MI or non-fatal stroke; time to first event, n = 883 events), and an atherothrombotic endpoint (fatal or non-fatal MI or fatal or non-fatal non-haemorrhagic stroke; time to first event, n = 284 events). Variables are ordered after value for total Wald χ2 after adding the laboratory variable, respectively.
For abbreviations and units, see Table 1.
Step 2
Table 4 shows the prognostic model for total mortality, after adding s/creatinine, ALAT, CK, TSH, ApoA-1, ApoB, and TGs to the basic model described in Table 2 (which only incorporated demographics and medical history). s/creatinine (which displaced age), EF, and BMI were the top three variables, when ordered by Wald χ2 value. ApoA-1 was the fifth most predictive variable on the basis of Wald χ2 value, displacing history of diabetes. Total Wald χ2 increased from 343 in Step 1 to 440 in Step 2. The results for the Step 2 models for all the other endpoints analysed are available on request (from the corresponding author).
Table 4.
Prognostic model for total mortality based on demographics and medical history (all variables listed in Table 2), and the following laboratory variables: ApoA-1, ApoB, s/creatinine, ALAT, CK, and TSH (Step 2)
| Variables | β-coeff. | SE | HR | 95% CI | Wald | P-value |
|---|---|---|---|---|---|---|
| s/creatinine/10 | 0.0782 | 0.0113 | 1.08 | 1.06–1.11 | 47.8 | <0.0001 |
| EF × 100 | −0.0321 | 0.0050 | 0.97 | 0.96–0.98 | 41.3 | <0.0001 |
| BMI, kg/ m2 | −0.0554 | 0.0088 | 0.95 | 0.93–0.96 | 39.7 | <0.0001 |
| Age/10 | 0.2584 | 0.0507 | 1.3 | 1.17–1.43 | 25.9 | <0.0001 |
| ApoA-1 | −06641 | 0.1321 | 0.52 | 0.40–0.67 | 25.3 | <0.0001 |
| Diabetes | 0.3089 | 0.0734 | 1.36 | 1.18–1.57 | 17.7 | <0.0001 |
| Heart rate, b.p.m./10 | 0.1175 | 0.0293 | 1.13 | 1.06–1.19 | 16.1 | <0.0001 |
| NYHA class | 0.2859 | 0.0751 | 1.33 | 1.15–1.54 | 14.5 | <0.0001 |
| Triglycerides | −0.1102 | 0.0350 | 0.90 | 0.84–0.96 | 9.9 | 0.0016 |
| Intermittent claud. | 0.2826 | 0.0900 | 1.33 | 1.11–1.58 | 9.9 | 0.0017 |
| CABG | −0.2971 | 0.0969 | 0.74 | 0.61–0.90 | 9.4 | 0.0022 |
| SBP/10 | −0.455 | 0.0214 | 0.96 | 0.92–1.00 | 4.5 | 0.033 |
| Myocardial infarction | 0.1377 | 0.0710 | 1.15 | 1.00–1.32 | 3.8 | 0.052 |
| Female sex | −0.1657 | 0.0902 | 0.85 | 0.71–1.01 | 3.4 | 0.066 |
| All variables total | 440 | <0.0001 |
Step 3
Table 5 shows the results of the prognostic model for total mortality, based on the addition of the biomarkers hsCRP and NT-proBNP to the model in Table 4 (i.e. demographics and history with basic biochemistry and ApoA-1 and ApoB added). Log NT-proBNP, age, and history of diabetes were the top three variables, when ordered by Wald χ2 value. Total Wald χ2 increased from 440 in Step 2 to 600 in Step 3, due to the addition of NT-proBNP. NT-proBNP was a very powerful predictive variable, with an individual Wald χ2 value of 167.
Table 5.
Prognostic model for total mortality based on demographics and medical history (all variables listed in Table 2, Step 1), and the following laboratory variables: ApoA-1, ApoB, s/creatinine, ALAT, CK, and TSH (Step 2, see Table 4), and hsCRP and NT-proBNP (Step 3)
| Variables | β-coeff. | SE | HR | 95% CI | Wald | P-value |
|---|---|---|---|---|---|---|
| Log NT-proBNP | 0.4681 | 0.0362 | 1.60 | 1.49–1.71 | 167 | <0.0001 |
| Age/10 | 0.2305 | 0.0513 | 1.26 | 1.14–1.39 | 20.2 | <0.0001 |
| Diabetes | 0.2689 | 0.0736 | 1.31 | 1.13–1.51 | 13.3 | 0.0003 |
| EFx100 | −0.0178 | 0.0052 | 0.98 | 0.97–0.99 | 11.5 | 0.0007 |
| BMI kg/ m2 | −0.0300 | 0.0089 | 0.97 | 0.95–0.99 | 11.3 | 0.0008 |
| CABG | −0.3210 | 0.0974 | 0.73 | 0.60–0.88 | 10.9 | 0.0010 |
| Female sex | −0.2945 | 0.0898 | 0.75 | 0.63–0.89 | 10.8 | 0.0010 |
| Atrial fibrillation | 0.2597 | 0.0794 | 1.30 | 1.11–1.52 | 10.7 | 0.0011 |
| NYHA class | 0.2428 | 0.0751 | 1.28 | 1.10–1.48 | 10.5 | 0.0012 |
| ApoA-1 | −0.4057 | 0.1310 | 0.67 | 0.52–0.86 | 9.6 | 0.0020 |
| s/creatinine/10 | 0.0353 | 0.0121 | 1.04 | 1.01–1.06 | 8.5 | 0.0035 |
| Intermittent claudication | 0.2420 | 0.0906 | 1.27 | 1.07–1.52 | 7.1 | 0.0076 |
| Heart rate b.p.m./10 | 0.0670 | 0.0296 | 1.07 | 1.01–1.13 | 5.1 | 0.024 |
| Myocardial infarction | 0.1396 | 0.0709 | 1.15 | 1.00–1.32 | 3.9 | 0.049 |
| All variables total | 600 | <0.0001 |
Complete results of the Step 3 analyses for all the other endpoints are available on request (from the corresponding author, see also Table 6).
Table 6.
First three variables for prognostic model (Step 3, see Table 5 for clarification) for all predefined endpoints (except total mortality, see Table 5), and for two post hoc defined endpoints (atherothrombotic endpoint defined as composite of non-fatal and fatal myocardial infarction and non-fatal and fatal non-haemorrhagic stroke; and composite of all-cause mortality and hospitalization for worsening heart failure; both as time to first event)
| Endpoint/variables | β-coeff. | SE | HR | 95% CI | Wald | P-value |
|---|---|---|---|---|---|---|
| Primary endpoint (n = 883)a | ||||||
| Log NT-proBNP | 0.4617 | 0.0370 | 1.59 | 1.48–1.71 | 155 | <0.0001 |
| Age/10 | 0.2388 | 0.0529 | 1.26 | 1.13–1.39 | 18.7 | <0.0001 |
| Atrial fibrillationb | 0.3409 | 0.0829 | 1.41 | 1.20–1.65 | 16.9 | <0.0001 |
| Total Wald χ2 | 477 | <0.0001 | ||||
| Atherothrombotic endpoint (n = 284)a | ||||||
| Log NT-proBNP | 0.2136 | 0.0624 | 1.24 | 1.10–1.40 | 11.7 | 0.0006 |
| Myocardial infarction | 0.3901 | 0.1330 | 1.48 | 1.14–1.92 | 8.6 | 0.0034 |
| Atrial fibrillationb | 0.4507 | 0.1605 | 1.57 | 1.15–2.15 | 7.9 | 0.0050 |
| Total Wald χ2 | 97.7 | <0.0001 | ||||
| Coronary endpoint (n = 741)a | ||||||
| Log NT-proBNP | 0.3846 | 0.0393 | 1.47 | 1.36–1.59 | 95.6 | <0.0001 |
| Atrial fibrillationb | 0.4275 | 0.0940 | 1.53 | 1.28–1.84 | 20.7 | <0.0001 |
| Diabetes mellitus | 0.2660 | 0.0816 | 1.31 | 1.11–1.53 | 10.6 | 0.0011 |
| Total Wald χ2 | 283 | <0.0001 | ||||
| Cardiovascular death (n = 725)a | ||||||
| Log NT-proBNP | 0.5508 | 0.0415 | 1.74 | 1.60–1.88 | 176 | <0.0001 |
| CABG | −0.4591 | 0.1168 | 0.63 | 0.50–0.79 | 15.5 | <0.0001 |
| Age/10 | 0.2130 | 0.0582 | 1.24 | 1.10–1.39 | 13.4 | 0.0003 |
| Total Wald χ2 | 515 | <0.0001 | ||||
| Sudden death (n = 407)a | ||||||
| Log NT-proBNP | 0.5235 | 0.0552 | 1.69 | 1.52-1.88 | 90.1 | <0.0001 |
| EF × 100 | −0.0230 | 0.0079 | 0.98 | 0.96–0.99 | 8.6 | 0.0034 |
| Age/10 | 0.1881 | 0.0779 | 1.21 | 1.04–1.41 | 5.8 | 0.016 |
| Total Wald χ2 | 246 | <0.0001 | ||||
| Death from heart failure (n = 230)a | ||||||
| Log NT-proBNP | 0.6859 | 0.0755 | 1.99 | 1.71–2.30 | 82.6 | <0.0001 |
| Age/10 | 0.3337 | 0.1031 | 1.40 | 1.14–1.71 | 10.5 | 0.0012 |
| Diabetes mellitus | 0.4533 | 0.1456 | 1.57 | 1.18–2.09 | 9.7 | 0.0018 |
| Total Wald χ2 | 296 | <0.0001 | ||||
| First CV hospitalization (n = 1452)a | ||||||
| Log NT-proBNP | 0.3078 | 0.0287 | 1.36 | 1.29–1.44 | 115 | <0.0001 |
| Intermittent claudication | 0.3424 | 0.0746 | 1.41 | 1.22–1.63 | 21.1 | <0.0001 |
| NYHA class | 0.2270 | 0.0604 | 1.26 | 1.12–1.41 | 14.1 | 0.0002 |
| Total Wald χ2 | 405 | <0.0001 | ||||
| First heart failure hosp. (n = 823)a | ||||||
| Log NT-proBNP | 0.5463 | 0.0404 | 1.73 | 1.60–1.87 | 183 | <0.0001 |
| Heart rate b.p.m./10 | 0.1579 | 0.0312 | 1.17 | 1.10–1.25 | 25.6 | <0.0001 |
| NYHA class | 0.3942 | 0.0824 | 1.48 | 1.26–1.74 | 22.9 | <0.0001 |
| Total Wald χ2 | 479 | <0.0001 | ||||
| All-cause mort/HF hosp. (n = 1376)a,c | ||||||
| Log NT-proBNP | 0.4939 | 0.0307 | 1.64 | 1.54–1.74 | 260 | <0.0001 |
| NYHA class | 0.3228 | 0.0626 | 1.38 | 1.22–1.56 | 26.6 | <0.0001 |
| Heart rate b.p.m./10 | 0.1155 | 0.0246 | 1.12 | 1.07–1.18 | 22.0 | <0.0001 |
| Total Wald χ2 | 701 | <0.0001 | ||||
Variables are ordered after value for Wald χ2 (total Wald χ2 for all variables included also given).
aNumber of endpoints included in prognostic model within brackets.
bCurrent at randomization.
cTime to first event.
CV, cardiovascular; hosp., hospitalization; mort., mortality; HF, heart failure. For other abbreviations and units, see Tables 1 and 2.
Step 3 for the other endpoints analysed
Table 6 presents the three most predictive variables, based on individual Wald χ2 values, for all endpoints examined in the Step 3 analyses. For each endpoint, NT-proBNP was the most powerful variable, accounting for a large part of the total Wald χ2. Complete results of the Step 3 analyses for all the endpoints are available on request (from the corresponding author).
C-statistics
For Step 1 the C-statistic (area under the ROC curve) for total mortality was 0.667, for Step 2 0.684, and for Step 3 0.719 (P-value for Step 1 vs. 2 and 2 vs. 3 both = 0.0001). Corresponding data for death due to heart failure were 0.742, 0.757, and 0.800 (P = 0.25 and 0.0002); and for the composite of all-cause mortality or hospitalization for worsening heart failure (time to first event) were 0.653, 0.666, and 0.701 (P = 0.002 and 0.0001, respectively).
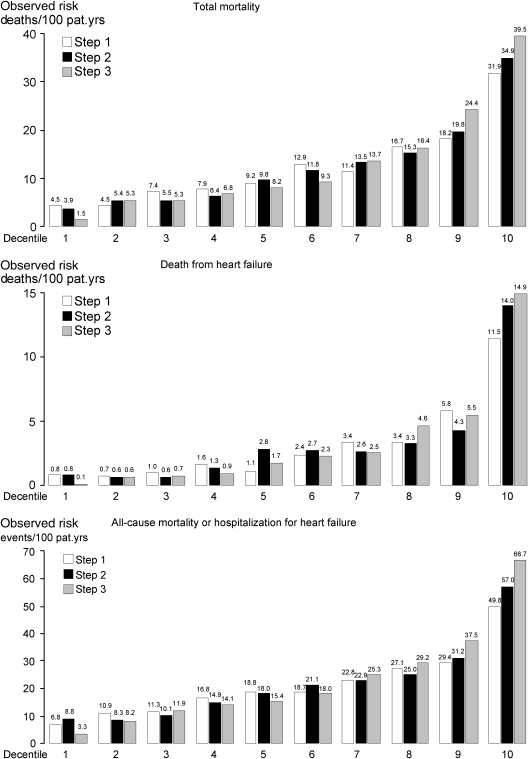
Observed risk by decentiles of predicted risk according to Step 1, 2, and 3 models
Figure 1 presents observed risk expressed as events per 100 patient-years of follow-up in decentiles of predicted risk according to Step 1, 2, and 3 models for the three endpoints total mortality (including 934 deaths), death from heart failure (230 deaths), and the composite of all-cause mortality or hospitalization from worsening heart failure (time to first event; 1376 events). The data illustrate that adding NT-proBNP in Step 3 leads to an observed risk that differs much more between decentile 1 and 10 compared with both Step 1 and 2. For total mortality, the ratio between observed risk decentile 10/decentile 1 is 7.1 for Step 1 (31.9/4.5 deaths/100 patient-years, Figure 1), 8.9 for Step 2, and 26.3 for Step 3. Corresponding data for death from heart failure is 14.4, 17.5, and 149; for the composite of all-cause mortality or hospitalization from worsening heart failure (time to first event) 7.3, 6.5, and 20.2; and for sudden death (including 407 deaths, not illustrated) 6.8, 6.5, and 27.8, respectively.
Figure 1.
Observed risk by decentiles of predicted risk according to the three different models in Step 1, 2, and 3 for total mortality (934 deaths, upper panel), death from heart failure (230 deaths, middle panel), and the composite of all-cause mortality or hospitalization for worsening heart failure (time to first event, 1376 events, lower panel), see text for comments.
Discussion
Although a number of prognostic models have already been described in patients with heart failure, the current series of models have a number of new features and, we believe, advantages.4–7,11–14
First, our models were derived in a large cohort of older patients with a mean age of 72 years, much closer to the age of patients with heart failure in the general population. This contrasts to the mean age of 65 years in the Seattle derivation cohort (and a mean age ranging between 53 and 64 years in four of the five validation cohorts) and 66 years in CHARM.4,5
Secondly, our patients received excellent contemporary pharmacological treatment, with the highest rate of use of beta-blockers (77%) reported in any large-scale, long-term, treatment trial in ambulatory patients with chronic heart failure, making our findings more relevant to current practice.
Thirdly, our models included routine biochemical measurements which were made in all participants in CORONA, which differentiates our models from prior examples such as the CHARM models where biochemical data were not used.5 This is important, given the predictive importance of simple measures such as creatinine and the more recent recognition that other routine tests, such as cholesterol, also provide useful prognostic information.11,14 We have confirmed and extended knowledge of the predictive value of lipids and lipoproteins in heart failure. We found that higher baseline LDL, HDL, apolA-1, ApoB, and TG were each associated with a better prognosis. Interestingly, we found that apolipoproteins, particularly ApoA-1, was more predictive than LDL or HDL cholesterol (or the LDL/HDL ratio), further emphasizing the different relationship between lipids/lipoproteins in patients with coronary heart disease and heart failure, compared with those with coronary heart disease alone.15 This finding requires verification in an independent patient cohort. If verified, it will raise questions about lipid/lipoprotein measurement in heart failure (which is the most useful test?) and disease mechanisms (why should ApoA-1 be the most predictive lipid measure?).
Finally, and most importantly, we examined the prognostic value of two new biomarkers in our patients, namely hsCRP and NT proBNP. The patho-physiological role of inflammation in heart failure has been the subject of debate. While many inflammatory markers are abnormal in patients with heart failure, and inflammation can cause heart failure in animal models, anti-inflammatory interventions in heart failure have not improved clinical outcomes so far.16–18 Interestingly, hsCRP was not an independent predictor of death from any cause in heart failure, in contrast to its prognostic importance in patients with coronary heart disease and no heart failure.19 However, consistent with prior studies in coronary heart disease, we did show that hsCRP was an independent predictor of our atherothrombotic composite endpoint.19 As with lipids, this finding further emphasizes the different epidemiology of heart failure and atherosclerotic disease uncomplicated by heart failure.
Measurement of natriuretic peptides was not routine when the Seattle and CHARM models were developed but has now become part of usual clinical practice in many centres. The prognostic importance of these peptides is also now clearly established.10,20 However, the independent and incremental prognostic information provided by natriuretic peptides in a large and otherwise well-characterized and representative patient cohort has not been extensively evaluated. For example, when CORONA was designed, we did not know whether natriuretic peptides really added much prognostic information to cheaper, simpler, and routine measurements such as creatinine in typically older patients with heart failure.8,9 We confirmed the value of s/creatinine, which in Step 2 of our model building, for all-cause mortality, had the largest Wald χ2 value (47.8 compared with EF which had the second highest value of 41.3).21,22 However, NT-proBNP surpassed all other variables, with a Wald χ2 value of 167. This finding is in keeping with some other recent analyses.23,24 The results from the evaluation of C-statistics in our study, and also the data by observed risk in the decentiles of predicted risk comparing Step 1, 2, and 3 showed that adding NT-proBNP in Step 3 clearly offered statistical proof that NT-proBNP improved the model.
Furthermore, the addition of NT-proBNP weakened the association between many of the other baseline variables, such as age and NYHA class, and outcome. Nevertheless, while NT-proBNP was a very powerful individual prognostic variable, it was only one of 14 independent predictors of all-cause mortality and the final model for this outcome had a total Wald χ2 value of 600 (addition of NT-proBNP increased the total Wald χ2 value from 400 to 600). Interestingly, although NT-proBNP may be regarded as an integrator of cardiac and renal dysfunction, both creatinine and EF remained in our final mortality model. This might be because natriuretic peptides also predict myocardial ischaemic events, although the evidence for this is conflicting and the number of events in our study is too small to draw firm conclusions.25,26 Nevertheless, while NT-proBNP is a valuable additional prognostic measure in heart failure, it is not the whole story, as has been suggested previously.27
Although exact comparisons are difficult, our model more closely resembles the CHARM than the Seattle models.4,5 The Seattle model incorporated non-randomized drug therapy (which was not included in either the CHARM or the current models) and a different set of laboratory variables than in the CORONA models.4,5 As in the CHARM models, diabetes was a powerful predictor of death; and age, BMI, and EF were also prominent. Both sets of models also included sex, NYHA class, and history of MI, atrial fibrillation/flutter, and heart rate.5 The Seattle model also contained many but not all of these variables.4 The most striking difference between the Seattle and the CHARM and CORONA models was diabetes, which was not an independent predictor in the Seattle model (and, compared with the CORONA model, creatinine was not an independent predictor in the Seattle model).4,5
An additional strength of our study was that we built models not just for all-cause mortality but also for non-fatal outcomes and composites of death and non-fatal outcomes (as well as modes of death). This is important because in older patients, death may not be the only (or even the most important) clinical outcome. Non-fatal outcomes are also important from a health-care burden and economic perspective and this type of analysis helps in understanding their pathogenesis and in identifying potential targets for preventive therapy. Interestingly, NT-proBNP emerged as the most important prognostic variable (as ranked by the Wald χ2 value) for each outcome, even though these reflected different types of disease activity (e.g. events related to atherothrombosis compared with those related to the failing myocardium). This was in contrast to other variables such as hsCRP as alluded to above.
Our study had a number of specific limitations as well as strengths. All patients had an ischaemic aetiology (as reported by the investigator). We did not make several biochemical (e.g. sodium) and haematological (e.g. haemoglobin) and other measurements which have been predictive of mortality in prior studies.28–31 Our findings have not been validated in an external cohort (although the similarity of many of our findings to those in CHARM suggests that they are likely to be valid).
Funding
AstraZeneca, Mölndal, Sweden sponsored the CORONA trial.
Conflict of interest: J.G.F.C., J.H.C., P.D., Å.H., J.K., J.J.V.M., J.V., F.W., and H.W. report having received consulting or advisory board fees from AstraZeneca. J.G.F.C., Å.H., J.K., M.K., J.J.V.M., J.V., and F.W. report having been paid lecture fees by AstraZeneca. Å.H. and J.J.V.M. report having received research grants from AstraZeneca. F.W. reports owning shares in AstraZeneca. J.W. is a former Senior Medical Advisor at AstraZeneca, now consultant. M.L. is an employee of AstraZeneca.
Appendix
The members of the CORONA Study Group are as follows: Executive Committee: P.D., Amphia Hospital, Breda, Netherlands; Å.H., Wallenberg Laboratory for Cardiovascular Research, Sahlgrenska Academy, University of Gothenburg, Sweden (Chairman of the Executive Committee); Lennart Jansson, AstraZeneca, Mölndal, Sweden (Study team leader); J.K., Department of Cardiology, Rikshospitalet University Hospital, Oslo, Norway; M.L., AstraZeneca. Mölndal, Sweden (AstraZeneca biostatistication); J.J.V.M., BHF Glasgow Cardiovascular Research Centre, University of Glasgow, UK; F.W., Wallenberg Laboratory for Cardiovascular Research, Sahlgrenska Academy, University of Gothenburg, Sweden; H.W., Nordic School of Public Health, Göteborg, Sweden (Independent biostatistician); J.W., Wallenberg Laboratory for Cardiovascular Research, Sahlgrenska Academy, University of Gothenburg, Sweden (Secretary of the Executive Committee). Writing Committee: P.D., Å.H., J.K., J.J.V.M. (Chairman of the Writing Committee), F.W., H.W., and J.W. Steering Committee: Chairman J.K.; Co-chair P.D. and Å.H. Members of the Steering Committee are the Executive Committee members and all National Co-ordinating Investigators (for names and affiliations, see reference 9). The Steering Committee also included one AstraZeneca monitor from each of the 21 participating countries (non-voting; for names see reference 9). Investigators: A list of all investigators is available in the on line version of the CORONA Design and Baseline publication at doi:10.1016/j.ejheart.2005.09.005. Data and Safety Monitoring Board: Henry Dargie, Scottish Advanced Heart Failure Service, Glasgow Royal Infirmary, Glasgow, Scotland (Chairman); David DeMets, Department of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA (DSMB biostatistician); Rory Collins, Clinical Trial Service Unit, University of Oxford, Oxford, UK; Jan Feyzi, Department of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA (SDAC biostatistician); Barry Massie, Veterans Affairs Medical Center and University of California San Francisco, San Francisco. Independent Endpoint Committee: Bengt-Olov Fredlund, Department of Emergency and Cardiovascular Medicine, Sahlgrenska University Hospital Östra, Göteborg University, Göteborg, Sweden; Mikael Holmberg, Department of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; Katarina Saldeen, Department of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; Ola Samuelsson (Secretary), Department of Nephrology, Department of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; and Karl Swedberg, Department of Emergency and Cardiovascular Medicine, Sahlgrenska University Hospital Östra, Göteborg University, Göteborg, Sweden (Chairman).
References
- 1.MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation. 2000;102:1126–1131. doi: 10.1161/01.cir.102.10.1126. [DOI] [PubMed] [Google Scholar]
- 2.Schaufelberger M, Swedberg K, Köster M, Rosén M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–307. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW. Predicting life expectancy in heart failure. JAMA. 2008;299:2566–2567. doi: 10.1001/jama.299.21.2566. [DOI] [PubMed] [Google Scholar]
- 4.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 5.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 6.Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN Val-HeFT Investigators. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 7.May HT, Horne BD, Levy WC, Kfoury AG, Rasmusson KD, Linker DT, Mozaffarian D, Anderson JL, Renlund DG. Validation of the Seattle Heart Failure Model in a community-based heart failure population and enhancement by adding B-type natriuretic peptide. Am J Cardiol. 2007;100:697–700. doi: 10.1016/j.amjcard.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 8.Kjekshus J, Dunselman P, Blideskog M, Eskilson C, Hjalmarson A, McMurray JV, Waagstein F, Wedel H, Wessman P, Wikstrand J CORONA Study Group. A statin in the treatment of heart failure? Controlled rosuvastatin multinational study in heart failure (CORONA): study design and baseline characteristics. Eur J Heart Fail. 2005;7:1059–1069. doi: 10.1016/j.ejheart.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 10.Pfister R, Diedrichs H, Schiedermair A, Rosenkranz S, Hellmich M, Erdmann E, Schneider CA. Prognostic impact of NT-proBNP and renal function in comparison to contemporary multi-marker risk scores in heart failure patients. Eur J Heart Fail. 2008;10:315–320. doi: 10.1016/j.ejheart.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 13.Heywood JT, Elatre W, Pai RG, Fabbri S, Huiskes B. Simple clinical criteria to determine the prognosis of heart failure. J Cardiovasc Pharmacol Ther. 2005;10:173–180. doi: 10.1177/107424840501000305. [DOI] [PubMed] [Google Scholar]
- 14.Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, Coats AJ, Anker SD. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 15.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S INTERHEART study investigators. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 16.Aukrust P, Gullestad L, Ueland T, Damås JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med. 2005;37:74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- 17.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 18.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E Pravastatin or Atorvastatin Evaluation Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 20.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–1593. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 21.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 22.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ Candesartan in Heart Failure: Assessment of Reduction in Mortality Morbidity (CHARM) Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 23.Olsson LG, Swedberg K, Cleland JG, Spark PA, Komajda M, Metra M, Torp-Pedersen C, Remme WJ, Scherhag A, Poole-Wilson P COMET Investigators. Prognostic importance of plasma NT-proBNP in chronic heart failure in patients treated with a beta-blocker: results from the Carvedilol Or Metoprolol European Trial (COMET) trial. Eur J Heart Fail. 2007;9:795–801. doi: 10.1016/j.ejheart.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN Val-HeFT Investigators. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 25.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, Landaas S, Rouleau JL, Domanski MJ, Hall C, Pfeffer MA, Braunwald E PEACE Investigators. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;50:205–214. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Kearney MT, Fox KA, Lee AJ, Prescott RJ, Shah AM, Batin PD, Baig W, Lindsay S, Callahan TS, Shell WE, Eckberg DL, Zaman AG, Williams S, Neilson JM, Nolan J. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. J Am Coll Cardiol. 2002;40:1801–1808. doi: 10.1016/s0735-1097(02)02490-7. [DOI] [PubMed] [Google Scholar]
- 29.Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, Burton PB. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 30.Brophy JM, Dagenais GR, McSherry F, Williford W, Yusuf S. A multivariate model for predicting mortality in patients with heart failure and systolic dysfunction. Am J Med. 2004;116:300–304. doi: 10.1016/j.amjmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Gradman A, Deedwania P, Cody R, Massie B, Packer M, Pitt B, Goldstein S. Predictors of total mortality and sudden death in mild to moderate heart failure. Captopril-Digoxin Study Group. J Am Coll Cardiol. 1989;14:564–570. doi: 10.1016/0735-1097(89)90093-4. [DOI] [PubMed] [Google Scholar]