Abstract
Although calcium phosphate cement (CPC) is promising for bone repair, its clinical use requires on site powder–liquid mixing. To shorten surgical time and improve graft properties, it is desirable to develop premixed CPC in which the paste remains stable during storage and hardens only after placement into the defect. The objective of this study was to develop premixed CPC with rapid setting when immersed in a physiological solution. Premixed CPCs were formulated using the following approach: Premixed CPC = CPC powder+nonaqueous liquid+gelling agent+hardening accelerator. Three premixed CPCs were developed: CPC–monocalcium phosphate monohydrate (MCPM), CPC–chitosan, and CPC–tartaric. Setting time for these new premixed CPCs ranged from 5.3 to 7.9 min, significantly faster than 61.7 min for a premixed control CPC reported previously (p<05). SEM revealed the formation of nano-sized needle-like hydroxyapatite crystals after 1 d immersion and crystal growth after 7 d. Diametral tensile strength for premixed CPCs at 7 d ranged from 2.8 to 6.4 MPa, comparable to reported strengths for cancellous bone and sintered porous hydroxyapatite implants. Osteoblast cells attained a normal polygonal morphology on CPC–MCPM and CPC–chitosan with cytoplasmic extensions adhering to the nano-hydroxyapatite crystals. In summary, fast-setting premixed CPCs were developed to avoid the powder–liquid mixing in surgery. The pastes hardened rapidly once immersed in physiological solution and formed hydroxyapatite. The cements had strengths matching those of cancellous bone and sintered porous hydroxyapatite and non-cytotoxicity similar to conventional non-premixed CPC.
Keywords: Premixed calcium phosphate cement, Rapid setting, Hydroxyapatite, Strength, Cell culture cytotoxicity, Bone repair
1. Introduction
Several calcium phosphate cements have been developed since the proposal of apatitic calcium phosphates as restorative materials in 1982 and the first self-setting calcium phosphate cement reported in 1986 [1,2]. Calcium phosphate cements generally consist of a powder and an aqueous liquid, which are mixed to form a paste [2–8]. The paste is placed into a defect as a substitute for the damaged part of bone [9–14]. One calcium phosphate cement, referred to as CPC [2,12,13], consists of tetracalcium phosphate (TTCP, Ca4[PO4]2O) and dicalcium phosphate anhydrous (DCPA, CaHPO4). The CPC paste intimately adapts to the bone cavity even for irregularly shaped cavities. CPC forms hydroxyapatite in an aqueous environment at body temperature, hence it is more similar to biological apatites than sintered hydroxyapatite formed at high temperatures [15]. As a result, CPC is not only bioactive, non-cytotoxic and osteoconductive, it is also bioresorbable and can be replaced by new bone [9–13].
One drawback is that, in clinical use, the surgeon needs to have the CPC powder and liquid mixed properly and thoroughly in a short period of time [16]. The surgeon then needs to place the paste into the defect within a prescribed time before the paste hardens. The requirement of on-site powder–liquid mixing increases the surgical time. It may also raise concerns about insufficient and inhomogeneous mixing thus compromising the implant strength, and inconsistencies between operators causing unpredictable variations in graft performance.
To circumvent such problems, it is desirable to develop premixed CPC in which the paste is prepared in advance under well-controlled conditions and will not harden in the package or in a syringe. This was achieved in a previous study [16] where the CPC powder was mixed with a nonaqueous but water-miscible liquid. This water-free, glycerol-containing CPC paste was stable and did not harden, because CPC hardens only when exposed to an aqueous environment. After this paste was placed in a physiological solution (or in a defect site with exposure to water from the surrounding physiological solution), exchange of glycerol-aqueous solution occurred, leading to CPC hardening [16].
However, a major shortcoming with premixed CPC in the previous study was that it took an hour or more for the paste to harden when immersed in a physiological solution [16]. Such a long setting time could cause problems clinically because of the cement’s inability to support stresses within this time period [17]. Therefore, it would be desirable to develop a rapid-setting premixed CPC in order to provide geometrical integrity and mechanical strength from the initial stage after placement in a defect site.
Accordingly, the objective of the present study was to develop new premixed CPCs that are capable of rapid hardening when immersed in a physiological solution. In addition, the cytotoxicity of the new formulations was assessed to test the hypothesis that the fast-setting premixed CPCs, containing various gelling agents and hardening accelerators, have no adverse effect on the attachment and viability of osteoblast cells.
2. Materials and methods
2.1. Premixed CPC compositions
TTCP powder was synthesized from a solid-state reaction between equimolar amounts of CaHPO4 (DCPA) and CaCO3 (J. T. Baker Chemical, Phillipsburg, NJ), which were mixed and heated at 1500 °C for 6 h in a furnace (Model 51333, Lindberg, Watertown, WI). All the chemicals used in fabricating the premixed CPCs had a purity level of reagent grade unless otherwise noted. The heated mixture was ball milled and sieved to obtain TTCP particles with sizes ranging from approximately 1 to 80 μm, with median = 17 μm. DCPA powder had particle sizes ranging from 0.4 to 3 μm, with median = 1 μm. The TTCP and DCPA powders were mixed at mass fractions of 73% TTCP and 27% DCPA to form the CPC powder.
Premixed CPCs with fast-setting were formulated by using the following approach:
The purpose of using a nonaqueous liquid was to mix the powders into a paste. The purpose of including a gelling agent was to improve the paste cohesiveness. The purpose of incorporating a hardening accelerator was to achieve fast setting. Following this approach, the following three premixed CPCs were developed.
CPC– MCPM
Glycerol was selected as the nonaqueous liquid following a previous study [16] because glycerol is nontoxic and biocompatible, can serve as a lubricant, and has been used in beverages, chewing gum and gelatin foods [18]. Glycerol is also water-miscible which, when the CPC–glycerol paste was immersed in water, allowed glycerol–water exchange to occur, resulting in cement hardening [16]. The liquid consisted of 100% of glycerol (J. T. Baker Chemical, Phillipsburg, NJ). The powder phase consisted of mass fractions of 79.5% CPC powder mixed with 20% monocalcium phosphate monohydrate (MCPM, Ca(H2PO4)2 · H2O, Monsanto Company, St. Louis, MO) and 0.5% hydroxypropyl methylcellulose (HPMC, Sigma Chemical, St. Louis, MO). MCPM was used as a hardening accelerator. It dissolved quickly in water and formed phosphoric acid and small crystals of dicalcium phosphate dihydrate in preliminary studies, thus promoting hydroxyapatite formation and paste hardening. HPMC is a derivative of cellulose, one of the most commonly occurring polysaccharides. It was used here as a gelling agent because it is known for its gelling ability to form viscous solutions, thus improving the washout resistance of the cement [16,19]. The powder:liquid mass ratio was 4:1. This ratio and the fractions of MCPM and HPMC were selected based on preliminary results with the requirement for fast setting while achieving a workable paste viscosity. This premixed CPC was referred to as ‘‘CPC–MCPM’’.
CPC– tartaric
The powder consisted of 100% CPC powder. The liquid consisted of mass fractions of 62.2% of poly(propylene glycol), 37.5% d-tartaric acid, and 0.3% HPMC. Poly(propylene glycol) (2700, Aldrich, Milwaukee, WI) was used as the nonaqueous liquid instead of glycerol. In preliminary studies using glycerol, the premixed paste was not stable and showed hardening, because d-tartaric acid was able to dissolve in glycerol to react with the CPC powder. When poly (propylene glycol) was used, unwanted reaction was prevented and a stable premixed paste was obtained. Poly(propylene glycol) was selected because it has been used as a defoaming agent in processed beet sugar and yeast with no known toxicity [18]. D-tartaric acid (J. T. Baker Chemical, Phillipsburg, NJ) was used as a hardening accelerator because it reacts with calcium to form calcium tartarate, thus imparting fast hardening. A powder:liquid ratio of 4:1 was used. This premixed CPC was designated as ‘‘CPC–tartaric’’.
CPC– chitosan
The powder consisted of 100% CPC powder. The liquid consisted of mass fractions of 59.5% glycerol, 0.5% Ca(OH)2, and 40% chitosan malate. Ca(OH)2 (Fisher Scientific, Fair Lawn, NJ) was used as a hardening accelerator to cause an increase in pH resulting in chitosan gelling. Chitosan malate (technical grade, VANSON, Redmond, WA) was used as a gelling agent because it imparted washout resistance to the paste in preliminary studies, and because chitosan and its derivatives are natural biopolymers that are biocompatible, biodegradable and osteoconductive [20–23]. A lower powder:liquid ratio of 2:1 was used to avoid paste dryness for this premixed CPC, which was denoted ‘‘CPC–chitosan’’.
A premixed CPC developed previously [16] was used as a control for hardening and strength measurements. The powder consisted of 100% of the same CPC powder; the liquid consisted of 69.45% glycerol, 0.55% HPMC, and 30% Na2HPO4 (Abbott Laboratories, North Chicago, IL) [16]. A powder:liquid ratio of 4:1 was used for this premixed CPC, which was designated as ‘‘premixed control’’.
2.2. Hardening time and washout resistance
The powder and liquid of each premixed CPC were mixed using a spatula to form a cohesive paste that was filled into a mold of 6 mm diameter and 3 mm depth [16]. Each specimen in the mold was sandwiched between two fritted glass slides (pore size = 25–50 μm, thickness = 3.5 mm, ACE Glass, Vineland, NJ). The assembly was then immersed in a simulated physiological solution (1.15 mmol/L Ca, 1.2 mmol/L P, 133 mmol/L NaCl, 50 mmol/L Hepes, buffered to a pH of 7.4; homemade, reagent grade) [23] stored in a humidor with 100% relative humidity at 37 °C. The use of the porous fritted glass was to allow the nonaqueous liquid–water exchange, thereby causing the CPC to harden. The hardening time was measured by using the Gilmore needle method with a load of 453.5 g and a tip diameter of 1.06 mm [24]. A cement specimen was considered set when the needle loaded onto the specimen surface failed to leave a perceptible indentation. The time measured from the paste being immersed in the physiological solution to this point was used as the setting time. This method was used to test CPC–MCPM, CPC–tartaric, and premixed control.
For CPC–chitosan, the Gilmore needle method was not used because chitosan-containing cement was relatively soft even after setting [19,23]. Therefore, another method was used following previous studies on cements including non-premixed CPC–chitosan with water in the cement liquid [19]. The premixed CPC–chitosan paste was filled into the mold and immersed as described above. When the powder component of the specimen did not come off when scrubbed gently with fingers as described in previous studies [19,25], the setting reaction had occurred enough to hold the specimen together. The time measured from the paste being immersed to this point was used as the setting time for premixed CPC–chitosan [19,25].
For measurement of washout resistance, the premixed CPC paste was manually shaped into a ball and placed into the physiological solution. The material was considered to pass the washout resistance test if the paste ball did not visibly disintegrate in the solution [16,19].
2.3. Conversion to hydroxyapatite
Powder X-ray diffraction (XRD) analysis was used to examine the CPC conversion to hydroxyapatite [23,26]. Specimens of 6 mm diameter and 3 mm thickness were immersed in the physiological solution for 1, 3 and 7 d, and then dried and milled into powder by mortar and pestle. A 4 × 3 full factorial design was thus tested with four materials (CPC–MCPM, CPC–chitosan, CPC–tartaric, and premixed control) and the three immersion times. The XRD patterns were recorded with a powder X-ray diffractometer (Rigaku, Danvers, MA) using graphite-monochromatized copper Kα radiation (λ = 0.154 nm) generated at 40 kV and 40 mA [23]. For the estimation of hydroxyapatite conversion, a series of samples that contained known amounts of hydroxyapatite were prepared using 100% converted CPC and known amounts of unreacted CPC powder (e.g. mass fractions of 25% hydroxyapatite and 75% unreacted CPC powder, etc.). Using the X-ray patterns of these samples, a standard curve that describes the relationship between the mass fractions of hydroxyapatite and the intensities of (0 0 2) peak of hydroxyapatite was constructed. Then for the experimental CPC specimens, the hydroxyapatite conversion was obtained using the standard curve and the measured (0 0 2) peak intensity of the experimental CPC specimen. All data were collected in a continuous scan mode (1° 2θmin−1, step time 0.6 s, step size 0.01°).
2.4. Mechanical properties
Specimens of 6 mm diameter and 3 mm thickness [16] were immersed in the physiological solution for 1, 3 and 7 d. This constituted a 4 × 3 full factorial design with four materials (CPC–MCPM, CPC–chitosan, CPC–tartaric, and premixed control) and three immersion times. Diametral tensile strength was measured on a computer-controlled Universal Testing Machine (model 5500R, Instron, Canton, MA) at a crosshead speed of 1 mm/min [16].
2.5. Premixed CPC– cell interactions
Double-staining of cells Attached on CPCs
Because cell culture toxicity assays are the international standard for biocompatibility screening [27], in vitro cell culture was performed to evaluate the cytotoxicity of the cements. MC3T3-E1 mouse osteoblast cells (Riken, Hirosaka, Japan) were cultured following established protocols [28–31]. Cells were cultured at 37 °C and 100% humidity with 5% CO2 (volume fraction) in α modified Eagle’s minimum essential medium (Cell-culture grade, Biowhittaker, Walkersville, MD). The medium was supplemented with 10% volume fraction of fetal bovine serum (FBS, Cell-culture grade, Gibco, Rockville, MD) and 60 mg/mL kanamycin sulfate (Cell-culture grade, Sigma, St. Louis, MO), and changed twice weekly. The cultures were passaged with 2.5 g/L trypsin containing 1 mmol/L EDTA (Cell-culture grade, Gibco, Rockville, MD) once per week. Cultures of 90% confluent cells were trypsinized, washed and suspended in fresh media.
Five materials were tested: CPC–MCPM, CPC–chitosan, CPC–tartaric, conventional CPC, and tissue culture polystyrene (TCPS) control. The reason for using the conventional CPC (with water as liquid at powder:liquid = 4:1) as a control for the cell culture was because of its known non-cytotoxicity. The conventional CPC is being marketed as BoneSourceTM which has received FDA approval for neurosurgical and maxillo-facial indications [12]. The cement specimens for cell attachment study were bar-shaped with dimensions of 3 mm × 4 mm × 12 mm, similar to those used in a previous study [31]. These specimens were immersed in the physiological solution for 3 d and then sterilized by autoclaving at 121 °C for 20 min [30]. Five specimens of each material were tested (n = 5). Fifty thousand osteoblast cells diluted into 2 mL of media were added to each well containing a specimen or to an empty well of TCPS and incubated for 1 d [29–31]. Cells were then stained and viewed by epifluorescence microscopy (Eclipse TE300, Nikon, Melville, NY). Staining of cells was done for 5 min with 1 mL of cell media (with serum) containing 2 μmol/L calcein-AM and 2 μmol/L ethidium homodimer-1 (Reagent grade, both from Molecular Probes, Eugene, OR). Calcein-AM is a nonfluorescent, cell-permeant fluorescein derivative, which is converted by cellular enzymes into cell-impermeant and highly fluorescent calcein. Calcein accumulates inside live cells having intact membranes causing them to fluoresce green. Ethidium-homodimer-1 enters dead cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to their DNA causing the nuclei of dead cells to fluoresce red. Double-staining cells anchored on the specimens allows simultaneous examination of both live and dead cells [29–31]. To estimate the live cell density, two randomly chosen fields of view were photographed from each specimen using 100 × magnification. Each field of view was photographed through a green filter and red filter to yield 4 pictures from each specimen. Five specimens were examined for each of the five materials above. This yielded a total of 100 images. Each of the images was printed and the cells were counted. The percent of live cells was calculated as the number of live cells/(the number of live cells+the number of dead cells).
Extraction and cell viability
A flask of 80% confluent MC3T3-E1 osteoblast cells was passaged and cells were seeded into 24-well plates with 10,000 cells per well in 2 mL of media. The same five materials described above were tested. Each specimen was immersed in a well with 2 mL of fresh medium (without cells) and extracted overnight in the incubator to accumulate any possible harmful leach-out in the medium. Each specimen had dimensions of approximately 3 mm × 4 mm × 6 mm yielding a volume similar to that of a previous extraction study [32]. Six bars were tested for each material (n = 6). On the second day of the experiment, the medium from each well containing the cells was removed and replaced with the 2 mL of extraction medium from one of the specimens. The cells were incubated in the extracts for 3 d, photographed by using digital photography with an inverted phase contrast microscope (Nikon TE300, Melville, NY), and then prepared for the cell viability assay.
Quantitative cell viability was measured by using the Wst-1 assay which is a colorimetric assay of cellular dehydrogenase activity where absorbance at 450 nm is proportional to the amount of dehydrogenase activity in the cell [29–33]. Wst-1 refers to 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (Reagent grade, Dojindo, Gaithersburg, MD). Cells cultured in the extracts were rinsed with 1 mL of Tyrode’s Hepes buffer. One millilitre of Tyrode’s Hepes buffer (140 mmol/L NaCl, 0.34 mmol/L Na2HPO4, 2.9 mmol/L KCl, 10 mmol/L Hepes, 12 mmol/L NaHCO3, 5 mmol/L glucose, pH 7.4; home-made using reagent grade salts and buffers) and 0.1 mL of Wst-1 solution (5 mmol/L Wst-1 and 0.2 mmol/L 1-methoxy PMS in water) were then added to each well. After a 2 h incubation, a 0.2 mL aliquot from each well was transferred to a 96-well plate and absorbance was measured with a platereader (Wallac 1420 Victor2, PerkinElmer Life Sciences, Gaithersburg, MD). Blank wells that contained Tyrode’s Hepes buffer and Wst-1 solution were also prepared; the blank value was subtracted from each of the experimental values as background [29–32].
2.6. SEM and statistics
A scanning electron microscope (SEM, JEOL 5300, Peabody, MA) was used to examine the specimens. Cells cultured for 1 d on cements were rinsed with saline, fixed with 1% volume fraction of glutaraldehyde, subjected to graded alcohol dehydrations, rinsed with hexamethyldisilazane, and sputter coated with gold.
One standard deviation was used as the estimated standard uncertainty of the measurements. These values should not be compared with data obtained in other laboratories under different conditions. Two- and one-way ANOVA were performed to detect significant effects. Tukey’s multiple comparison was used at a family confidence coefficient of 0.95.
3. Results
Anti-washout and rapid setting
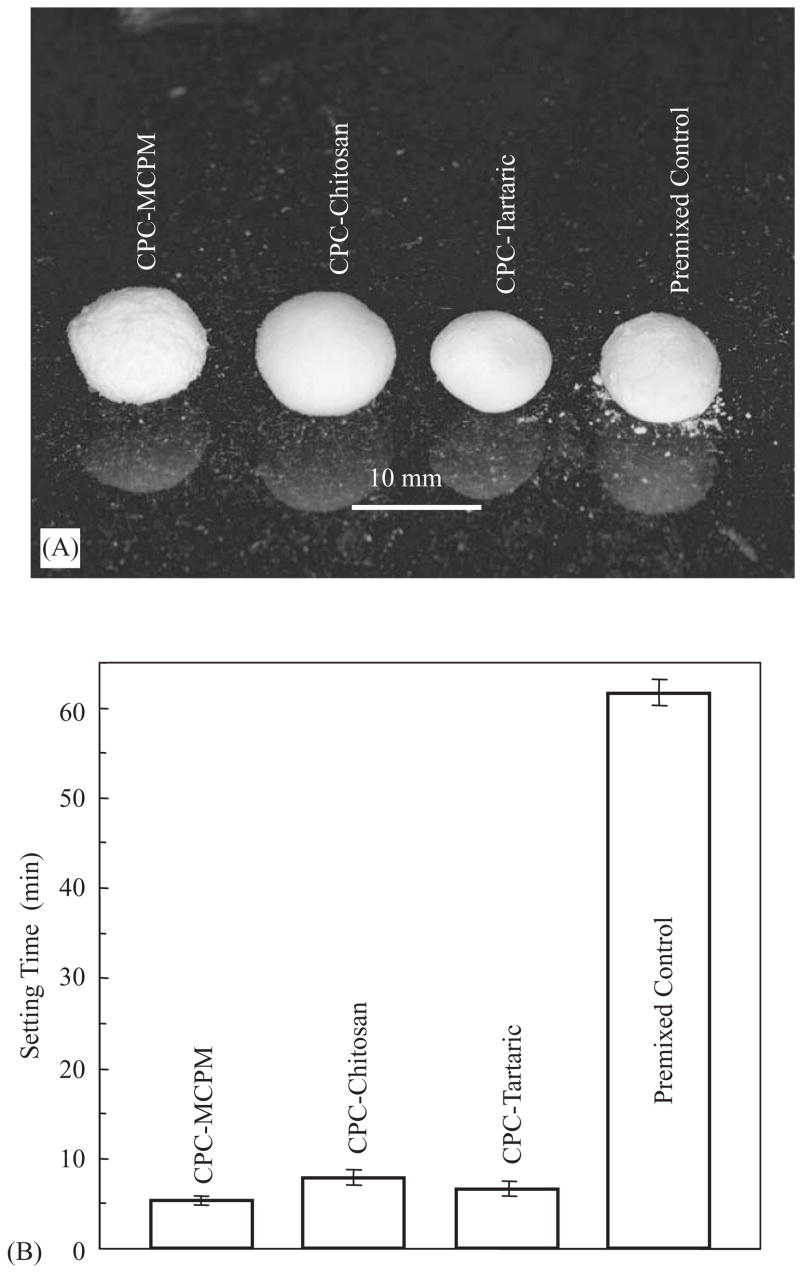
Fig. 1A shows the washout resistance results for the cements. Slight disintegration was observed for premixed control, manifested by the small debris under the paste ball. No noticeable disintegration was observed for the other premixed CPCs. All four premixed CPCs set into hard solid balls. Following previous studies [16,19], the sample was considered to pass the washout resistance test if the paste did not visibly disintegrate. The results here showed that CPC–MCPM, CPC–chitosan and CPC–tartaric were resistant to washout, while premixed control was marginally resistant to washout.
Fig. 1.
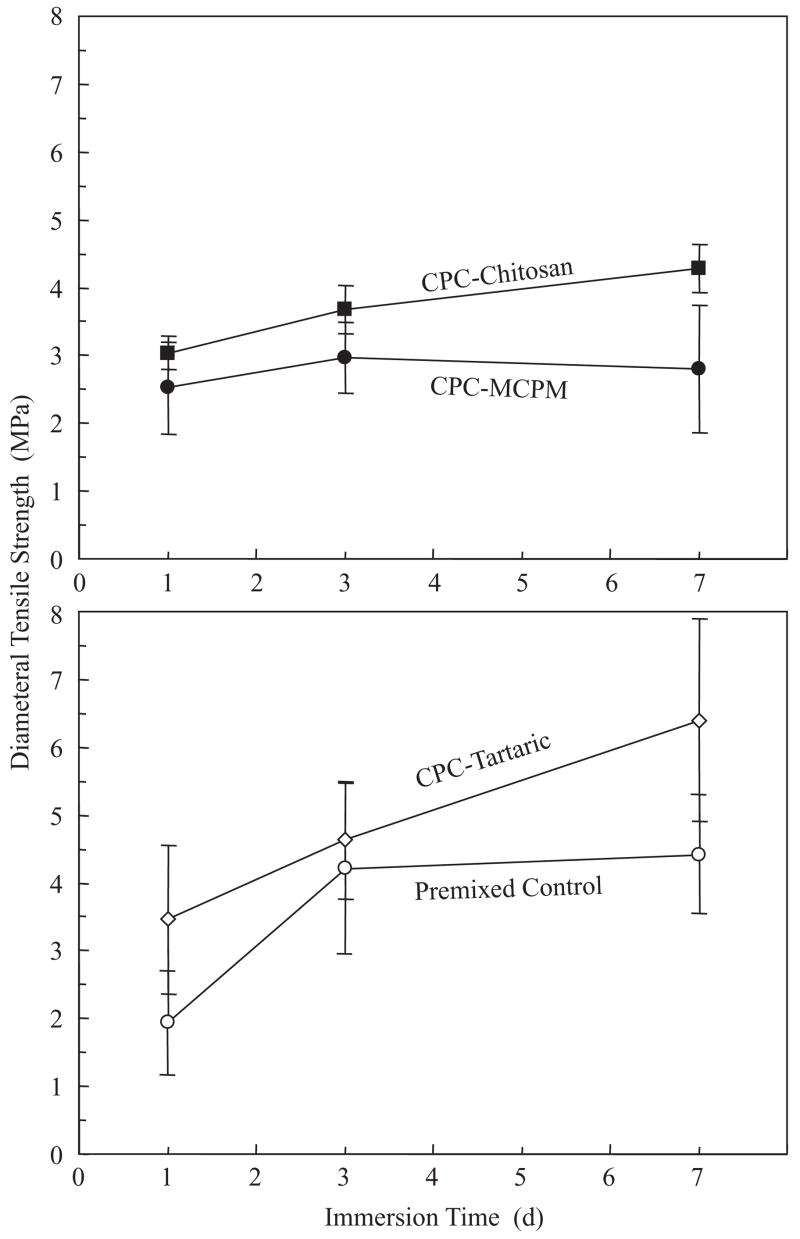
(A) Washout resistance. The picture was taken 10 min after the paste balls were immersed. While slight disintegration was observed for premixed control, no disintegration occurred for the new premixed CPCs. Observations made 30 min and 24 h later were the same as (A), with no further disintegration from premixed control. (B) The times it took for the paste to harden in a physiological solution were much shorter than that for premixed control. Each value is the mean of four measurements with the error bar showing one standard deviation (mean±sd; n = 4).
Fig. 1B plots the setting time results. CPC–MCPM and CPC–tartaric took (5.3±0.5) and (6.5±0.8) min to harden, respectively, not significantly different from each other (Tukey’s at family confidence coefficient of 0.95). These times were significantly shorter than (7.9±0.8) min for CPC–chitosan. All these three pre-mixed CPCs had significantly shorter hardening times than (61.7±1.5) min for the premixed control.
Mechanical properties
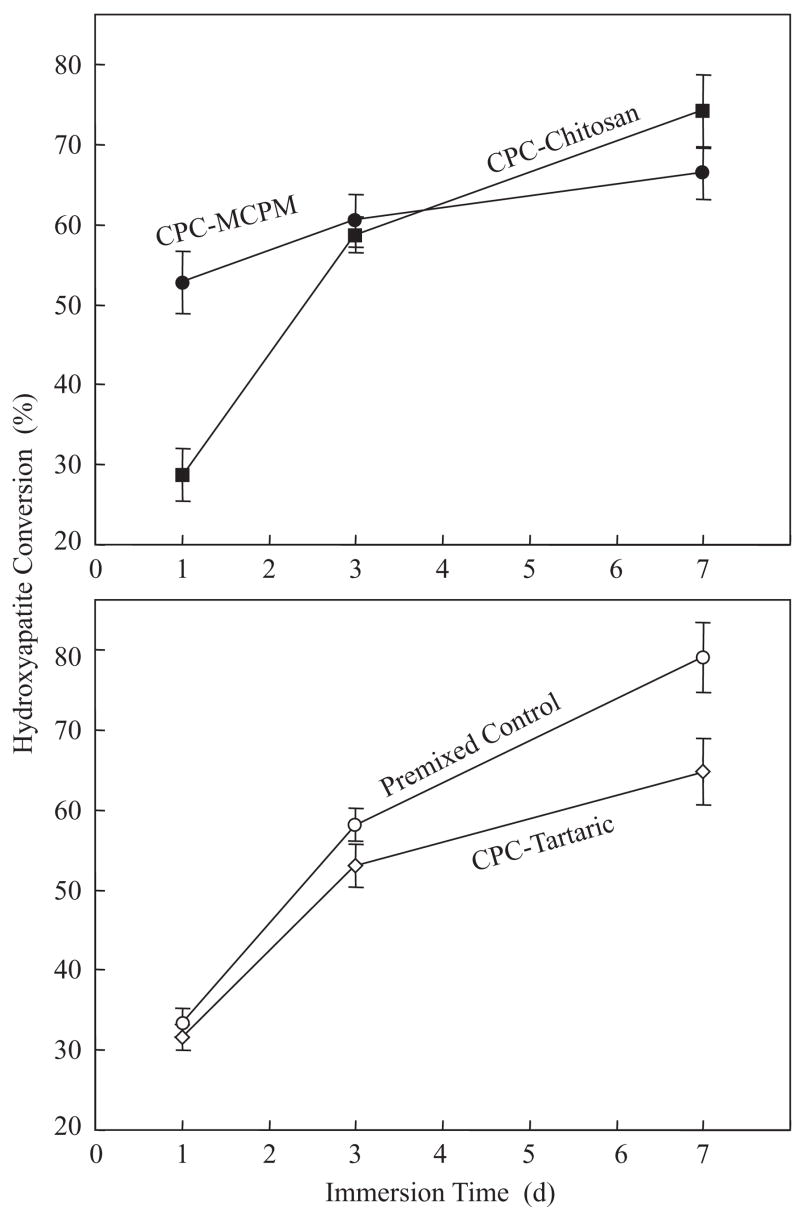
Diametral tensile strength (Fig. 2) of premixed CPC increased significantly (p<0.05) from 1 to 7 d except for CPC–MCPM. The strength of CPC–tartaric at 7 d was (6.4±1.5) MPa, significantly higher than all other materials (p< 0.05). The strengths of CPC–chitosan and premixed control at 7 d were (4.3±0.3) MPa and (4.5±0.8) MPa, respectively; both were higher than (2.8±0.9) MPa of CPC–MCPM (p<0.05).
Fig. 2.
Diametral tensile strength. The four materials were separated into two plots for clarity. Each value was mean±sd; n = 6. The strength of CPC–chitosan, CPC–tartaric and premixed control all increased significantly from 1 to 7 d (p<0.05); that of CPC–MCPM was not significantly changed.
Conversion to hydroxyapatite
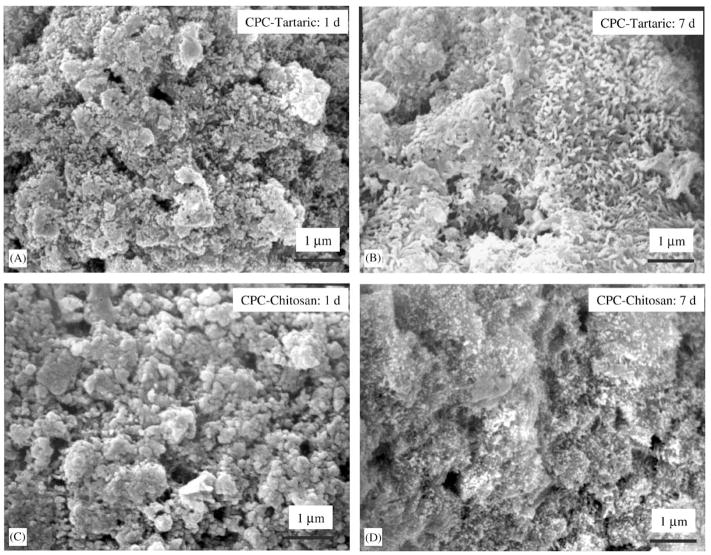
The percentage of conversion to hydroxyapatite is plotted in Fig. 3. At 1 d, CPC–MCPM had a conversion of (52.5±3.6)%, significantly higher than all other materials (p<0.05). At 7 d, CPC–chitosan and premixed control had conversions of (73.6±4.1)% and (78.5±3.5)%, respectively; both were significantly higher than those for CPC–MCPM and CPC–tartaric (p<0.05).
Fig. 3.
Percentage (%) of CPC converted to hydroxyapatite. The conversion increased rapidly from 1 to 7 d for CPC–chitosan, CPC–tartaric and premixed control. CPC–MCPM had a relatively high conversion at 1 d, with a mild increase at 7 d.
SEM micrographs of fracture surfaces of CPC–MCPM are shown in Fig. 4. Relatively small hydroxyapatite crystals were observed after 1 d immersion (A). These needle-like crystals had a length of approximately 0.5–1 μm and a diameter of about 0.1 μm. At 7 d, both a mixture of needle-like crystals and medium-sized platelets (B) and large platelets (C) were observed. The large platelets were about 1–2 μm in width and 0.2 μm in thickness.
Fig. 4.
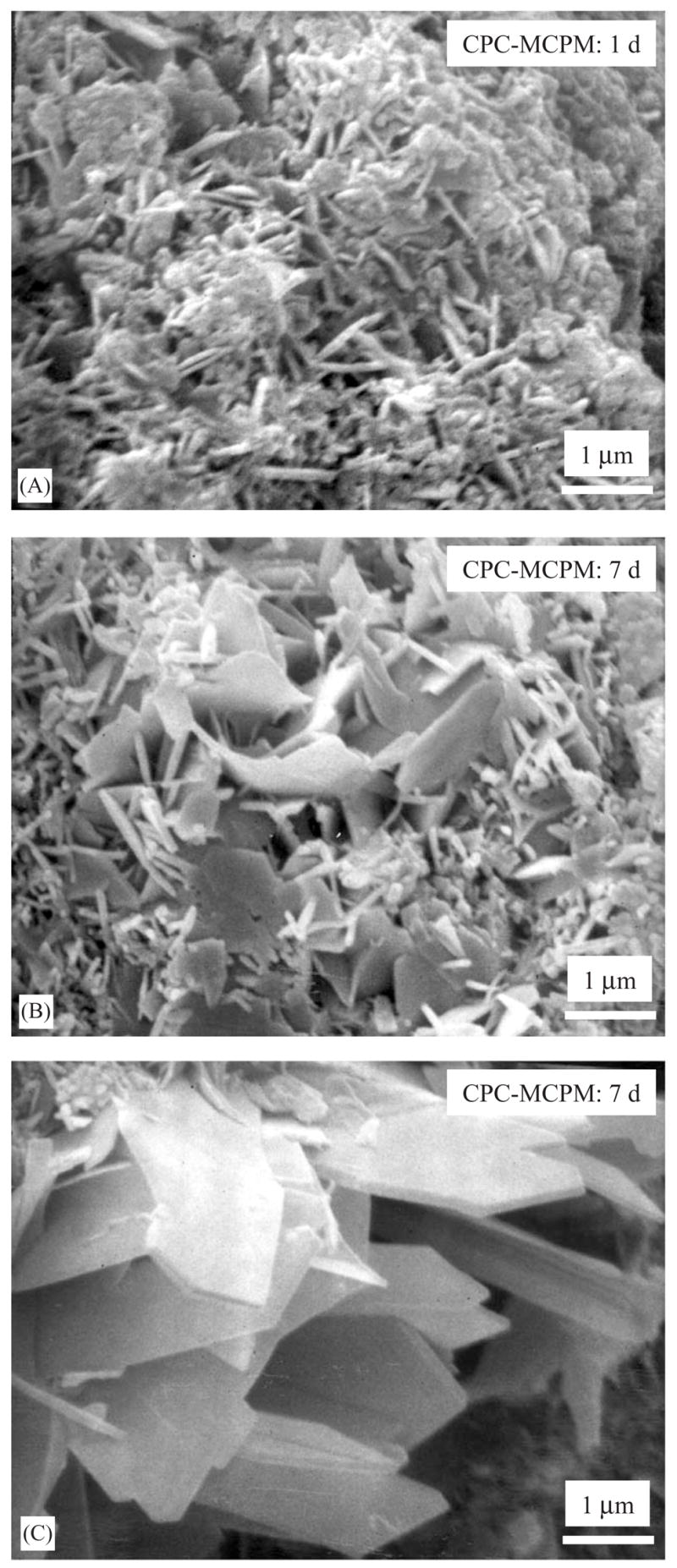
CPC–MCPM specimens were immersed in a physiological solution, then fractured and the fracture surfaces were examined with SEM. (A) After 1 d, small hydroxyapatite crystals of needle shapes were observed with a length of about 0.5–1 mu;m and a diameter of about 0.1 mu;m. After 7 d, a mixture of needle-like crystals and medium-sized platelets were observed in some areas in the fracture surface (B). In other areas of the fracture surface, relatively large platelet crystals were observed (C).
Fig. 5 shows fracture surfaces of (A) CPC–tartaric at 1 d, (B) CPC–tartaric at 7 d, (C) CPC–chitosan at 1 d, and (D) CPC–chitosan at 7 d. Small crystals with sizes of the order of 0.1 μm were observed in the 1 d specimens in (A). The crystals appeared to have grown larger in the 7 d specimens in (B), with diameters of about 0.1 μm and lengths up to 0.3 μm. No crystals were visible for CPC–chitosan at 1 d (C); however, by 7 d, numerous but very small crystals were observed (D).
Fig. 5.
(A) CPC–tartaric after 1 d immersion showing small hydroxyapatite crystals with sizes of the order of 0.1 μm. (B) CPC–tartaric at 7 d showing that the crystals have grown larger with diameters of about 0.1 μm and lengths up to 0.3 μm. (C) CPC–chitosan at 1 d with no noticeable crystals. (D) CPC–chitosan at 7 d showing numerous small hydroxyapatite crystals.
Cell live/dead staining
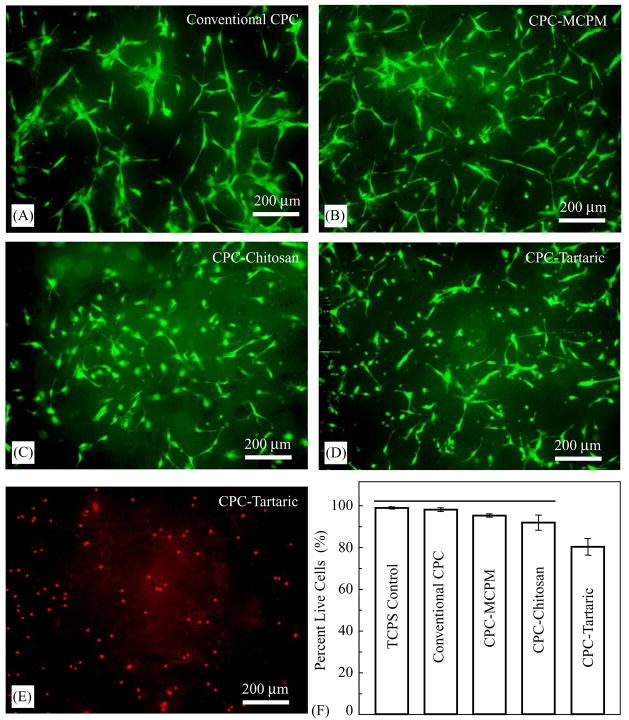
Cells cultured for 1 d are shown in Fig. 6. Live cells, stained green, appeared to have adhered and attained a normal polygonal morphology on the specimens. Dead cells, stained red, are shown in (E) on CPC–tartaric. Dead cells were very few and were similar on TCPS, conventional CPC, CPC–MCPM and CPC–chitosan (not shown in Fig. 6). However, many more dead cells were observed on CPC–tartaric (Fig. 6E). The percent of live cells was plotted in (F). CPC–tartaric had a significantly lower percentage of live cells than all the other materials (p<0.05).
Fig. 6.
Cells cultured for 1 d and double stained with live cells staining green and dead cells staining red. (A) Live cells on conventional non-premixed CPC (a known biocompatible control). (B) CPC–MCPM. (C) CPC–chitosan. (D) CPC–tartaric. Dead cells were very few on conventional CPC, CPC–MCPM, and CPC–chitosan. More dead cells were observed on CPC–tartaric (E). In (F), the percent of live cells was plotted. The tissue culture polystyrene, a biocompatible control, was designated as TCPS. Each value is mean ± sd, n = 5. Horizontal line indicates values that are not significantly different.
Cell attachment to premixed CPCs
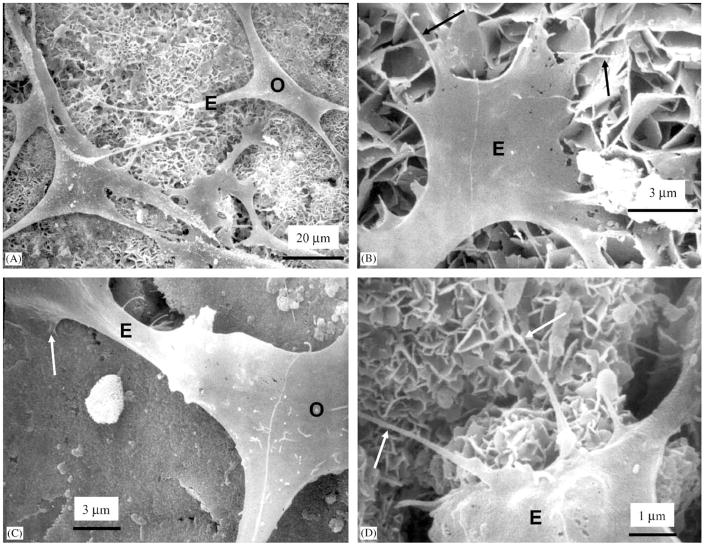
SEM micrograph in Fig. 7A shows osteoblasts (O) cultured for 1 d on CPC–MCPM. The cells had developed cytoplasmic extensions (E) with lengths ranging from about 20 to 50 μm that attached to the specimen surface. These cytoplasmic extensions are regions of the cell plasma membrane that contain a meshwork or bundles of actin-containing microfilaments which permit the movement of the migrating cells along a substratum [34]. Higher magnification in Fig. 7B of the tip of the cytoplasmic extension revealed smaller secondary extensions (arrows) that were attached to the hydroxyapatite crystals in CPC–MCPM. In Fig. 7C, an osteoblast (O) was firmly attached to CPC–chitosan (arrow). At a higher magnification in Fig. 7D for CPC–chitosan, the tip of a cytoplasmic extension had sprouted secondary extensions (arrows) of diameters of about 0.1 mu;m that were attached to the hydroxyapatite crystals.
Fig. 7.
Cells cultured for 1 d on (A–B) CPC–MCPM and (C–D) CPC–chitosan. (A) Cells had adhered and attained a normal polygonal morphology on the specimens and had developed cytoplasmic extensions (E). (B) Higher magnification of the tip of the cytoplasmic extension showed smaller secondary extensions (arrows) that were attached to the hydroxyapatite crystals. (C) Osteoblast (O) was attached to CPC–chitosan (arrow). (D) Higher magnification showed that the tip of a cytoplasmic extension had sprouted secondary extensions (arrows) attaching to hydroxyapatite crystals.
Extraction and cell viability
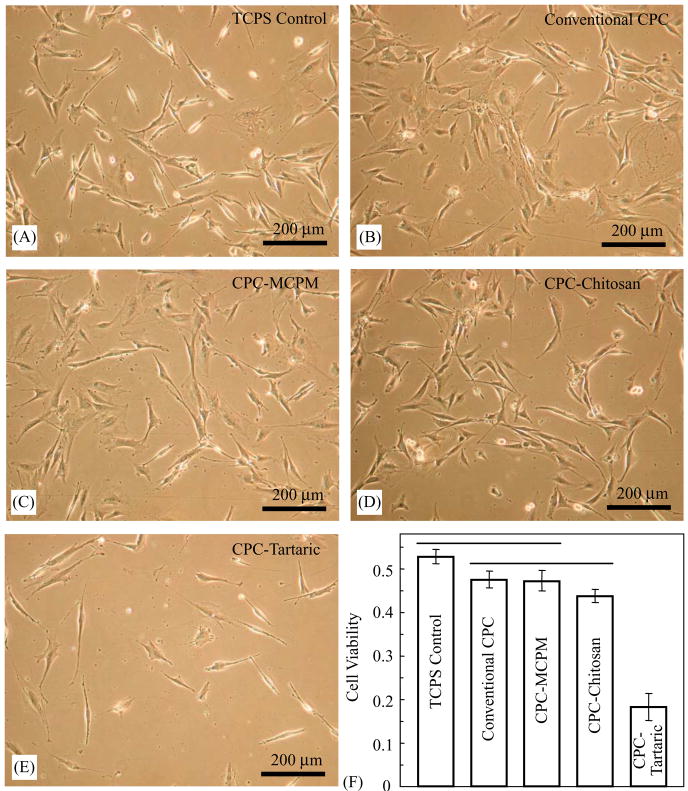
Cells cultured in CPC–MCPM and CPC–chitosan extracts for 3 d displayed a normal, spread and polygonal morphology similar to the controls (Fig. 8). CPC–tartaric had a much lower cell density (Fig. 8E). This was consistent with the quantitative cell viability results in (F), where the Wst-1 assay showed that extract from CPC–tartaric caused a significant drop in cell viability (p<0.05). Cell viability of CPC–chitosan had a mild decrease compared to TCPS. Both CPC–MCPM and CPC–chitosan had statistically similar (p>0.1) cell viability as conventional CPC (a known non-cytotoxic cement).
Fig. 8.
(A–D) Cells cultured for 3 d in CPC–MCPM and CPC–chitosan extracts displayed a normal polygonal morphology similar to TCPS and conventional CPC (known to be non-cytotoxic). (E) CPC–tartaric had a much lower cell density, consistent with the quantitative cell viability results in (F). Each value in (F) is mean ± sd, n = 6. CPC–MCPM and CPC–chitosan had statistically similar (p>0.1) cell viability as conventional CPC.
4. Discussion
Novel premixed CPC compositions were developed to eliminate the need for on-site powder–liquid mixing during surgery and minimize implant performance variations due to insufficient or inhomogeneous mixing. While the premixed control was marginally resistant to washout in a physiological solution, all three new premixed CPCs exhibited excellent washout resistance (Fig. 1). It should be noted that the washout test had no agitation to the solution, while there is fluid flow in vivo. While this test was able to distinguish pastes that showed washout from those pastes that showed no washout [19], future tests should better mimic the in vivo flow situation. The setting time was shortened from more than an hour for the control to 5.3–7.9 min for the new premixed CPCs. Such fast setting should enable the graft to attain significant strength and geometrical integrity within a short period of time postoperatively. When conventional CPC was mixed and implanted subcutaneously in a previous study [6], it failed to set and elicited a severe inflammatory response. Inflammation was correlated with the observation that the conventional CPC disintegrated, likely due to low initial mechanical strength [6]. The rapid setting of the new premixed CPC should help improve the initial mechanical strength, thereby avoid implant disintegration and accompanied inflammation.
After 1 d immersion, the hydroxyapatite conversion was the highest for CPC–MCPM (Fig. 3), lower for CPC–tartaric, and the lowest for CPC–chitosan. This was consistent with SEM observations showing numerous hydroxyapatite crystals in CPC–MCPM (Fig. 4A), small hydroxyapatite crystals in CPC–tartaric (Fig. 5A), and no noticeable crystals in CPC–chitosan (Fig. 5C). This was also consistent with a previous study on non-premixed CPC–chitosan showing that the incorporation of chitosan slowed the conversion to hydroxyapatite [23], likely because the chitosan paste coated the TTCP and DCPA particles thus delaying their reaction with each other. The conversion did eventually occur, as shown by a relatively high hydroxyapatite conversion for CPC–chitosan at 7 d. In addition, the incorporation of chitosan not only imparted washout resistance [19] but also increased the strength for non-premixed CPC–chitosan composites [23,35].
The strength of CPC–tartaric nearly doubled from 1 to 7 d, likely related to its doubling in hydroxyapatite conversion from 1 to 7 d. For CPC–chitosan, the hydroxyapatite conversion increased rapidly from 1 to 7 d, but its accompanying strength increase was moderate. This may be because that its strength contribution was largely from the chitosan matrix, and only to a small extent from the hydroxyapatite formation inside the chitosan matrix. For CPC–MCPM, while the hydroxyapatite conversion increased from 1 to 7 d, its strength was nearly unchanged. This may be because the increase from hydroxyapatite conversion was offset by the formation of large crystals (Fig. 4C). Large crystalline grain sizes were shown to decrease the material’s strength because the intrinsic flaw size also grows with the crystalline grain size [36,37]. The diametral tensile strength ranged from 2.5 to 6 MPa for the new premixed CPCs. Sintered porous hydroxyapatite implants had flexural strength of 2–11 MPa [38]. Cancellous bone had a tensile strength of about 3.5 MPa [39]. While the measurements are not identical and direct comparison cannot be made, these data suggest that the strength of premixed CPCs approached those of sintered porous hydroxyapatite implants and cancellous bone.
The mechanisms via which the new premixed CPCs hardened in a much shorter time than the premixed control can be explained as follows. The premixed control had Na2HPO4 as the hardening accelerator [16]. Its hardening mechanism was the reaction between TTCP and DCPA leading to the formation of hydroxyapatite. TTCP (Ca4(PO4)2O) and DCPA (CaHPO4) dissolved in water as Ca2+, PO43− and OH− ions, which then reprecipitated to form hydroxyapatite, Ca10 (PO4)6(OH)2. The Na2HPO4 crystals dissolved in water and increased the phosphate concentration, which in turn accelerated the setting reaction to form hydroxyapatite. The use of Na2HPO4 did not change the CPC setting mechanism, it only accelerated the reaction. In contrast, for the premixed CPC–chitosan, another faster reaction occurred besides the usual TTCP–DCPA reaction, resulting in faster-setting. In preliminary studies, when chitosan malate was dissolved, the paste became acidic. The addition of Ca(OH)2 increased the pH to above 7, causing the soft CPC–chitosan paste to transform to a hard mass. Hence the initial hardening of CPC–chitosan was caused not by the TTCP–DCPA conversion to hydroxyapatite (which was slower), but by the chitosan hardening due to a pH increase from the dissolution of Ca(OH)2 (which was faster). The usual TTCP–DCPA conversion to hydroxyapatite proceeded in a hardened chitosan matrix.
The hardening mechanism of CPC–tartaric was similar to that of CPC–chitosan: another faster reaction occurred besides the TTCP–DCPA conversion to hydroxyapatite. In preliminary studies, it was found that d-tartaric acid reacted with calcium to form calcium tartrate tetrahydrate (CaO6C4H4 · 4H2O) as the matrix of the cement, resulting in fast hardening [40]. Then, during further immersion, the TTCP–DCPA conversion to hydroxyapatite continued, as verified by the XRD analysis, Fig. 3. The fast setting mechanism of the premixed CPC–MCPM was also somewhat similar: another substance, DCPD (dicalcium phosphate dihydrate, CaHPO4 · 2H2O), was quickly formed besides the TTCP–DCPA conversion to hydroxyapatite. In a previous study [41], MCPM was observed to dissolve quickly in water. Most of the MCPM was converted to DCPD in 2 min, which was the shortest time that the sample could be frozen to stop the reaction for XRD analysis [41]. The dissolution of MCPM was followed by the precipitation of small crystals of DCPD throughout the paste [42]. These small DCPD particles likely served as seeds in CPC and imparted fast setting. This was consistent with an ongoing study using DCPD and TTCP to form hydroxyapatite with water as liquid, showing that the DCPD–TTCP hardening was nearly four times faster than the hardening for DCPA–TTCP without DCPD [43].
The conventional CPC was non-cytotoxic because its individual constituents (Ca4[PO4]2O and CaHPO4) and their reaction product (hydroxyapatite, or calcium-deficient hydroxyapatite if there is trace of TTCP left) were non-cytotoxic. Several compositions of CPC were shown to support cell attachment and proliferation [44–46]. Among the components used in the new premixed CPCs, glycerol is known to be non-cytotoxic and has been used in beverages [18]. HPMC is non-cytotoxic because it is a derivative of cellulose and is one of the commonly occurring polysaccharides. MCPM (Ca(H2PO4)2 · H2O) is comprised of calcium, phosphate and water. Calcium phosphate biomaterials are non-cytotoxic because the main inorganic constituent of bone, hydroxyapatite, is comprised of calcium and phosphate [47,48]. Therefore, from the compositional point of view, it is of no surprise that CPC–MCPM was non-cytotoxic to osteoblast cells in the present study. The same can be said for CPC–chitosan because chitosan and its derivatives are natural biopolymers and are non-cytotoxic [20–22].
CPC–tartaric yielded a low percentage of live cells. The percentage of live cells for CPC–tartaric was only mildly lower (80.2% live cells, Fig. 6F), while its cell viability was substantially lower (34% viability, Fig. 8F), compared to TCPS. This is likely because some of the dead cells on CPC–tartaric may have detached from the specimens and floated away, yielding a higher percentage of live cells. Furthermore, some of the cells may be alive but not healthy, with reduced enzymatic activity and hence a lower cell viability. To examine whether the low cell viability was related to poly(propylene glycol) or d-tartaric acid, CPC–tartaric specimens were immersed in copious water for four weeks to completely leach out its water-miscible poly(-propylene glycol). Cell studies on these specimens yielded nearly the same cell viability as the 3 d specimens. Hence the low cell viability was probably related to the d-tartaric acid in CPC–tartaric. However, both poly(propylene glycol) and tartaric acid have been used in several types of food with no known toxicity [18]. It is possible that in a closed static culture system, the cells may be more sensitive to the implant than in a circulating, dynamic and regenerative system in vivo. Further studies are needed to investigate whether or not CPC–tartaric is biocompatible in animal models. For the other premixed CPCs shown to be rapid-setting and non-cytotoxic, further research should focus on fabricating macroporous scaffolds [49,50] for cell infiltration and bone ingrowth [51] and animal studies [15,52].
5. Conclusions
New premixed CPCs were developed that were capable of rapid-setting, resisted washout, hardened while being immersed in a physiological solution, and formed hydroxyapatite. These premixed CPCs: (1) avoid the powder–liquid mixing in surgery thereby shorten the surgical time; (2) allow the paste to be mixed in advance under well-controlled conditions thus avoid insufficient and inhomogeneous mixing; (3) will not harden in the package or in a syringe, and will harden rapidly in aqueous environment with physiological solution; and (4) eliminate the requirement for the surgeon to mix and finish the placement into the defect within a prescribed time before the paste hardens. In the form of a paste capable of fitting complex cavity shapes without machining as required for sintered hydroxyapatite, the new compositions possessed strength approaching those of cancellous bone and sintered porous hydroxyapatite implants. Both premixed CPC–MCPM and premixed CPC–chitosan were as non-cytotoxic as conventional non-premixed CPC control. The method of ‘‘CPC powder+nonaqueous liquid+gelling agent+hardening accelerator’’ was demonstrated to be successful in developing rapid-setting premixed CPC, and may have applicability to the development of other direct-filling and injectable biomaterials.
Acknowledgments
We thank Drs. F.C. Eichmiller, S.H. Dickens and N.R. Washburn for discussions and A.A. Giuseppetti for experimental assistance. This study was supported by USPHS Grants DE14190 (Xu) and DE11789 (Chow), NIST, and the ADAF.
Footnotes
Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States.
Disclaimer
Certain commercial materials and equipment are identified in this paper to specify experimental procedures. In no instance does such identification imply recommendation by NIST or the ADA Foundation or that the material identified is necessarily the best available for the purpose.
References
- 1.LeGeros RZ, Chohayeb A, Shulman A. Apatitic calcium phosphates: possible restorative materials. J Dent Res. 1982;61:343. [Google Scholar]
- 2.Brown WE, Chow LC. A new calcium phosphate water setting cement. In: Brown PW, editor. Cements research progress. Westerville, OH: American Ceramic Society; 1986. pp. 352–79. [Google Scholar]
- 3.Ginebra MP, Fernandez E, De Maeyer EAP, Verbeeck RMH, Boltong MG, Ginebra J, Driessens FCM, Planell JA. Setting reaction and hardening of an apatite calcium phosphate cement. J Dent Res. 1997;76:905–12. doi: 10.1177/00220345970760041201. [DOI] [PubMed] [Google Scholar]
- 4.Constantz BR, Barr BM, Ison IC, Fulmer MT, Baker J, McKinney L, Goodman SB, Gunasekaren S, Delaney DC, Ross J, Poser RD. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J Biomed Mater Res (Appl Biomater) 1998;43:451–61. doi: 10.1002/(sici)1097-4636(199824)43:4<451::aid-jbm13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Knaack D, Goad MEP, Aiolova M, Rey C, Tofighi A, Chakravarthy P, Lee DD. Resorbable calcium phosphate bone substitute. J Biomed Mater Res (Appl Biomater) 1998;43:399–409. doi: 10.1002/(sici)1097-4636(199824)43:4<399::aid-jbm7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto Y, Ishikawa K, Takechi M, Toh T, Yuasa T, Nagayama M, Suzuki K. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J Biomed Mater Res (Appl Biomater) 1999;48:36–42. doi: 10.1002/(sici)1097-4636(1999)48:1<36::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Barralet JE, Gaunt T, Wright AJ, Gibson IR, Knowles JC. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res (Appl Biomater) 2002;63:1–9. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, Nakasu M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials. 2002;23:1091–101. doi: 10.1016/s0142-9612(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 9.Friedman CD, Costantino PD, Jones K, Chow LC, Pelzer HJ, Sisson GA. Hydroxyapatite cement: II, Obliteration and reconstruction of the cat frontal sinus. Arch Otolaryngol Head Neck Surg. 1991;117:385–9. doi: 10.1001/archotol.1991.01870160039005. [DOI] [PubMed] [Google Scholar]
- 10.Costantino PD, Friedman CD, Jones K, Chow LC, Sisson GA. Experimental hydroxyapatite cement cranioplasty. Plast Reconstr Surg. 1992;90:174–91. [PubMed] [Google Scholar]
- 11.Shindo ML, Contantino PD, Friedman CD, Chow LC. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119:185–90. doi: 10.1001/archotol.1993.01880140069012. [DOI] [PubMed] [Google Scholar]
- 12.Friedman CD, Costantino PD, Takagi S, Chow LC. Bone-SourceTM hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res (Appl Biomater) 1998;43:428–32. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Chow LC. Calcium phosphate cements: chemistry, properties, and applications. Mater Res Symp Proc. 2000;599:27–37. [Google Scholar]
- 14.Apelt D, Theiss F, El-Warrak AO, Zlinszky K, Bettschart-Wolfisberger R, Bohner M, Matter S, Auer JA, von Rechenberg B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials. 2004;25:1439–51. doi: 10.1016/j.biomaterials.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 15.Takagi S, Chow LC, Markovic M, Friedman CD, Costantino PD. Morphological and phase characterizations of retrieved calcium phosphate cement implants. J Biomed Mater Res (Appl Biomater) 2001;58:36–41. doi: 10.1002/1097-4636(2001)58:1<36::aid-jbm50>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Takagi S, Chow LC, Hirayama S, Sugawara A. Premixed calcium-phosphate cement pastes. J Biomed Mater Res (Appl Biomater) 2003;67B:689–96. doi: 10.1002/jbm.b.10065. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa K, Miyamoto Y, Takechi M, Toh T, Kon M, Nagayama M, Asaoka K. Non-decay type fast-setting calcium phosphate cement: hydroxyapatite putty containing an increased amount of sodium alginate. J Biomed Mater Res. 1997;36:393–9. doi: 10.1002/(sici)1097-4636(19970905)36:3<393::aid-jbm14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Winter R. A consumer’s dictionary of food additives. New York: Crown Publishers; 1978. p. 123.p. 192.p. 229. [Google Scholar]
- 19.Xu HHK, Takagi S, Quinn JB, Chow LC. Fast-setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. J Biomed Mater Res. 2004;68A:725–34. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 20.Machida Y, Nagai T, Abe M, Sannan T. Use of chitosan and hydroxypropyl chitosan in drug formulations to effect sustained release. Drug Dis Deliv. 1986;1:119–30. [PubMed] [Google Scholar]
- 21.Muzzarelli RAA, Biagini G, Bellardini M, Simonelli L, Castaldini C, Fraatto G. Osteoconduction exerted by methylpyrolidinone chitosan in dental surgery. Biomaterials. 1993;14:39–43. doi: 10.1016/0142-9612(93)90073-b. [DOI] [PubMed] [Google Scholar]
- 22.Muzzarelli RAA. Amphoteric derivatives of chitosan and their biological significance. In: Skjak-Brak G, Anthonsen T, Sandford P, editors. Chitin and Chitosan. New York: Elsevier Applied Science; 1989. pp. 87–99. [Google Scholar]
- 23.Xu HHK, Quinn JB, Takagi S, Chow LC. Processing and properties of strong and non-rigid calcium phosphate cement. J Dent Res. 2002;81:219–24. [PubMed] [Google Scholar]
- 24.ADA Specification No. 9 for Dental Silicate Cement. Guide to dental materials and devices. 7. American Dental Association: 19741975. pp. 194–202. [Google Scholar]
- 25.Cherng A, Takagi S, Chow LC. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J Biomed Mater Res. 1997;35:273–7. doi: 10.1002/(sici)1097-4636(19970605)35:3<273::aid-jbm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Fukase Y, Eanes ED, Takagi S, Chow LC, Brown WE. Setting reactions and compressive strengths of calcium phosphate cement. J Dent Res. 1990;69:1852–6. doi: 10.1177/00220345900690121201. [DOI] [PubMed] [Google Scholar]
- 27.International Standards Organization. ISO 10993-5. Biological evaluation of medical devices—Part 5: tests for in vitro cytotoxicity. Geneva, Switzerland: International Standards Organization; 1999. [Google Scholar]
- 28.Attawia MA, Uhrich KE, Botchwey E, Langer R, Laurencin CT. In vitro bone biocompatibility of poly(anhydride-co-imides) containing pyromellitylimidoalanine. J Orthopaed Res. 1996;14:445–54. doi: 10.1002/jor.1100140315. [DOI] [PubMed] [Google Scholar]
- 29.Simon CG, Jr, Khatri CA, Wight SA, Wang FW. Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly(lactide-co-glycolide) microspheres. J Orthop Res. 2002;20:473–82. doi: 10.1016/S0736-0266(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 30.Xu HHK, Smith DT, Simon CG., Jr Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials. 2004;25:4615–26. doi: 10.1016/j.biomaterials.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 31.Xu HHK, Simon CG., Jr Self-hardening calcium phosphate composite scaffold for bone tissue engineering. J Orthopaedic Res. 2004;22:535–43. doi: 10.1016/j.orthres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Simon CG, Jr, Antonucci JM, Skrtic D. In vitro cytotoxicity of amorphous calcium phosphate composites. J Bioactive Compatible Polym. doi: 10.1177/0883911506064476. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P-G. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull. 1993;41:1118–22. [Google Scholar]
- 34.Darnell J, Lodish H, Baltimore D. Molecular cell biology. 2. New York: Freeman and Company; 1990. pp. 890–1. [Google Scholar]
- 35.Xu HHK, Quinn JB, Takagi S, Chow LC. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials. 2004;25:1029–37. doi: 10.1016/s0142-9612(03)00608-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu HHK, Wei L, Padture NP, Lawn BR, Yeckley RL. Effect of microstructural coarsening on Hertzian contact damage in silicon nitride. J Mater Sci. 1995;30:869–78. [Google Scholar]
- 37.Xu HHK, Jahanmir S. Effect of microstructure on damage tolerance in grinding dental glass-ceramics. J Mater Res. 1998;13:2231–6. [Google Scholar]
- 38.Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13:94–117. [Google Scholar]
- 39.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 40.Chow LC, Takagi S, Liao L. Rapid-hardening pre-mixed calcium phosphate cement pastes (abstract # 844) J Dent Res (special issue A) 2003:82. [Google Scholar]
- 41.Chow LC, Takagi S, Ishikawa K. Formation of hydroxyapatite in cement systems. In: Brown PW, Constantz B, editors. Hydroxyapatite and related materials. Boca Raton, FL: CRC Press; 1994. pp. 127–37. [Google Scholar]
- 42.Mirtchi AA, Lemaître J, Munting E. Calcium phosphate cements: action of setting regulators on the properties of the β–tricalcium phosphate-monocalcium phosphate cements. Biomaterials. 1989;10:634–8. doi: 10.1016/0142-9612(89)90120-8. [DOI] [PubMed] [Google Scholar]
- 43.Burguera E, Xu HHK, Takagi S, Chow LC. High strength hydroxyapatite cement based on dicalcium phosphate dehydrate for bone repair. J Biomed Mater Res. 2004;71A:272–82. [Google Scholar]
- 44.Ehara A, Ogata K, Imazato S, Ebisu S, Nakano T, Umakoshi Y. Effects of α–TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization. Biomaterials. 2003;24:831–6. doi: 10.1016/s0142-9612(02)00411-8. [DOI] [PubMed] [Google Scholar]
- 45.Yuasa T, Miyamoto Y, Ishikawa K, Takechi M, Momota Y, Tatehara S, Nagayama M. Effects of apatite cements on proliferation and differentiation of human osteoblasts in vitro. Biomaterials. 2004;25:1159–66. doi: 10.1016/j.biomaterials.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Knabe C, Berger G, Gildenhaar R, Meyer J, Howlett CR, Markovic B, Zreiqat H. Effect of rapidly resorbable calcium phosphates and a calcium phosphate bone cement on the expression of bone-related genes and proteins in vitro. J Biomed Mater Res. 2004;69A:145–54. doi: 10.1002/jbm.a.20131. [DOI] [PubMed] [Google Scholar]
- 47.LeGeros RZ, LeGeros JP. Dense hydroxyapatite. In: Hench LL, Wilson J, editors. An introduction to bioceramics. New Jersey: World Scientific; 1993. pp. 139–80. [Google Scholar]
- 48.Hing KA, Best SM, Bonfield W. Characterization of porous hydroxyapatite. J Mater Sci: Mater Med. 1999;10:135–45. doi: 10.1023/a:1008929305897. [DOI] [PubMed] [Google Scholar]
- 49.Xu HHK, Quinn JB, Takagi S, Chow LC, Eichmiller FC. Strong and macroporous calcium phosphate cement: effects of porosity and fiber reinforcement on mechanical properties. J Biomed Mater Res. 2001;57:457–66. doi: 10.1002/1097-4636(20011205)57:3<457::aid-jbm1189>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 50.Xu HHK, Quinn JB. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials. 2002;23:193–202. doi: 10.1016/s0142-9612(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 51.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50:50–8. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara A, Fujikawa K, Kusama K, Nishiyama M, Murai S, Takagi S, Chow LC. Histopathologic reaction of a calcium phosphate cement for alveolar ridge augmentation. J Biomed Mater Res. 2002;61:47–52. doi: 10.1002/jbm.10010. [DOI] [PubMed] [Google Scholar]