Abstract
The hippocampus is especially sensitive to the effects of gestational and neonatal iron deficiency, even after iron repletion. This study compared the effects of iron deficiency, maintained from gestational day 2 to postnatal day (P)7, on “delay” and “trace” fear conditioning. Only the latter paradigm is critically dependent on the dorsal hippocampus. In different groups of rats, fear conditioning commenced either prior to puberty (P28 or P35) or after puberty (P56). Fear conditioning was measured using fear-potentiated startle. Both delay and trace fear conditioning were diminished by iron deficiency at P28 and P35. Hippocampal expression of the plasticity-related protein PKC-gamma was increased through trace fear conditioning, but reduced at P35 in the iron deficient group. Trace fear conditioning was enhanced by prior iron deficiency in the P56 group. This unanticipated finding in iron-repleted adults is consistent with the effects of developmental iron deficiency on inhibitory avoidance learning, but contrasts with the persistent deleterious long-term effects of a more severe iron deficiency protocol, suggesting that degree and duration of iron deficiency affects the possibility of recovery from its deleterious effects.
Keywords: PKC, startle, trace conditioning, hippocampus, development
1. Introduction
Neonatal iron deficiency commonly results from premature birth or from maternal conditions during gestation, including iron deficiency, hypertension, or diabetes mellitus. Untreated, it may result in short-term (Siddappa et al., 2004) and long-term (DeBoer et al., 2005) cognitive deficits, as well as concomitant behavioral, attentional, and emotional problems (Lozoff et al., 2000; Wachs et al., 2005).
Work on rodent models indicates that perinatal iron deficiency produces anatomical, physiological, and molecular changes in the brain that are both regionally and biochemically specific (Rao et al., 2003). For example, cytochrome c oxidase activity, an iron-dependent marker of energy metabolism, is unchanged in some regions and reduced by >40% in others (de Deungria et al., 2000). One of the most severely affected brain regions is the hippocampus, a structure vital for memory and for neuroendocrine and emotional responses to stress. In contrast, the amygdala, another limbic structure critically involved in memory and emotion, is less vulnerable to the energy-depleting effects of early iron deficiency. This does not necessarily imply that amygdala function is entirely spared, because the amygdala is richly innervated through the monoaminergic system, which is itself dysregulated by iron deficiency (Beard and Connor, 2003). Nevertheless, the relative contributions of the hippocampus and amygdala to changes in emotional regulation and memory following iron deficiency have only recently begun to be elucidated (McEchron et al., 2005).
The current study investigated the effects of gestational and early neonatal iron deficiency in the rat on amygdala- and hippocampus-dependent memory processes by comparing the behavioral outcomes of delay and trace fear conditioning procedures. These two Pavlovian conditioning paradigms differ only in whether presentations of the conditioned stimulus (CS; e.g., tone) and unconditioned stimulus (US; e.g., footshock) overlap (delay conditioning) or are separated by a brief interval (trace conditioning). By virtue of the insertion of this brief interval trace, but not delay, fear conditioning is dependent on the dorsal portion of the hippocampus (Burman et al., 2006; Chowdhury et al., 2005; Trivedi and Coover, 2006). In contrast, the amygdala plays a central role in Pavlovian fear conditioning more generally (Davis, 2006; Fanselow and Gale, 2003; LeDoux, 2003). In view of the relatively severe effects of iron deficiency on hippocampal structure and function during development, we predicted that trace fear conditioning would be especially vulnerable to disruption following gestational iron deprivation.
One of the reasons that fear conditioning lends itself well to the study of memory processes in juvenile rats is that fear can be acquired rapidly and can be measured reliably at a relatively early age (Barnet and Hunt, 2005; Moye and Rudy, 1987). In the current study, three sessions of trace and delay fear conditioning commenced in different groups of perinatally iron-sufficient and iron-deficient rats at postnatal day (P)28, P35, and P56. The dams were fed an iron-depleted diet from the second day of gestation until P7. Following this protocol, brain weight and iron concentration in the offspring are still reduced by 20% on P28 (Rao et al., 2003), but are fully recovered by P65 (Jorgenson et al., 2003). Interestingly, however, detrimental effects on biochemical markers, dendritic structure, electrophysiology, and gene regulation in the hippocampus persist even after complete iron repletion (i.e., after 56 days postnatal age) (Carlson et al., 2007; Jorgenson et al., 2003; Jorgenson et al., 2005). Thus, comparing the strength of fear conditioning that commenced at P28 and P35, or P56 allowed us to examine whether learning and memory, like hippocampus structure and physiology, are affected by prenatal and perinatal iron deficiency, even after the iron status of the brain has undergone a full recovery.
Consistent with this possibility, McEchron et al. (2005) have reported that rats exposed to a severely iron deficient diet prenatally and in early life were impaired in trace fear conditioning trained on P60. In that study, however, an iron deficient diet was initiated only 10 days prior to birth and maintained throughout postnatal brain development (until P31), exposing the brain to severe iron deficiency in a different developmental time period. Stead et al., (2006) recently demonstrated that predominantly proliferative genes are expressed until P7 and primarily differentiative genes at and after P14 in hippocampus, cortex and hypothalamus (Stead et al., 2006). Thus, it remains to be seen whether earlier induction and cessation of iron deficiency produces similarly enduring effects in associative learning tasks.
In both conditioning paradigms, and at all three ages, fear was measured as the increase in magnitude of the startle reflex seen when startle was elicited acoustically in the presence of a CS previously paired with shock. Among the advantages of “fear-potentiated startle” as a measure of fear is the fact that the startle reflex is elicited in both the presence and absence of a fear-inducing CS. This means that any effects of dietary treatment on fear would affect startle only in the presence of the CS and thus could be differentiated from a nonspecific, motor or sensory deficit, which would affect startle indiscriminately in both the presence and absence of fear-inducing stimulation. This within-subject control is particularly important in light of evidence that iron deficiency can reduce myelination and axonal conduction speed and can produce long-term deficits in motor function (Algarin et al., 2003; Angulo-Kinzler et al., 2002).
In addition to the behavioral measures, we also quantified hippocampal expression of the gamma isoform of the serine/threonine kinase Protein Kinase C (PKC). Through its role in phosphorylation of the transcription factor cyclic AMP-Response Element Binding Protein (CREB) (Roberson et al., 1999), PKC contributes to the maintenance and expression of long-term potentiation (LTP), a widely accepted cellular substrate for learning and memory. In the immature animal, hippocampally dependent spatial learning results in an increase in protein expression of PKC isoform gamma in CA1 pyramidal neurons, particularly in the dendritic field of the stratum radiatum (Wu and Wang, 2002). This potentiation of PKC activity can be interrupted during development pharmacologically (Wu and Wang, 2002) or by neonatal hypoxic-ischemic insult (Braaksma et al., 1999). Thus, we expected that trace fear conditioning and iron deficiency would have opposite effects on expression of the PKC-gamma signal to CREB in CA1, increasing and decreasing it respectively.
2. Results
2.1. Pup survival
The pups of one iron-deficient (ID) dam were all stillborn. A second gave birth to only 3 pups, which were euthanized. Hence 17 iron-sufficient (IS) and 18 ID dams remained for further analyses. There were no significant differences in the number of male and female pups born to ID and IS dams, Median (range) male-female ratios = 1.1 (4.5) and 1.6 (5.1), respectively, Mann-Whitney U, p>.1. ID dams gave birth to fewer live pups than IS dams, Medians (ranges) = 11 (6) and 12 (5), respectively, a difference that was statistically significant, Mann-Whitney U, p < .05. Additionally, there were significantly more deaths in ID litters compared to IS litters prior to weaning, Medians (ranges) = 3 (3) and 0 (0), respectively, Mann-Whitney U, p<.05. ID and IS litters did not differ with respect to the day by which all pups’ eyes were open, Medians (ranges) = 15.0 (1.6) and 14.9 (1.6), respectively, Mann-Whitney U, p >.1.
2.2. Body weight
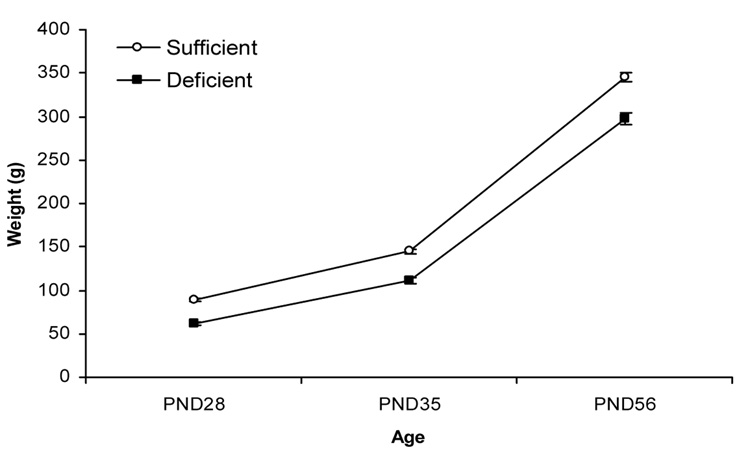
Both ID and IS subjects showed growth curves, but ID animals were lighter than IS animals at all three ages (see Table 1). This was confirmed by a 2 (diet) × 3 (age) ANOVA, which indicated significant effects of diet and age, F(1,99) = 122.5, p < .001, F(2,99) = 2088.3, p < .001, respectively. The diet × age interaction was also significant, F(2,99) = 834, p < .05. In proportional terms, the severity of the deficit in body weight in ID animals diminished as a function of age. Thus, mean weights of ID groups was 31.1%, 23.0%, and 13.7% below that of their IS peers at P28, P35, and P56 respectively.
Table 1.
Birth and Pre-Weaning Statistics: Median (Range)
| Sufficient | Deficient | |
|---|---|---|
| # Live Births | 12.00 (5.00) | 11.00 (6.00)* |
| M:F Ratio | 1.60 (5.12) | 1.09 (4.50) |
| Pre-weaning Deaths | 0.00 (0.00) | 0.00 (3.00)* |
| Day of Eye Opening | 14.88 (1.63) | 15.00 (1.63) |
p<.05 compared with sufficient rats (Mann-Whitney U test)
2.3. Iron status
The model induced significant iron deficiency at P7 as indexed by a 47% reduction in hematocrit and a 62% reduction in brain iron concentration. The deficiencies had completely resolved by P56 (see Table 2).
Table 2.
Iron status at P7 and P56
| Hematocrit (%) | Brain Iron (mcg/g dry weight) | |||||
|---|---|---|---|---|---|---|
| Sufficient | Deficient | p-value | Sufficient | Deficient | p-value | |
| P7 | 32 ± 3 | 17 ± 1 | <0.001 | 50.5 ± 6.3 | 19.3 ± 3.4 | <0.001 |
| P56 | 46 ± 3 | 47 ± 3 | 0.72 | 40.8 ± 5.3 | 38.1 ± 2.8 | 0.48 |
Values are means ± SD
2.4. Baseline startle responding
All behavioral measures were tested using a between-subjects design, with diet (ID and IS) and age at the start of fear conditioning training (P28, P35, and P56) as the independent variables. Startle responding on the second startle pretest session increased as a function of age, but not of diet (see Table 3). These observations were confirmed by statistical analyses. A 2 (diet) × 2 (P28, P35) ANOVA revealed a main effect of age, F(1,66) = 6.0, p < .05, but no significant main effect of diet or diet × age interaction, ps > .1. ID and IS animals also did not differ in baseline startle levels at P56, p > .1.
Table 3.
Baseline Startle (mean ± SEM arbitrary units)
| Sufficient | Deficient | |
|---|---|---|
| P28 | 15.86 ± 1.32 | 12.91 ± 0.59 |
| P35 | 18.31 ± 4.68 | 17.07 ± 3.16 |
| P56 | 27.63 ± 2.80 | 32.34 ± 3.97 |
2.5. Shock reactivity
Reactivity to footshock was lower in ID than in IS rats, and in older than in younger rats, with particularly high reactivity in the oldest (P56) iron-sufficient animals (see Table 4). A 2 (diet) × 2 (age) analysis of variance (ANOVA) on P28 and P35 rats revealed a main effect of diet F(1,66) = 9.0, p < .01, but no significant main effect of diet, or diet × age interaction, ps > .1. In contrast, formerly ID rats showed significantly lower shock reactivity at P56, t(33) = 3.2, p < .01.
Table 4.
Shock Reactivity (mean ± SEM arbitrary units)
| Sufficient | Deficient | |
|---|---|---|
| P28 | 30.90 ± 5.10 | 25.19 ± 1.54 |
| P35 | 42.62 ± 4.68 | 39.01 ± 3.16 |
| P56 | 148.97 ± 21.20 | 72.61 ± 7.82 |
2.6. Fear conditioning
Delay fear conditioning was poorer in ID animals than in IS animals across all three ages, but particularly at the younger two ages. Consistent with these observations, a 2 (diet) × 3 (age) ANOVA indicated a significant main effect of diet, F(1,45) = 3.94, p < .05, but no effect of age or diet × age interaction, ps > .1.
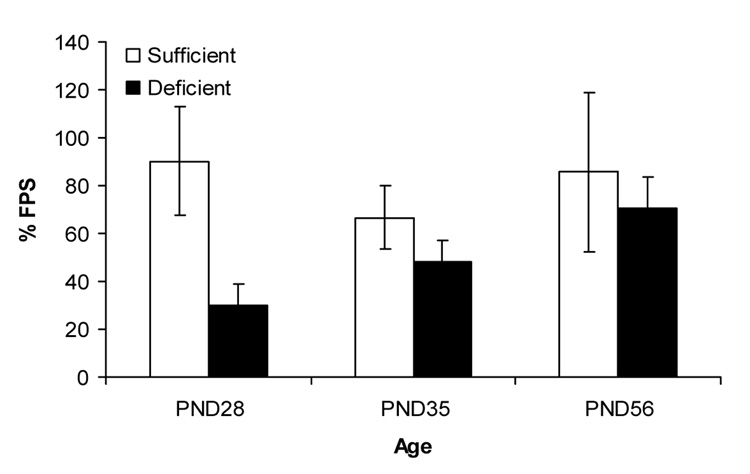
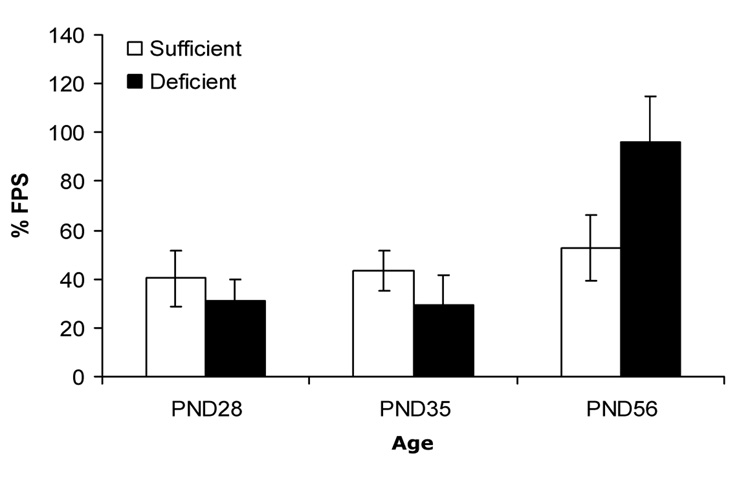
In animals given trace fear conditioning training, the pattern of differences between IS and ID animals across the three ages was quite different. Fear-potentiated startle was somewhat poorer in the iron-deficient P28 and P35 groups than in the age-matched iron-sufficient groups. However, fear-potentiated startle was substantially greater in the ID animals than in IS animals when training began at P56. These observations were confirmed by a 2 (diet) × 3 (age) ANOVA, which revealed a significant main effect of age, F(2,48) = 6.01, p < .005, and diet × age interaction F(2,48) = 3.15, p < 0.05. The main effect of diet was not significant, p >.1.
2.7. PKC-gamma expression at P35
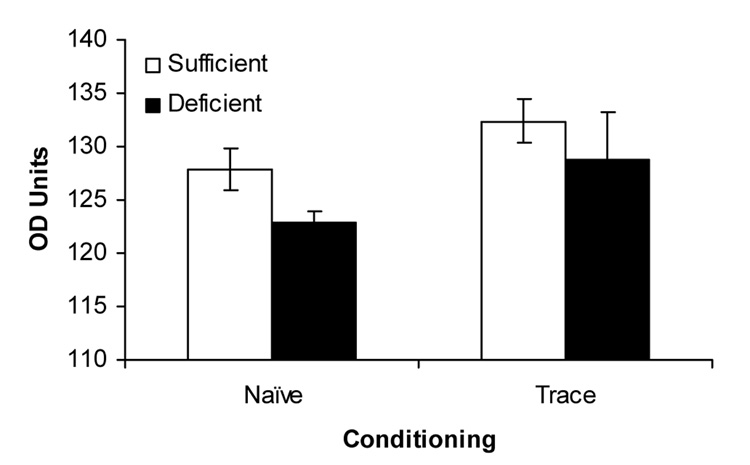
As shown in the representative photomicrographs (Figure 4), the primary antibody caused staining of PKC-gamma-containing neurons in CA1 of the hippocampus. PKC-gamma expression in hippocampal area CA-1 at baseline in naïve animals was greater in the IS animals than in the ID animals, F(1,16) = 12.14, p < 0.005; Figure 5). Trained animals from the trace paradigm demonstrated greater PKC-gamma expression than naïve animals, F(1,16) = 4.69, p < .05). There was no significant diet by condition interaction. There were no differences in CA-1 PKC-gamma expression between ID and IS animals in the delay group (data not shown; p > .1).
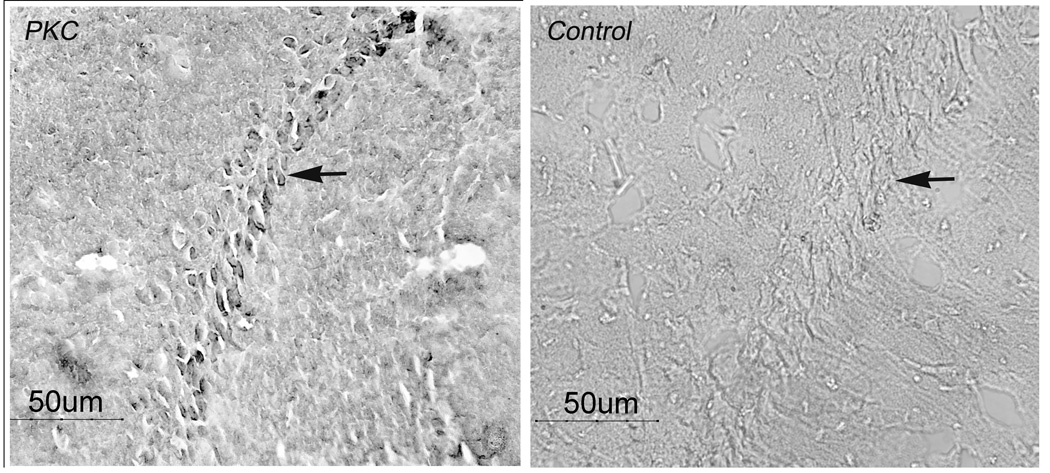
Figure 4.
Positive PKC staining (arrow, left panel) in pyramidal cells of hippocampal area CA1 and absence of staining in antibody control (arrow, right panel).
Figure 5.
Hippocampal area CA1 PKC-gamma activity at P35 in naïve and trace-conditioned IS and ID groups. In rats not previously exposed to fear conditioning, levels of PKC-gamma activity were higher in IS than in ID rats. Across diet manipulations, rats trained for trace fear conditioning displayed a higher level of PKS-gamma activity than naïve rats.
3. Discussion
The hippocampus, a structure critical for many forms of learning and memory, is particularly sensitive to the effects of prenatal and neonatal iron deficiency, and some of these effects persist long after brain iron levels have normalized (Jorgenson et al., 2003; Jorgenson et al., 2005). We hypothesized that prenatal/neonatal iron deficiency would affect rats’ subsequent ability to acquire trace fear conditioning, a form of learning that requires the dorsal hippocampus, while sparing delay fear conditioning, a form of learning that does not depend on the dorsal hippocampus. Unexpectedly, however, both delay and trace fear conditioning were impaired in preadolescent (P28 and P35) rats. These deficits did not extend into adulthood, by which time iron repletion was complete. In fact, at P56, the offspring of iron-deficient mothers actually showed enhanced trace fear conditioning compared to control rats. Thus, prenatal/neonatal iron deficiency produced a general impairment in fear conditioning in preadolescent rats, but a specific potentiation of dorsal hippocampus-dependent fear conditioning in early adulthood.
There are at least two plausible explanations for the attenuation of delay and trace fear conditioning seen at P28 and P35. The first is that the impairment was related to a disruption of amygdala function. At first blush this may appear unlikely, because amygdala metabolism is only mildly disturbed, at least at P10, by the same diet employed here (de Deungria et al., 2000). However, it is worth noting that fear conditioning and memory consolidation are impaired by disruption of dopaminergic (Nader and LeDoux, 1999) and noradrenergic neurotransmission (Hatfield and McGaugh, 1999) respectively in the amygdala. Hence, even in the absence of direct metabolic disturbances in the amygdala, fear conditioning in this study may have been affected in this study via the well-documented effects of iron deficiency on the brains’ catecholamine systems (Burhans et al., 2005).
The second possibility is that the impairment in both delay and trace conditioning was attributable to a loss of function in the ventral hippocampus. The rodent hippocampus can be divided into three rostrocaudal neuroanatomical segments that are segregated in terms of their inputs, outputs, and interconnectivity. Most investigations of the hippocampus’s role in fear conditioning, including trace fear conditioning, have focused on its septal (i.e., dorsal) portion, (Quinn et al., 2002; Quinn et al., 2008). However, it is the temporal (i.e., ventral) pole of the hippocampus that is most closely associated with the amygdala, and recent evidence indicates that damage to this area impacts fear conditioning in general (Bast et al., 2001; Kjelstrup et al., 2002; Richmond et al., 1999; Trivedi and Coover, 2004), including delay fear conditioning trained and tested using parameters similar to those of the current study (Burman et al., 2006). Thus, fear conditioning is unlikely ever to occur entirely independently of the hippocampus, but rather independently of its septal pole. Because there is no reason to assume that iron deficiency differentially affects portions of the hippocampus along its rostrocaudal axis, it is quite plausible that delay fear conditioning is disrupted due to the effects of iron deficiency on ventral hippocampus function.
Although the involvement of the amygdala cannot be ruled out, the pattern of expression of PKC-gamma protein observed post-mortem in the P35 trace-conditioned animals is at least consistent with the possibility of hippocampal involvement in the observed fear conditioning deficits. Thus, expression of this plasticity-related protein was higher in trace-conditioned than in naïve, age-matched controls, but was reduced in previously iron deficient animals. While this study assessed PKC staining in the hippocampus because of the targeted nature of the behavior being tested, it is likely that PKC would be affected in other areas of the brain as well. To resolve the issue of the relative impact of iron deficiency on hippocampus-dependent versus amygdala-dependent memory, further research is required to establish whether these alterations in protein expression are specific to trace fear conditioning, and whether they occur in the amygdala, as well as in the hippocampus.
The recovery of fear conditioning seen in adult animals is congruent with the trajectory of brain iron and hematocrit content, which had reached normal levels by P56. Our behavioral data might suggest, therefore, that the impairments in learning seen at earlier time points were related to current iron status rather than to the long-term sequelae of metabolic dysfunction occurring during periods of gestation and infancy. In contrast, McEchron et al. (2005) (McEchron et al., 2005) found that late gestational and early-life iron deficiency impaired trace fear conditioning trained as late as P60, at which time point hematocrit levels were normal. Despite a number of differences between the two studies, their contrasting outcomes are most likely attributable to differences in iron deficiency regimens. Whereas the rats in the current study were returned to an iron-rich diet starting on P7, a low-iron diet was maintained in the earlier study until P31. This extension of iron deficiency throughout the juvenile preadolescent period may have been crucial in inducing long-term changes in proteins closely associated with differentiation and plasticity (Carlson et al., 2007), and in dendritogenesis, which is maximal between P15 and P25 (Pokorny and Yamamoto, 1981). In strikingly parallel findings, striatum-dependent learning is similarly spared in adult rats exposed to iron deficiency until P7 (Schmidt et al., 2007), but not in adult rats exposed to iron deficiency throughout the period of late brain development. Taken together, the behavioral data derived from these disparate learning paradigms are consistent in suggesting that the cognitive deficits produced by prenatal iron deficiency may be recoverable through later iron supplementation, whereas the deficits induced through postnatal, extra-uterine iron deficiency are more liable to be irreversible.
An alternative explanation of the divergent effects of iron deficiency on trace fear in this and the McEchron et al (2005) study is that a putative deficit in trace fear conditioning was mitigated by our adoption of more extensive fear conditioning procedures. Indeed, such a pattern has been reported in spatial learning (Schmidt et al., 2007), where deficits in formerly ID rats were manifested only in the earliest phase of training. However, this interpretation could only account for an absence of differences in the relative strengths of delay and trace conditioning, but not for the frank enhancement of trace fear conditioning in previously ID adults that was observed in the current study.
The most striking aspect of the current findings was, perhaps, that trace fear conditioning was increased in formerly iron-deficient adult rats. Iron deficiency has a profound effect on brain, and specifically, hippocampal metabolism as evidenced by decreased cytochrome c oxidase (de Deungria et al., 2000) and altered intracellular phosphocreatine:creatine ratio (Rao et al., 2003) during the period of early iron deficiency. Others have postulated the induction of a relative hypothyroid state by iron deficiency (Beard et al., 1998) that would also slow metabolic rate during development. This downregulation of metabolism during late fetal and early neonatal life may not only influence ongoing morphologic development and function of the brain while iron deficient but also induce long-term genomic and metabolic changes in a manner similar to how gestational protein-energy restriction that results in intrauterine growth restriction or choline administration during pregnancy modifies metabolism setpoints in the offspring for life (Gluckman and Hanson, 2004; Meck and Williams, 2003). These changes likely involve epigenetic mechanism of methylation and histone modification. While the effect of iron deficiency on methylation and acetylation of genes has not been specifically studied, there is circumstantial evidence that those processes are affected. For example, Carlson et al report in another article in this special issue that iron deficiency upregulates APP, Htatip, and appb1 expression in the hippocampus. The protein products of these genes combine to form a complex that translocates to the nucleus, inducing acetyl transferase activity (Cao and Sudhof, 2001).
Iron is required to support highly metabolic activities such as dendritogenesis (Jorgenson et al., 2003) and synaptic plasticity (Carlson et al., 2007). The genes that regulate these processes are active in late gestation and early postnatal life and include those in the mTOR (mammalian target of rapamycin) pathway and vamp1 suggesting that they may be most malleable at that time (Carlson et al., 2007). They are responsive to gestational iron status and their regulatory setpoints may be set during this time period, metabolically adapting to the low iron environment (Meck and Williams, 2003). While there are other potential reasons for the enhanced performance of the formerly iron deficient animals at P56 in the current study, it is possible that iron treatment with standard amounts of iron actually represents a state of supplementation to a system trained in early life to function (albeit suboptimally) when far less iron is available. The result of provision of this critical metabolic substrate could be to enhance efficient energy production and thus synaptic plasticity to experience.
Interestingly, the unanticipated finding of stronger trace fear conditioning is actually consistent with an earlier literature showing an enhancement of inhibitory avoidance learning in previously iron deficient rats (Findlay et al., 1981; Weinberg et al., 1979; Weinberg et al., 1980). Long-term memory of inhibitory avoidance learning, like trace fear conditioning, is dependent on both the dorsal and ventral hippocampus (Martinez et al., 2002). Thus, collectively, these studies may suggest that early life iron deficiency produces a long-term enhancement in the expression of dorsal hippocampus-dependent fear.
4. Experimental Procedure
4.1. Animals
Thirty-seven timed-pregnant albino, Charles River-derived, Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually on a 12-hour light-dark cycle. Litters were culled to 8 on P3, retaining as many male pups as possible. One hundred and five male rat pups were studied in total. Animals were weighed on day 3 at culling and assessed for eye opening from P14–P17. Rats were otherwise left undisturbed except for routine cage changes until weaning at P21. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications No. 8023, revised 1996) and the Guidelines for the Humane Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee at the University of Minnesota.
4.2. Dietary conditions
Twenty dams were assigned to the ID condition (3–6 mg elemental iron/kg; formula TD 80396, Harlan-Teklad) and 17 dams were assigned to the IS condition (198 mg elemental iron/kg). From gestational day 2 though P7, dams in the ID group were fed the low-iron diet, while dams in the IS group received the standard iron-supplemented diet. Aside from diet, there were no differences in the treatment of IS and ID litters. Both ID and IS animals were given ad libitum access to water and their respective diets. Following P7, all pups received the standard iron-supplemented chow. After weaning on P21, pups continued to be group-housed with free access to water and the iron-sufficient diet.
4.3. Apparatus and Stimuli
Animals were tested in four identical 7.5 × 9 × 17-cm stabilimeter devices, each located within a ventilated, sound-attenuated chamber. Each stabilimeter consisted of a Plexiglas cage, which rested on a platform supported by four compression springs. To maximize the sensitivity of the equipment for animals differing widely in body mass, different platforms containing springs with a lower or higher spring constants were used to test smaller rats (P28 and P35 groups) and larger rats (P56 group) respectively. Cage movement resulted in displacement of a Type 338B35 accelerometer (PCB Piezotronics, Depew, NY) attached to the top of each cage. The resultant voltage of the accelerometer was proportional to the velocity of the cage displacement. This signal was amplified by a signal-processing unit (# 482820 PCB Piezotronics). An InstruNet 100b board (GW Instruments, Somerville, MA) interfaced to a Macintosh G3 microcomputer digitized the voltage output of the accelerometer on a scale of 0 – 1000 units. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 200 ms after onset of the startle stimulus. High frequency speakers (Radio Shack Supertweeters, range 5–40 kHz, #40–1310b) located 5 cm behind each cage delivered the startle stimuli. The startle stimuli were 50-ms (rise-decay: 5 ms) bursts of white noise (low pass, 22 kHz) at 95 and 105 dB. The ventilation fans of the sound-attenuating chamber elevated background noise to 60–65 dB. The foot shock was a 0.8-mA constant current scrambled shock, delivered by a shock generator (#SGS-004, by BRS-LVE, Bellsville, ME) through the four bars that made up the bottom of the stabilimeter. Shock intensity was measured with a 1-kΩ resistor across a differential channel of an oscilloscope in series with a 100-kΩ resistor connected between two floor bars in each cage. Current was defined as the RMS voltage across the resistor where mA = 0.707 × 0.5 × peak-to-peak voltage. Shock reactivity was defined as the peak accelerometer voltage that occurred during the first 500 ms after onset of the shock. The CS was a 4-s (trace conditioning) or a 7.5-s (delay conditioning), band pass-filtered noise, raised to a sound pressure level 5 dB above background noise (65–70 dB), with high and low cut-offs set at 4 kHz and 24 dB per octave attenuation. The noise was generated by the computer and delivered through a low frequency speaker (Radio Shack woofer, Model # 40–1024A) situated 15 cm from the cage. Previous research demonstrated no unconditioned effects of the CS on the startle response (Burman and Gewirtz, 2004).
4.4. Startle Habituation Sessions
In order to acclimate the rats to the apparatus and startle stimuli, and to measure levels of baseline startle, the naïve rats underwent two days of startle testing prior to training. After a five-minute acclimation period, they were presented with 40 startle stimuli, seven at each of two intensities (95 and 105 dB). The various stimulus intensities were presented in a semi-random order with a 30-s interstimulus interval. The two startle habituation sessions were conducted on the two days prior to the start of training, i.e., starting on P26, 33, and 54 for the P28, 35, and 56 groups respectively. At this point, IS and ID animals were assigned to delay or trace conditioning groups with similar levels of baseline startle. To ensure equivalent genetic heterogeneity between the groups, assignment was conducted with the constraint that no more than one animal from each IS or ID litter was assigned to the same (i.e., P28/P35/P56 × delay/trace) group.
4.5. Fear Conditioning Procedure
After baseline startle sessions, acquisition was conducted over three successive days, starting the day after the second startle habituation session. Thus, training commenced in different groups of IS and ID animals on P28, P35, and P56. On each day, rats were presented with 16 tone-shock pairings after a five-minute acclimation period. The interval between shocks was variable with a mean of 2.75 min (range: 2.0 – 3.5 min). Because the CS-US interval is the critical feature for fear expression in both trace and delay conditioning (Burman and Gewirtz, 2004), the current experiments compared trace conditioning to delay conditioning matched in terms of the CS-US onset interval rather than CS duration. Thus for delay conditioning, the 0.5-s shock overlapped and co-terminated with the 7.5-s CS whereas for trace conditioning, the onset of the shock occurred 3 s after offset of the 4-s CS. Although fear conditioning studies have most commonly used 20–30- s trace intervals (e.g., Huerta et al., 2000; McEchron et al., 1998; Quinn et al., 2002) some studies have employed briefer trace intervals (Crestani et al., 2002; Misane et al., 2005; Trivedi and Coover, 2006). A 3-s interval was chosen for this study to ensure robust trace fear-potentiated startle (Burman and Gewirtz, 2004) and to reduce the influence of context conditioning (Marlin, 1981).
4.6. Testing procedure
A similar testing procedure has been described previously (Burman and Gewirtz, 2004). The test session occurred 24 hours after completion of acquisition. Testing consisted of a five-minute acclimation period, followed by 30 startle stimuli (15 at each of 95 and 105 dB), delivered in an intermixed sequence, to provide a measure of baseline startle magnitude. This sequence was followed immediately by presentation of the test trials. The startle stimuli were presented either alone (startle-alone trials), 3.5 s after CS onset, or 7 s after CS onset. There were five of each of the two CS-containing test trials and ten of the startle-alone test trials, presented in a pseudorandom order. The interval between successive startle stimuli was 30 s throughout the session. After testing, the rats were overdosed with sodium pentobarbital and perfused intracardically with saline followed by 5% sucrose-fomalin solution, for subsequent immunohistochemical procedures (data not shown).
4.7. Biochemical Analysis
Separate cohorts of 4 rats raised on identical dietary protocols as the behavioral subjects were deeply anesthetized with sodium pentobarbital administered intraperitoneally. Iron status was assessed on P7 as this represented the end of the ID diet phase and thus constituted maximal iron deficiency and on P56. Blood for hematocrit was collected by cardiac puncture. The tubes were spun at 3,000×g for 20 min and measured with a standard hematocrit reader. The animals were trans-cardially perfused with phosphate buffered saline (PBS, pH 7.4) until the effluent was clear. Following decapitation, the brains were removed and frozen at −80°. Whole brain iron concentrations were assayed by atomic absorption spectroscopy as previously described (Rao et al., 1999). Values were expressed as µmol of elemental iron/g wet tissue weight.
4.8. Histochemical Analysis
As in previous studies (de Deungria et al., 2000; Jorgenson et al., 2003; Siddappa et al., 2002), 5 brains at P35 from the trace-conditioned and a set of never-trained naïve animals were excised and post-fixed with sucrose-formalin solution at 4°C for 24 hours. Brains were cryoprotected by serial sucrose incubations (20%, 30%, 40%) and embedded in tissue medium. Immunostaining density of PKC-gamma was assessed in coronal sections (12 and 20 µm thick) with di-aminobenzidine tetrahydrochloride after application of the primary rabbit anti-rat phosphorylated PKC-gamma antibody (Santa Cruz Biotechnology, Santa Cruz CA) at a 1:100 dilution and application of a universal secondary antibody (ABC kit, Vector Corp, Burlingame, CA). For immunohistological controls, the primary antibody was omitted and the tissue sections were incubated with the primary antibody diluent consisting of Tris Buffered Saline (pH 7.4) containing 10 % horse serum and 0.2% Triton X-100 overnight at 4 °C. The images were captured by digital camera at a consistent light intensity and stored on a Dell Dimension 8200 computer in Adobe Photoshop (version 6) and assessed using ACT-1 software (version 2.20; Nikon, Inc. Melville, NY). The anatomic regions of interest were identified as in previous studies (Jorgenson et al., 2003; Siddappa et al., 2003) and were assessed from their most anterior to their most posterior aspect in the P35 brain. CA1 was sampled in a minimum of 3 and a maximum of 5 planes and each brain was sampled at least in the most anterior and most posterior aspects. The hippocampus was assessed from 0.0 to 2.6 mm anterior to the inter-auricular line with CA1 assessed from its medial border to 2 mm laterally. Quantitation of immunoreactivity in hippocampal field CA1 used unbiased stereology as described before (Jorgenson et al., 2003; Siddappa et al., 2003). The mean optical density (OD) for each section was assessed in Photoshop, which assigned values ranging from 0 (white) to 254 (black). A histogram for each section was generated both prior to capture to ensure that values did not top out at pure black and after capture for quantitation. After setting a threshold (114) for background and non-specific staining, the total number of pixels with staining between 114 and 254 was recorded in an Excel spreadsheet. The mode OD was recorded for each section, averaged across animal, and expressed as a single value for the animal.
4.9. Statistics
Statistics were performed using SPSS. Several parameters associated with the health and survival of ID and IS litters (i.e., number of live births, male/female ratio, pre-weaning deaths, and days to eyes open) in the behavioral experiment were compared using Mann-Whitney U tests. Body weights, measured on the day on which behavioral training commenced (i.e., P28, P35, or P56), were analyzed by analysis of variance (ANOVA), using a 2 × 3 (diet × age) between-subjects design. Because a different measurement device was used for the P28 and P35 groups versus the P56 group (see above), comparisons of shock reactivity (a measure of nociception) and baseline startle could not be made across the different age groups. Thus, IS and ID groups were compared at P28 and P35 using a 2 × 2 (diet × age) ANOVA. At P56, a t-test was used to compare the IS and ID groups. Percent fear-potentiated startle was calculated by comparing mean startle obtained on each CS trial type to mean startle obtained on startle-alone trials [i.e., (CS-startle – startle alone)/startle alone × 100%]. Fear-potentiated startle data from delay and trace conditioning groups were analyzed separately using 2 × 3 (diet × age) between-subjects design ANOVAs. The data could be compared across the three ages, despite large differences in baseline startle and in the device used to measure it, because the percent measure of fear-potentiated startle used here is insensitive to large variations in baseline startle (Walker & Davis, 2000). Hematocrits and brain iron concentrations were compared between groups across time by repeated-measures ANOVA. A two-way ANOVA was used to assess the effect of diet (ID vs IS) and condition (trace vs naïve) on PKC-gamma staining density.
Figure 1.
Perinatal iron deficiency resulted in reduced body weights (g; mean ± SEM) across all ages of behavioral testing.
Figure 2.
Delay fear conditioning (% fear-potentiated startle) was not affected by age of the rats, but was reduced by ID across all three ages (mean ± SEM).
Figure 3.
Trace fear conditioning (% fear-potentiated startle) increased with age. There was an interaction between age of behavioral testing and diet: formerly ID rats ID displayed greater trace fear conditioning at P56 than IS rats (mean ± SEM).
Acknowledgments
This work was supported by the National Institutes of Health (HD-29421; DA-07097.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53:217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002;70:85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learn Behav. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Beard JL, Brigham DE, Kelley SK, Green MH. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J Nutr. 1998;128:1401–1408. doi: 10.1093/jn/128.8.1401. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Braaksma MA, Douma BR, Nyakas C, Luiten PG, Aarnoudse JG. Delayed neuronal migration of protein kinase Cgamma immunoreactive cells in hippocampal CA1 area after 48 h of moderate hypoxemia in the near term ovine fetus. Brain Res Dev Brain Res. 1999;114:253–260. doi: 10.1016/s0165-3806(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Timing of fear expression in trace and delay conditioning measured by fear-potentiated startle in rats. Learn Mem. 2004;11:205–212. doi: 10.1101/lm.66004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol. 2005;47:525–531. doi: 10.1017/s0012162205001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Findlay E, Ng KT, Reid RL, Armstrong SM. The effect of iron deficiency during development on passive avoidance learning in the adult rat. Physiol Behav. 1981;27:1089–1096. doi: 10.1016/0031-9384(81)90375-9. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Marlin NA. Contextual associations in trace conditioning. Anim Learn Behav. 1981;9:519–523. [Google Scholar]
- Martinez I, Quirarte GL, Diaz-Cintra S, Quiroz C, Prado-Alcala RA. Effects of lesions of hippocampal fields CA1 and CA3 on acquisition of inhibitory avoidance. Neuropsychobiology. 2002;46:97–103. doi: 10.1159/000065419. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev Psychobiol. 1987;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res Bull. 1981;7:121–130. doi: 10.1016/0361-9230(81)90076-9. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008 doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR, Georgieff MK. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53:800–807. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006;26:345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Neurotoxic lesions of the dorsal and ventral hippocampus impair acquisition and expression of trace-conditioned fear-potentiated startle in rats. Behav Brain Res. 2006;168:289–298. doi: 10.1016/j.bbr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Levine S, Dallman PR. Long-term consequences of early iron deficiency in the rat. Pharmacol Biochem Behav. 1979;11:631–638. doi: 10.1016/0091-3057(79)90254-5. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Dallman PR, Levine S. Iron deficiency during early development in the rat: behavioral and physiological consequences. Pharmacol Biochem Behav. 1980;12:493–502. doi: 10.1016/0091-3057(80)90179-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang L. The effects of antiepileptic drugs on spatial learning and hippocampal protein kinase C gamma in immature rats. Brain Dev. 2002;24:82–87. doi: 10.1016/s0387-7604(02)00012-8. [DOI] [PubMed] [Google Scholar]