Summary
Convergent evidence suggests that serotonin 5-HT1A receptor (5-HT1AR) agonists reduce L-DOPA-induced dyskinesia by auto-regulating aberrant release of L-DOPA-derived dopamine (DA) from raphestriatal neurons. However, recent findings indicate that 5-HT1AR stimulation also modifies D1 receptor (D1R)-mediated dyskinesia and rotations implicating a previously unexplored extra-raphe mechanism. In order to characterize the contribution of the striatum to these effects, rats with medial forebrain bundle DA lesions were tested for abnormal involuntary movements (AIMs) and rotations following striatal microinfusions of the 5-HT1AR agonist ±8-OH-DPAT and systemic D1R agonist treatment with SKF81297. Additional rats with multi-site striatal DA lesions were tested for motor disability following systemic or intrastriatal ±8-OH-DPAT with or without systemic SKF81297. In rats with medial forebrain bundle lesions, striatal infusions of ±8-OH-DPAT dose-dependently reduced AIMs while conversely increasing rotations. In rats with striatal lesions, ±8-OH-DPAT alone, both systemic and intrastriatal administration, optimally reversed motor disability. Collectively, these results support an important functional interaction between 5-HT1AR and D1R in the striatum with implications for the improved treatment of Parkinson’s disease.
Keywords: 5-HT1A receptor, D1 receptor, Dopamine, Dyskinesia, L-DOPA, Parkinson’s disease
Introduction
Chronic dopamine (DA) replacement therapy with L-3,4-dihydroxyphenylalanine (L-DOPA) for Parkinson’s disease (PD) patients often results in abnormal and excessive movements known as L-DOPA-induced dyskinesia (LID; Jankovic, 2005). Evidence suggests that LID is in large part due to aberrant, extra-physiological stimulation of supersensitive striatal D1 (D1R) and D2 (D2R) receptors following DA depletion (Pavese et al., 2006; Cenci, 2007; Westin et al., 2007). Striatal D1R appear to be particularly important given their expression and signaling are markedly increased in dyskinetic animals and humans (Cenci et al., 1998; Gerfen et al., 2002; Aubert et al., 2005; Guigoni et al. 2007), and D1R agonists stimulate dyskinesia in both experimental and clinical populations (Rascol et al., 2001; Delfino et al., 2007; Dupre et al., 2007).
Following DA depletion, serotonergic neurons of the raphe nuclei are thought to actively convert exogenously administered L-DOPA into DA and release it into the striatum in an unregulated fashion (Tanaka et al., 1999; Maeda et al., 2003; Carta et al., 2007). The ability of serotonin 1A receptor (5-HT1AR) agonists to reduce LID in animal models (Lundblad et al., 2005; Tomiyama et al., 2005; Ba et al., 2006; Dekundy et al., 2007; Eskow et al., 2007) and human PD patients (Kannari et al., 2002; Bara-Jimenez et al., 2005) has been predominantly attributed to stimulation of 5-HT1A inhibitory autoreceptors within the raphe nuclei, which may normalize the amount of DA delivered to the striatum (Bibbiani et al., 2001; Carta et al., 2007; Eskow et al., 2007). However, several studies suggest that extra-raphe mechanisms also play a role in diminishing LID, including populations of 5-HT1AR within forebrain regions like the motor cortex and striatum (Santiago et al., 1998; Mignon & Wolf, 2005). For example, striatal 5-HT1AR binding increases following chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treatment in primates (Frechilla et al., 2001), and 5-HT1AR agonists can enhance rotational activity (Matsubara et al., 2006) and alleviate dyskinesia (Iravani et al., 2006) induced by direct DA receptor agonists. Our lab recently demonstrated that systemic 5-HT1AR agonist administration reduced dyskinesia induced by D1R stimulation while dramatically enhancing contralateral rotations in DA-depleted rats (Dupre et al., 2007), implicating a more direct interaction between 5-HT1AR and D1R within the striatum.
The aim of the current study, therefore, was to determine the effects of striatal 5-HT1AR stimulation on D1R-mediated behaviors in hemiparkinsonian rats. Using microinfusion techniques, the full 5-HT1AR agonist (±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide (±8-OH-DPAT) was administered into the striatum prior to systemic injection of the D1R agonist SKF81297. Dyskinesia was measured using the abnormal involuntary movements (AIMs) scale (Lundblad et al., 2002) and contralateral rotations were tallied. Motor performance was rated in a separate group of animals using the forepaw adjusting steps (FAS) test (Chang et al., 1999). The present results indicate that striatal 5-HT1AR stimulation reduces D1R-mediated dyskinesia while enhancing rotations, and improves motor performance in DA-depleted rats. These novel findings confirm an important interaction between striatal 5-HT1AR and D1R with significant implications for the treatment of PD and LID.
Methods
Animals
Adult male Sprague-Dawley rats were used (225–250 g upon arrival; Taconic Farms, Hudson, NY, USA). Rats were kept in plastic cages (22 cm high, 45 cm deep and 23 cm wide) and given free access to food (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was kept on a 12 hr light/dark cycle (light on at 0700 hr) and maintained at 22–23°C. The guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academic Press 1996; NIH publication number 85–23, revised 1996) were maintained throughout the study.
Experiment 1: Striatal 5-HT1AR stimulation on D1R-mediated dyskinesia and rotations
Medial forebrain bundle 6-hydroxydopamine lesion and cannulae implantation surgeries
The first study sought to determine the effects of striatal 5-HT1AR stimulation on D1R agonist-induced dyskinesia and contralateral rotations. In order to mimic more severe DA depletion typical of late-stage PD, medial forebrain bundle (MFB) DA lesions were utilized.
One week after arrival, rats (n=40) in this experiment received unilateral DA lesions of the left MFB. Each rat was administered desipramine HCl (25 mg/kg, ip; Sigma, St. Louis, MO, USA) 30 minutes prior to surgery in order to protect norepinephrine (NE) neurons. Rats were anesthetized with inhalant isoflurane (2–3%; Sigma) in oxygen (2.5 L/min) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The following coordinates relative to bregma were used for the site of injection: AP, −1.8 mm; ML, +2.0 mm; DV, −8.6 mm, with the incisor bar positioned at 5.0 mm below interaural line (Paxinos & Watson, 1998). After drilling a small hole in the skull above the site of injection, a 10 μl Hamilton syringe attached to a 26 gauge needle was lowered into the target. At that point, 4 μl of 6-hydroxydopamine hydrobromide (6-OHDA; 3 μg/μl; Sigma), dissolved in 0.9% sodium chloride (NaCl) + 0.1% ascorbic acid, was injected at a rate of 2 μl/min. The needle was withdrawn 5 min later. During the same surgery, rats were chronically implanted with bilateral chronic 22 gauge intracranial guide cannulae (C313G/SPC; Plastics One, Roanoke, VA). Bilateral cannulation was chosen in order to control for any differences between the two cerebral hemispheres, including variables pertaining to surgery, injection, damage, and pharmacology. With the incisor bar located 5.0 mm below the interaural line, cannulae were positioned above the dorsal striatum using the coordinates: AP, +0.4 mm; ML, ±2.9 mm; DV, −3.6 mm, relative to bregma (Paxinos & Watson, 1998). Cannulae were fixed in place using liquid and powder dental acrylic (Lang Dental, Wheeling, IL). At the completion of surgery, guide cannulae were fitted with 28 gauge inner stylets (Plastics One) to maintain patency. Animals were single housed, placed in clean cages and allowed to recover with ad lib food and water. Five min pre-surgery and 1 hr and 1 day post-surgery, rats received an injection of Buprenex (buprenorphine HCl; 0.03 mg/kg, ip; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) as analgesic treatment. Fruit was also provided and rats were monitored and handled twice per week for 3 weeks post-surgery in order to ensure full recovery and acclimation to experimenters. During each handling time, rats were loosely towel-wrapped and dummy cannulae were removed for 5 min to allow for habituation to the microinjection procedure.
Pharmacological treatments
Three weeks after 6-OHDA lesions of the MFB and bilateral striatal cannulations, rats in the first experiment received injections of the D1R agonist R(+)-SKF-81297 hydrobromide (SKF81297; 0.8 mg/kg, sc; Sigma), dissolved in 20% dimethyl sulfoxide (DMSO) in 0.9% NaCl, on 3 separate occasions 2–3 days apart in order to sensitize D1R (Pollack & Yates, 1999; Dupre et al., 2007). Contralateral rotations and AIMs were observed immediately after injections. Rats displaying an axial, limb, and orolingual AIMs (ALO AIMs; see description below) score of ≥10 by the 3rd day of D1R priming were retained for further study (n=36), which corresponded with at least 95% DA depletion upon HPLC analysis of striatal tissue samples.
The pharmacological treatment regimen commenced 2 days after the last day of SKF81297 priming, and test days were separated by a period of 2–3 days. Using a randomized between-subjects design, rats were assigned to equally dyskinetic groups and received 1 of 6 intrastriatal pretreatments bilaterally: Vehicle (0.9% NaCl), various doses of the full 5-HT1AR agonist ±8-OH-DPAT (5, 10, or 15 μg; Sigma), the 5-HT1AR antagonist N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY100635; 5 μg; Sigma), or combined ±8-OH-DPAT (15 μg) + WAY100635 (5 μg). Preliminary test infusions with cresyl violet dye in a separate set of rats ensured the placement and volume of the infusion were appropriate. During infusion, rats were lightly restrained with a towel and 16 mm 30 gauge injectors (Plastics One) were slowly lowered to extend 1 mm past the end of the guide cannulae. Once injectors were in place, drugs were infused at a rate of 0.5 μl/min for a total of 2.0 μl by a microinfusion pump (Harvard Apparatus, Boston, MA) that held two-10 μl Hamilton syringes attached to plastic tubing (PE20 Tygon tubing; Plastics One) and the injectors. Following infusions, injectors remained in place for 4 additional min. In order to limit damage at the target site, infusions were limited to 3 per animal. Five min following intrastriatal injections, rats received systemic injections of: Vehicle (20% DMSO, 0.9% NaCl, sc) or SKF81297 (0.8 mg/kg, sc; Dupre et al., 2007), after which rats were monitored for AIMs and rotations. Three days after completion of all pharmacological testing (approximately 3 weeks after the first day of priming), rats received systemic injections of SKF81297 (0.8 mg/kg, sc) as a post-test in order to ensure stable responses of AIMs and rotational behaviors.
Experiment 2: Systemic 5-HT1AR stimulation on motor performance
Striatal 6-OHDA lesion surgeries
In order to examine the effects of 5-HT1AR stimulation on motor performance, more moderate striatal 6-OHDA lesions were used in the last 2 experiments. This type of lesion mimics early to moderate-stage PD and induces motor impairment in the FAS test (see description below), while limiting the possibility of dyskinesia development and expression that may confound results on the FAS test.
One week after arriving, rats (n=17) in the second experiment received unilateral, multi-site 6-OHDA lesions of the left striata. Each rat was administered desipramine HCl (25 mg/kg, ip) 30 min prior to surgery in order to protect NE neurons. Rats were anesthetized with inhalant isoflurane (2–3%) in oxygen (2.5 L/min) and placed in a stereotaxic apparatus. The following coordinates relative to bregma were obtained from Kirik et al. (1998) and used for four-site DA terminal lesions: 1st site: AP, +1.3 mm; ML, +2.6 mm; DV, -5.0 mm; 2nd site: AP, +0.4 mm; ML, +3.0 mm; DV, −5.0 mm; 3rd site: AP, −0.4 mm; ML, +4.2 mm; DV, −5.0 mm; 4th site: AP, −1.3 mm; ML, +4.5 mm; DV, −5.0 mm, with the incisor bar positioned at 5.0 mm below interaural line (Paxinos & Watson, 1998). After drilling a small hole in the skull above the site of injection, a 10 μl Hamilton syringe attached to a 26 gauge needle was lowered into the target. At that point, 2 μl of 6-OHDA (8 μg), dissolved in 0.9% NaCl + 0.1% ascorbic acid, was injected at a rate of 1 μl/min. The needle was withdrawn 2 min later, and the preceding steps were repeated for each striatal site. At completion, stainless steel wound clips were used to close the surgical site. Animals were placed in clean cages and allowed to recover with ad lib food and water. As analgesic treatment, 5 min pre-surgery and 1 hr and 1 day post-surgery, rats received an injection of Buprenex (0.03 mg/kg, ip). Wound clips were removed 3–4 days post-surgery. Fruit was also provided and rats were monitored and handled twice per week for 3 weeks to ensure full recovery and acclimation to experimental procedures.
Pharmacological treatments
Two weeks after intrastriatal 6-OHDA lesions, SKF81297-naïve rats in the second experiment were trained and tested for lesion-induced motor disability using the FAS. One week later the pharmacological treatment regimen began. Using a randomized within-subjects design, rats (n=17) received pretreatments of: Vehicle or ±8-OH-DPAT (0.1 or 1.0 mg/kg, sc), followed 5 min later by injections of: Vehicle or SKF81297 (0.8 mg/kg, sc). Based on previous D1R-mediated effects (Dupre et al., 2007), the FAS test was completed for each rat 60 min after SKF81297 treatment, and test days were separated by 2–3 days.
Experiment 3: Striatal 5-HT1AR stimulation on motor performance
Striatal 6-OHDA lesion and cannulae implantation surgeries
One week following arrival, rats (n=15) in the third experiment received unilateral, multi-site 6-OHDA lesions of the left striata as explained in the second experiment. During the same surgery, rats were chronically implanted with bilateral chronic 22 gauge intracranial guide cannulae within the striatum, using the same coordinates and procedure described in the first experiment. Animals were then single housed in clean cages and allowed to recover with ad lib food and water. As analgesic treatment, rats received an injection of Buprenex (0.03 mg/kg, ip) 5 min pre-surgery and 1 hr and 1 day post-surgery. Fruit was also provided to facilitate recovery and animals were handled twice per week until testing commenced.
Pharmacological treatments
Two weeks after intrastriatal 6-OHDA lesions, SKF81297-naïve rats in the third experiment were trained and tested for lesion-induced motor disability using the FAS. One week later, using a randomized between-subjects design and the same microinjection procedure described in the first experiment, rats (n=15) were subjected to bilateral intrastriatal infusions of: Vehicle or ±8-OH-DPAT (15 μg), followed 5 min later by treatment injections of: Vehicle or SKF81297 (0.8 mg/kg, sc). The FAS test was completed for each rat 60 min after SKF81297 treatment and test days were separated by 2–3 days.
Behavioral analyses
Abnormal Involuntary Movements
Rats were monitored for AIMs using a procedure similarly described in Dupre et al. (2007). The AIMs model of dyskinesia utilizes distinct behavioral measures and demonstrates face validity with known anti-dyskinetic compounds (Lundblad et al., 2002; Taylor et al., 2005; Dekundy et al., 2007). AIMs can also be maintained over repeated testing by separating experimental days after initial priming (Bishop et al., 2006; Taylor et al., 2006). On test days (0900–1400 hr), rats were individually placed in plastic trays (60 × 75 cm) 5 min prior to pretreatments. Following SKF81297 injection, a trained observer blind to treatment condition assessed each rat for exhibition of axial, limb, and orolingual AIMs (inter-rater reliability=0.98). In addition, contralateral rotations, defined as complete 360° turns away from the lesioned side of the brain, were tallied. Dystonic posturing of the neck and torso, involving positioning of the neck and torso in a twisted manner directed toward the side of the body contralateral to the lesion, were referred to as ‘axial’ AIMs. ‘Limb’ AIMs were defined as rapid, purposeless movements of the forelimb located on the side of the body contralateral to the lesion. ‘Orolingual’ AIMs were composed of repetitive openings and closings of the jaw and tongue protrusions. The movements were considered abnormal as they occurred at times when the rats were not chewing or gnawing on food or other objects. Every 10th min for 2 hr, rats were observed for 2 consecutive min. Rats were rated for AIMs during the 1st min and rotational behavior in the 2nd min. During the AIMs observation periods (beginning 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 and 120 min postinjection), a severity score of 0–4 was assigned for each AIMs category: 0, not present; 1, present for <50% of the observation period (i.e. 1–29 s); 2, present for >50% or more of the observation period (i.e. 30–59 s); 3, present for the entire observation period (i.e. 60 s) and interrupted by a loud stimulus (a tap on the wire cage lid), or 4, present for the entire observation period but not interrupted by a loud stimulus. Thus, the theoretical maximum score for each type of AIM was 48 (4 × 12 periods) although observed scores were never this severe. The scores of the 3 AIMs subcategories (axial, limb, and orolingual; ALO AIMs) were summed (with a maximum potential of 144) and rotations were tallied for the entire 2 hr period.
Forepaw adjusting steps
The FAS test (Olsson et al., 1995; Schallert et al., 2000) has been extensively utilized as a measure of forelimb akinesia, demonstrating sensitivity to DA loss and reversal of deficit by DA replacement therapy, stem cell transplantation, and surgical intervention (Chang et al., 1999; Cho et al., 2006). Using a procedure similar to that described in Eskow et al. (2007), the number of adjusting steps taken by the forelimb in order to compensate for lateral movement were counted to determine the effects of lesion and drug treatment on motor performance. Rats were moved laterally across a table at a steady rate of 90 cm/10 s. The rear part of the torso and the hindlimbs were lifted from the table and 1 forepaw was held by the experimenter so as to bear weight on the other forepaw. Each stepping test consisted of 6 trials for each forepaw, alternating between directions both forehand (defined as compensating movement toward the body) and backhand (defined as compensating movement away from the body) on the table. Data was derived by summing steps (forehand and backhand) of the lesioned forelimb and dividing them by the sum of steps (forehand and backhand) of the intact forelimb. This calculation yields a percentage of the intact side indicating the degree of forepaw disability.
Histology and Neurochemical Analyses
Tissue dissection and cryostat sectioning
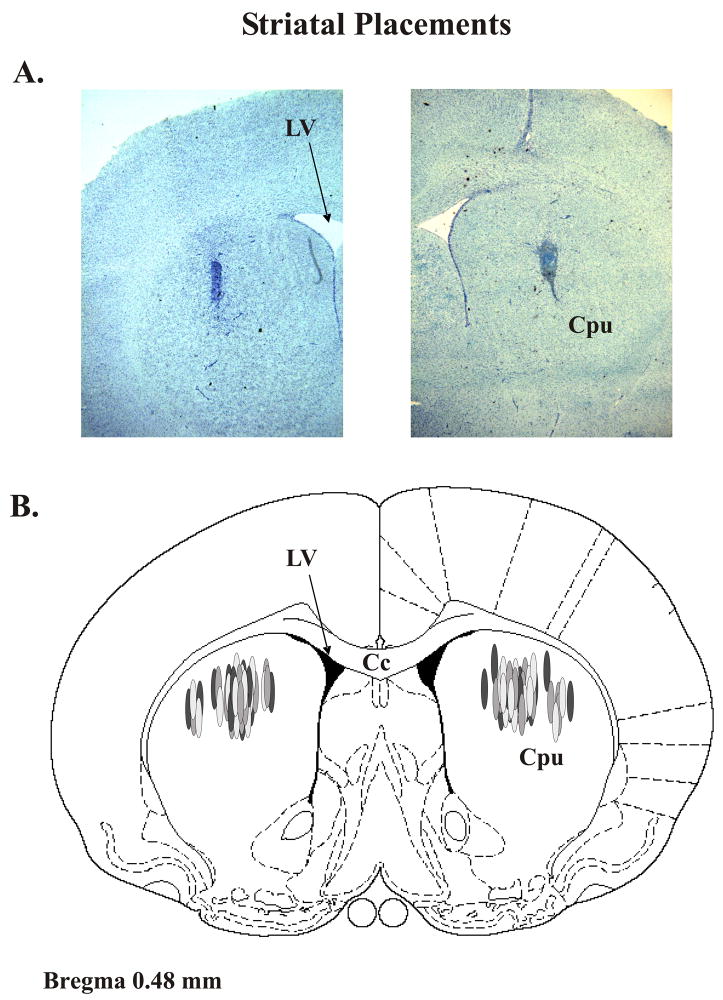
When experiments were completed, rats were sacrificed by decapitation and brains were immediately removed. To determine the level of DA depletion, posterior striata from rats in the first and third studies and whole striata from rats in the second study were freshly dissected, frozen at −80°C and later a subset of animals for each study were subjected to monoamine analysis using high performance liquid chromatography with electrochemical detection (HPLC-ED). Striata from rats in the first and third studies were also examined for verification of striatal placements. To accomplish this, during dissection anterior and central striata were removed and rapidly frozen in methylbutane (−30°C) and stored at −20°C. Cresyl violet (FD Neurotechnologies, Baltimore, MD) staining was used to determine injection sites and extent of gliosis from cryostat-generated 20 mm coronal sections containing injection sites that were postfixed with 4% paraformaldehyde (Fisher Scientific, Hanover Park, IL). Fig. 1A contains representative sections of left and right striata portraying the target site for drug injection. A schematic representation of coronal brain section identifying injection sites for all rats included in the microinfusion studies is shown in Fig. 1B. All rats that completed these studies were found to have injector placements within the dorsocentral or dorsolateral aspects of the striatum.
Figure 1. Striatal Injector Placements.
(A) Representative cresyl violet-stained striatal sections portraying injector typical placement. (B) Schematic representation of coronal rat brain section taken from Paxinos and Watson (1998). Shaded ovals depict the distribution of striatal microinfusion sites in all rats used in the current study (n=51). Relevant anatomical structures: Cc corpus callosum; Cpu caudate putamen; LV lateral ventricle.
High-performance liquid chromatography
Reverse-phase high performance liquid chromatography coupled to electrochemical detection (HPLC-EC) was performed on striatal tissue, obtained from all rats, according to protocol of Kilpatrick et al. (1986), a method for semiautomated catecholamine and indoleamine analysis with coulometric detection. The system included an ESA autoinjector (Model 542; Chelmsford, MA, USA), an ESA solvent delivery system (582), an external pulse dampener (ESA), an ESA column and a MD-150X3.2 (150 × 3.2 mm, 3 μm packing) column (ESA). Samples were homogenized in ice-cold perchloric acid (0.1 M) with 1% ethanol and 0.02% EDTA. The homogenates were spun for 30 min at 14,000 g with the temperature maintained at 4°C. Aliquots of supernatant were then analyzed for abundance of DA, 5-HT, NE, 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindole-3-acetic acid (5-HIAA). Samples were separated using a mobile phase composed of sodium phosphate (monobasic, anhydrous), 100 mM; EDTA, 0.05 mM; octane sulphonic acid, 1.4 mM; and acetonitrile, 9% adjusted to pH 3.0 with o-phosphoric acid. A coulometric detector configured with three electrodes (Coulochem III; ESA) measured the content of monoamines and metabolites. An ESA model 5020 guard cell (+300 mV) was positioned prior to the autoinjector. The analytical cell (ESA model 5011A; first electrode at −100 mV, second electrode at +250 mV) was located immediately after the column. The second analytical electrode emitted signals that were recorded and analyzed by EZChrom Elite software via Scientific Software Inc. module (SS420x). The final oxidation current values were compared to known standards 10−6–10−9, adjusted to striatal tissue weights, and expressed as nanograms (ng) of monoamine or metabolite per milligram (mg) tissue (mean ± SEM).
Data analyses
Monoamine and metabolite levels in the striatum were analyzed using paired t-tests (comparing intact vs. lesioned striata). The development and stability of ALO AIMs and rotations were analyzed with repeated-measures non-parametric Friedman ANOVA and repeated-measures parametric ANOVA, respectively. Significant differences between days were revealed with Wilcoxon post hoc analyses for ALO AIMs and planned comparison post hoc tests for rotations. Treatment effects (expressed as means ± SEM) for ALO AIMs and rotations were analyzed by employing non-parametric Kruskal-Wallis tests and two-way ANOVAs, respectively. Significant differences between treatments were determined by Mann-Whitney post hoc comparisons for ALO AIMs, and planned comparison post hoc tests for rotations. One-way ANOVAs and planned comparison post hoc tests were employed for analyses of FAS results. Analyses were performed with the use of Statistica software ’98 (Statsoft Inc., Tulsa, OK, USA) and alpha was set at p<0.05.
Results
Monoamine & metabolite levels
A subset of animals for each study was examined for HPLC for verification of DA depletion. In the first experiment (n=12), unilateral 6-OHDA injection into the medial forebrain bundle produced significant reductions in lesioned striatal DOPAC (t11=11.39; p<0.05) and DA levels (t11=11.17; p<0.05), 93.9% and 98.8% respectively, compared to intact striatum. The denervated side also showed an increased DOPAC/DA turnover rate (+441.4%) compared to control (t11=4.29; p<0.05). There were no significant differences between intact and lesioned striata for NE, 5-HIAA, 5-HT, or 5-HIAA/5-HT turnover rate in the first experiment.
For animals in the last 2 experiments (n=12), unilateral 6-OHDA injections into the striatum produced moderate but significant reductions in lesioned striatal DOPAC (t11=3.63; p<0.05) and DA levels (t11=6.11; p<0.05), 35.5% and 54.6% respectively, compared to intact striatum. There were also increases in lesioned striatal 5-HIAA (t11=2.42; p<0.05), +13.3%, and DOPAC/DA turnover (t11=2.613; p<0.05), +57.7%, compared to control. Finally, there were no significant differences between intact and lesioned striata for any other monoamine measure.
Development and stability of dyskinesia and rotations upon D1R agonist treatment
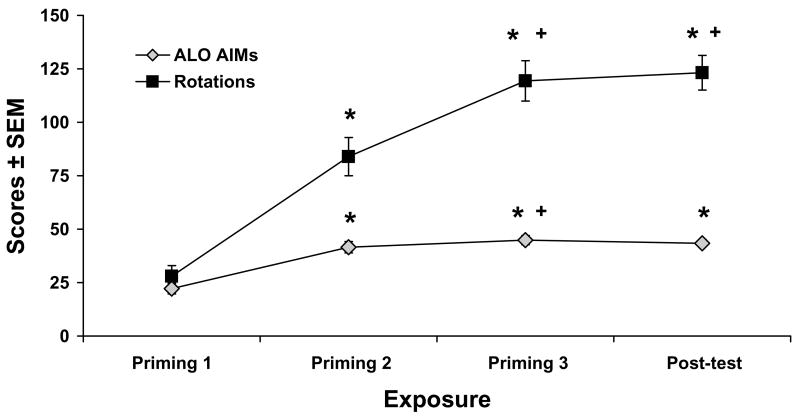
In order to address the development and stability of ALO AIMs and contralateral rotations upon D1R agonist treatment with SKF81297 (0.8 mg/kg) for rats in the first experiment, ALO AIMs and rotations were recorded during the 3 days of priming and a post-test with SKF81297 treatment was included following completion all pharmacological manipulations. Repeated-measures ANOVAs revealed main effects of time for both ALO AIMs (χ2 (3)=50.99, p<0.001) and contralateral rotations (F(3,105)=61.91; p<0.001). As demonstrated in Fig. 2, post hoc analyses revealed that both ALO AIMs and rotations significantly increased over the 3 days of priming, where the 2nd day of priming was significantly different from the 1st day, and the 3rd day was significantly different from the first 2 days (all p<0.05). Importantly, behaviors were stable based on post-test ALO AIMs and rotation scores that were not significantly different from the 3rd day of priming (both p<0.05).
Figure 2. Development and stability of dyskinesia and contralateral rotations upon D1R agonist treatment.
Rats (n=36) in the first experiment received treatment injections of the D1R agonist SKF81297 (0.8 mg/kg, sc) on 3 separate occasions 2–3 days apart in order to sensitize D1R. ALO AIMs and contralateral rotations were observed immediately after injections and summed over 2 hr. In order to determine stability of these behaviors, a post-test with SKF81297 was included following completion of pharmacological treatments. Lines depict mean scores ± SEM of ALO AIMs and contralateral rotations. Effects over time were determined by repeated-measures non-parametric Friedman ANOVA for ALO AIMs and repeated-measures parametric ANOVA for rotations. Post hoc analyses denote significant differences between exposure times. * p < 0.05 vs Priming 1; + p < 0.05 vs Priming 2
Experiment 1: Striatal ±8-OH-DPAT reduces D1R-mediated dyskinesia while increasing contralateral rotations
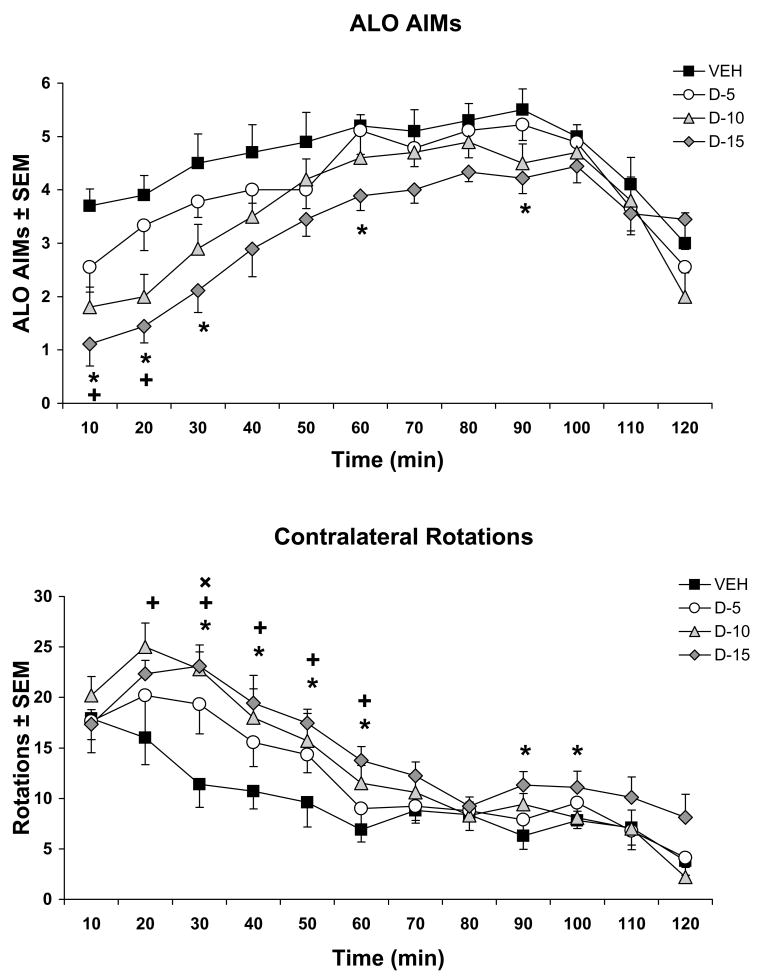
In order to determine the effects of striatal 5-HT1AR stimulation on D1R-mediated behaviors, various doses of the 5-HT1AR agonist ±8-OH-DPAT were infused bilaterally within the striatum, followed by systemic injections of the D1R agonist SKF81297 (0.8 mg/kg, sc), and AIMs and contralateral rotations were monitored. Main effects of ±8-OH-DPAT on SKF81297-induced ALO AIMs were found at the 10th (H3=16.40; p<0.01), 20th (H3=17.22; p<0.01), 30th (H3=12.03; p<0.01), 60th (H3=8.76; p<0.04) and 90th min (H3=8.52; p<0.04). As shown in Fig. 3A, post hoc analyses of revealed that the 10 μg dose of ±8-OH-DPAT significantly reduced ALO AIMs compared to Vehicle at the 10th and 20th min, while the 15 μg dose significantly attenuated ALO AIMs compared to Vehicle at the 10th, 20th, 30th, 60th, and 90th min (all p<0.05).
Figure 3. Striatal ±8-OH-DPAT reduces D1R-mediated dyskinesia while increasing contralateral rotations.
Five min after intrastriatal, bilateral microinfusions of vehicle (VEH) or the 5-HT1AR agonist ±8-OH-DPAT (D; 5, 10, 15 μg/side), rats (n=9–10/treatment group) received treatment with the D1R agonist SKF81297 (0.8 mg/kg, sc). Lines depict the treatment means for (A) ALO AIMs ± SEM and (B) contralateral rotations ± SEM for medial forebrain bundle 6-OHDA-lesioned rats every 10 min for 2 hr. Main effects were determined by Kruskal-Wallis tests for ALO AIMs and two-way ANOVAs for rotations. Post hoc comparisons denote significant differences between treatments at the time points indicated.
* p < 0.05 for D-15 vs VEH; + p < 0.05 for D-10 vs. VEH; ×p < 0.05 for D-5 vs. VEH
The effects of ±8-OH-DPAT on D1R agonist-induced contralateral rotations revealed a significant main effect of treatment (F(3,34)=5.24; p<0.01) and a main effect of time (F(11,374)=43.47; p<0.001). More importantly, a significant interaction between treatment and time was found (F(33,374)=1.62; p<0.05). Subsequent post hoc analysis demonstrated that the low dose of ±8-OH-DPAT (5 μg) significantly enhanced SKF81297-induced rotations compared to Vehicle at the 30th min, while the 10 μg dose significantly increased rotations from the 20th–60th min when compared to Vehicle (Fig. 3B; all p<0.05). Finally, the 15 μg dose of ±8-OH-DPAT significantly enhanced rotations from the 30th–60th and 90th–100th min when compared to Vehicle (all p<0.05).
Co-administration of the 5-HT1AR antagonist WAY100635 reverses ±8-OH-DPAT’s effects on D1R-mediated behaviors
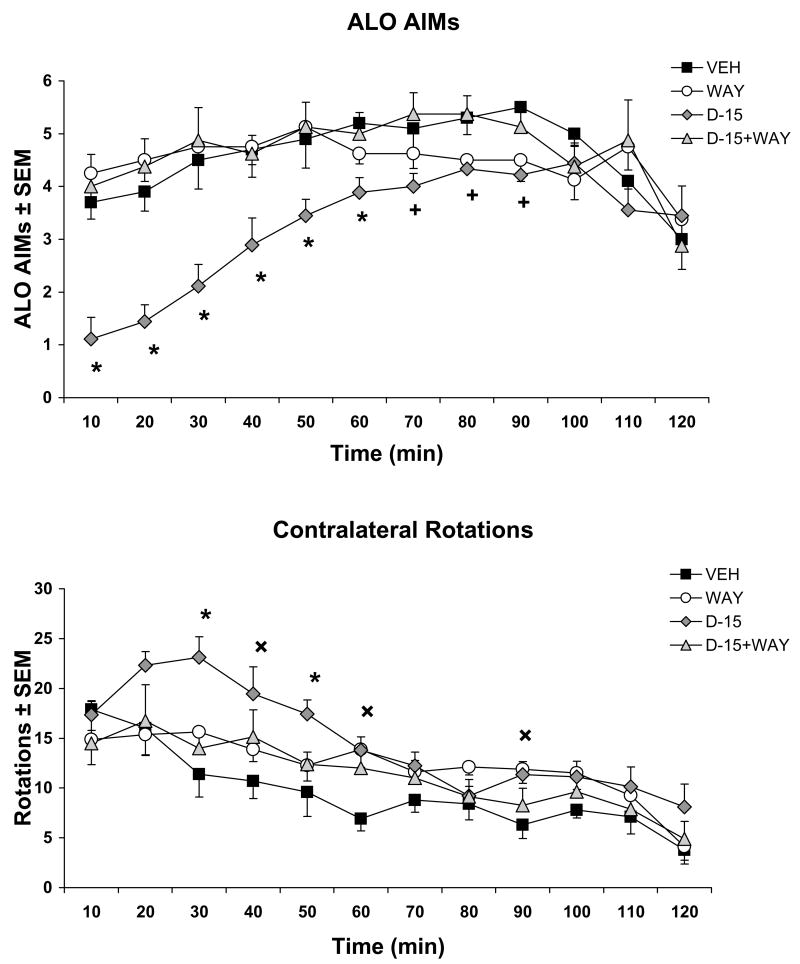
In order to ensure the receptor specificity of the aforementioned findings, the 5-HT1AR antagonist WAY100635 (5 μg) was co-infused intrastriatally with ±8-OH-DPAT (15 μg) prior to SKF81297 injections, after which ALO AIMs and rotations were observed. Analysis revealed main effects of treatment at the 10th (H3=16.94; p<0.01), 20th (H3=18.50; p<0.01), 30th (H3=15.21; p<0.01), 40th (H3=9.94; p<0.05), 50th (H3=11.70; p<0.01), 60th (H3=11.47; p<0.01), 70th (H3=8.80; p<0.05), 80th (H3=9.66; p<0.05), and 90th (H3=8.04; p<0.05) min. As shown in Fig. 4A, post hoc analyses revealed that, in support of previous findings, ±8-OH-DPAT treatment significantly reduced SKF81297-induced ALO AIMs compared to Vehicle from min 10–90 (all p<0.05). More importantly, co-administration of WAY100625 reversed the anti-dyskinetic effects of ±8-OH-DPAT during this time (all p<0.05).
Figure 4. Co-administration of the 5-HT1AR antagonist WAY100635 reverses ±8-OH-DPAT’s effects on D1R-mediated behaviors.
Rats (n=8–10/treatment group) received intrastriatal, bilateral microinfusions of vehicle (VEH), the 5-HT1AR antagonist WAY100635 (WAY; 5 μg/side), the 5-HT1AR agonist ±8-OH-DPAT (D; 15 μg/side), or ±8-OH-DPAT (15 μg/side) + WAY100635 (5 μg/side), followed 5 min later by treatment with the D1R agonist SKF81297 (0.8 mg/kg, sc). Lines indicate the treatment means for (A) ALO AIMs ± SEM and (B) contralateral rotations ± SEM for medial forebrain bundle 6-OHDA-lesioned rats every 10 min for 2 hr. Main effects were determined by Kruskal-Wallis tests and two-way ANOVAs for ALO AIMs and rotations, respectively. Post hoc comparisons indicate significant differences between treatments at the time points indicated.
* p < 0.05 for D-15 vs all; + p < 0.05 for D-15 vs. VEH and D-15+WAY; ×p < 0.05 for D-15 vs VEH only
The effects of ±8-OH-DPAT on D1R agonist-induced contralateral rotations revealed a significant main effect of treatment (F(3,31)=3.63; p<0.05) and a main effect of time (F(11,341)=26.57; p<0.001). A significant interaction between treatment and time was also found (F(33,341)=1.85; p<0.01). Post hoc analysis confirmed that ±8-OH-DPAT (15 μg) significantly enhanced SKF81297-induced rotations during the 30th-60th and 90th min when compared to Vehicle (Fig. 4B; all p<0.05). Moreover, in corroboration of a 5-HT1AR specific effect, co-infusion of WAY100635 reversed the ±8-OH-DPAT-induced increase in rotations at the 30th and 50th min (both p<0.05).
Experiment 2: Systemic ±8-OH-DPAT enhances motor performance in hemiparkinsonian rats
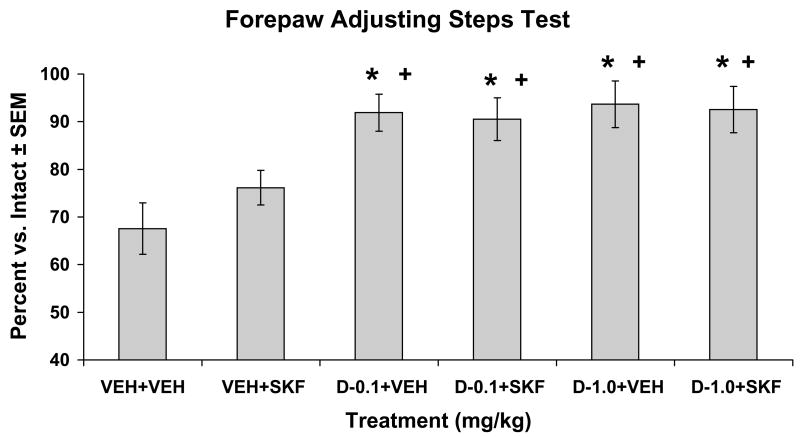
In order to address pro-rotational and thus possible anti-parkinsonian actions of ±8-OH-DPAT administration, striatally DA-lesioned, SKF81297-naïve rats were tested for motor performance using the FAS test (Table 1 and Fig. 5). Analysis of stepping indicated a significant effect of treatment (F(5,80)=7.17; p<0.05). Post hoc analyses revealed that while Vehicle + SKF81297 (0.8 mg/kg, sc) was not significantly different from Vehicle treatment alone, ±8-OH-DPAT (0.1 and 1.0 mg/kg, sc) + Vehicle and ±8-OH-DPAT (0.1 and 1.0 mg/kg, sc) + SKF81297 (0.8 mg/kg, sc) all improved stepping in the lesioned forepaw when compared to Vehicle or SKF81297 treatment alone (all p<0.05).
Table 1.
Mean values (SEM) of lesioned and intact forepaw adjusting steps in unilateral, striatal 6-OHDA-lesioned rats. Five min after pretreatments with systemic vehicle (VEH) or the 5-HT1AR agonist ±8-OH-DPAT (D; 0.1 or 1.0 mg/kg, sc) in the second experiment, or bilateral striatal microinfusions of vehicle (VEH) or ±8-OH-DPAT (D; 15 μg/side) in the third experiment, rats received systemic treatments of VEH or the D1R agonist SKF81297 (SKF; 0.8 mg/kg, sc) and were tested for forepaw adjusting steps 1 hr later.
| Experiment | Treatment | Lesioned | Intact | Percentage vs. Intact |
|---|---|---|---|---|
| 2 | VEH+ VEH (n=17) | 72.23 (6.83) | 105.23 (5.16) | 67.56 (5.41) |
| 2 | VEH+ SKF (n=17) | 77.35 (4.44) | 101.58 (3.22) | 76.15 (3.64) |
| 2 | D-0.1+ VEH (n=17) | 103.17 (5.07) | 113.11 (4.64) | 91.89 (3.90)*+ |
| 2 | D-0.1+ SKF (n=17) | 109.47 (6.44) | 121.00 (4.53) | 90.52 (4.46)*+ |
| 2 | D-1.0+ VEH (n=17) | 121.94 (6.16) | 131.05 (3.92) | 93.66 (4.87)*+ |
| 2 | D-1.0+ SKF (n=17) | 122.94 (6.23) | 134.17 (4.43) | 92.53 (4.87)*+ |
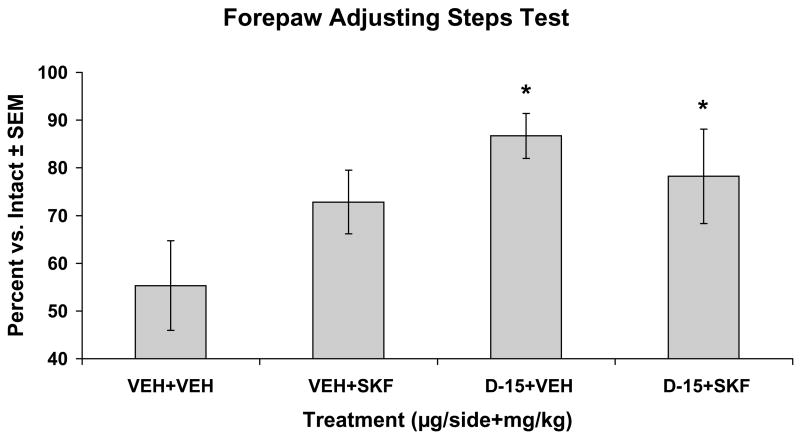
| 3 | VEH+ VEH (n=7) | 37.00 (7.41) | 64.85 (3.30) | 55.31 (9.37) |
| 3 | VEH+ SKF (n=8) | 53.75 (7.13) | 73.00 (5.68) | 72.83 (6.67) |
| 3 | D-15+ VEH (n=8) | 56.00 (8.86) | 63.50 (8.33) | 86.70 (4.68)* |
| 3 | D-15+ SKF (n=6) | 53.00 (12.12) | 70.16 (13.63) | 78.22 (9.89)* |
p < 0.05 vs VEH+VEH;
p < 0.05 vs. VEH+SKF
Figure 5. Systemic ±8-OH-DPAT enhances motor performance in hemiparkinsonian rats.
Five min after pretreatments with vehicle (VEH) or the 5-HT1AR agonist ±8-OH-DPAT (D; 0.1 or 1.0 mg/kg, sc), unilateral striatal 6-OHDA-lesioned rats (n=17) received treatments of VEH or the D1R agonist SKF81297 (SKF; 0.8 mg/kg, sc). Rats were tested for forepaw adjusting steps 1 hr later. Bars show the effects of treatment on motor performance in the FAS test expressed as a mean percentage of lesion vs. intact forepaw adjusting steps ± SEM. Effects of treatment were determined by one-way ANOVA and significant differences were established by post hoc planned comparisons.
* p < 0.05 vs VEH+VEH; + p < 0.05 vs. VEH+SKF
Experiment 3: Striatal ±8-OH-DPAT improves movement in hemiparkinsonian rats
In order to ascribe the systemic anti-parkinsonian effects of ±8-OH-DPAT to the striatum, SKF81297-naïve rats with striatal DA lesions and bilateral striatal cannulations were tested for motor performance using the FAS test (Table 1). Upon analysis, a significant effect of treatment was found (F(3,25)=3.61; p<0.05). As shown in Fig. 6, post hoc analysis demonstrated that ±8-OH-DPAT (15 μg) + Vehicle and ±8-OH-DPAT (15 μg) + SKF81297 (0.8 mg/kg, sc) improved stepping in the lesioned forepaw when compared to Vehicle treatment alone (both p<0.05).
Figure 6. Striatal ±8-OH-DPAT improves movement in hemiparkinsonian rats.
Unilateral striatal 6-OHDA-lesioned rats (n=6–8/treatment group) received intrastriatal, bilateral microinfusions of vehicle (VEH) or the 5-HT1AR agonist ±8-OH-DPAT (D; 15 μg/side), followed 5 min later by treatments with VEH or the D1R agonist SKF81297 (SKF; 0.8 mg/kg, sc). Rats were tested for forepaw adjusting steps 1 hr later. Bars depict the effects of treatment on motor performance in the FAS test expressed as a mean percentage of lesion vs. intact forepaw adjusting steps ± SEM. Treatment effects were determined by one-way ANOVA and post hoc planned comparisons established significant differences between treatment groups.
* p < 0.05 vs VEH+VEH
Discussion
The ability of 5-HT1AR agonists to reduce LID has been predominantly attributed to a tempering of the release of L-DOPA-derived DA from raphestriatal neurons. The current study clearly demonstrates an additional mechanism, revealing for the first time that the striatum is a central neuroanatomical site for both anti-dyskinetic and anti-parkinsonian actions of 5-HT1AR agonists. Direct striatal stimulation with the full 5-HT1AR agonist ±8-OH-DPAT reduced dyskinesia induced by the D1R agonist SKF81297, while increasing rotations and improving forepaw akinesia in DA-depleted rats. These novel findings support an important functional role between striatal 5-HT1AR and D1R in DA-depleted rats that could be exploited for the treatment of PD and LID.
Following DA depletion, the emergence of supersensitive striatal DA receptors largely contributes to the development and expression of LID (Pavese et al., 2006; Cenci, 2007; Westin et al., 2007). For example, in DA-lesioned animals, repeated exposure of DA receptor agonists increases striatal signal transduction, gene expression and DA-mediated behaviors to an extent not portrayed in controls (Cai et al., 2002; Bishop & Walker, 2003). Moreover, both D1R and D2R agonists produce dyskinesia (Rascol et al., 2001; Iravani et al., 2006; Taylor et al., 2006; Dupre et al., 2007), while D1R and D2R antagonists have been shown to block LID at the expense of L-DOPA’s efficacy (Monville et al., 2005; Taylor et al., 2005). Although both receptor subtypes appear to be involved in LID, striatal D1R are particularly important (Cenci et al., 1998; Gerfen et al., 2002; Guigoni et al., 2007; Westin et al., 2007). For example, striatal preprodynorphin (PPD) mRNA associated with the D1R-expressing direct pathway (Anderson & Reiner, 1990) is increased in dyskinetic rats and primates and reduced by a number of anti-LID treatments (Cenci et al., 1998; Queiroz et al., 2002; Mela et al., 2007). Furthermore, coadministration of a D1R antagonist with L-DOPA has recently been shown to completely block the development of L-DOPA-induced dyskinesia and associated molecular abnormalities in a rodent model of LID (Westin et al., 2007).
In addition to supersensitive striatal DA receptors, there is evidence for the involvement of the 5-HT system in LID. Following DA depletion, serotonergic raphe neurons are thought to compensate for the loss of DA neurons by converting L-DOPA into DA and releasing it into the striatum. However, this process is unregulated, leading to fluctuating stimulation of supersensitive striatal DA neurons associated with LID (Tanaka et al., 1999; Maeda et al., 2003; Carta et al., 2007). Several studies show that 5-HT1AR agonists reduce L-DOPA-induced motor complications (Tomiyama et al, 2005; Ba et al., 2006; Eskow et al., 2007) and attribute these effects to a raphestriatal-mediated mechanism whereby 5-HT1AR within the raphe nuclei attenuate the release of DA into the striatum (Bibbiani et al., 2001; Carta et al., 2007; Eskow et al., 2007).
Although this raphe-mediated 5-HT1AR mechanism has been supported, extra-raphe influences involving striatal 5-HT1AR have also been proposed (Santiago et al., 1998; Mignon & Wolf, 2005). 5-HT1AR populations exist in the striata of rat, primate, and human (Albert et al., 1990; Marazziti et al., 1994; Bezard et al., 2006) and in primates, striatal 5-HT1AR binding increases following chronic MPTP-treatment (Frechilla et al., 2001). The functionality of this population has been supported by findings that 5-HT1AR agonists can enhance rotations but alleviate dyskinesia induced by direct DA receptor agonists (Iravani et al., 2006; Matsubara et al., 2006). We recently found that systemic administration of the 5-HT1AR agonist ±8-OH-DPAT reduced D1R agonist-induced dyskinesia while enhancing rotations, implicating an important functional role between 5-HT1AR and D1R (Dupre et al., 2007). In line with these effects, 5-HT1AR stimulation has been shown to reduce overactive striatal D1R-mediated signaling and D1R-mediated behaviors associated with the expression of LID (Queiroz et al., 2002; Tomiyama et al., 2005).
Because of the potential interaction between striatal 5-HT1AR and D1R, the current study investigated the effects of intrastriatal administration of the 5-HT1AR agonist ±8-OH-DPAT on D1R-mediated behaviors. As shown in Fig. 3A, ±8-OH-DPAT infused locally into the striatum produced a dose-dependent decrease in ALO AIMs induced by the D1R agonist SKF81297, which was blocked by co-administration with the 5-HT1AR antagonist WAY100635 (Fig. 4A). None of the doses of ±8-OH-DPAT or WAY100635 when given without SKF81297 induced ALO AIMs (data not shown), indicating the observed effects were dependent on stimulation of both 5-HT1AR and D1R. Moreover, in corroboration with previous systemic findings (Dupre et al., 2007), contralateral rotations were dose-dependently enhanced by intrastriatal administration of ±8-OH-DPAT plus systemic SKF81279 when compared to SKF81297 alone (Fig. 3B). These rotational effects were blocked by co-administration of WAY100635 (Fig. 4B), and neither ±8-OH-DPAT nor WAY100635 induced rotations without SKF81297. Unlike previous studies using L-DOPA (Tomiyama et al, 2005; Ba et al., 2006; Carta et al., 2007; Eskow et al., 2007), apomorphine (Matsubara et al., 2006), the D2/3 receptor agonist pramipexole (Iravani et al., 2006), and the partial 5-HT1AR agonists buspirone (Eskow et al., 2007) and tandospirone (Kannari et al., 2002), the current study utilized the specific D1R agonist SKF81297 and the full 5-HT1AR agonist ±8-OH-DPAT, making these anti-dyskinetic and pro-rotational effects unique to the D1R and 5-HT1AR and representing a functional interaction between these two receptor subtypes at the level of the striatum.
In support of previous research suggesting anti-parkinsonian actions of 5-HT1AR agonists (Mignon & Wolf, 2005; Matsubara et al., 2006; Mignon & Wolf, 2007), the augmentation of D1R-mediated rotations produced by ±8-OH-DPAT in the current study may represent a beneficial, motor-enhancing effect. However, since the interpretation of rotational behavior is somewhat controversial (Lane et al., 2005) and the current findings may represent competing motor behaviors, the FAS test was utilized to examine the implied anti-parkinsonian effects. In order to mimic moderate-stage PD and prevent dyskinetic movements from confounding our results, more moderate striatal 6-OHDA lesions similar to previous reports (Kirik et al., 1998) were used. Rats with striatal lesions (approximately 55% DA-depletion) showed significant forepaw disability in the FAS test. In contrast with previous findings (Olsson et al., 1995), motor disability was not reversed using SKF81297 (0.8 mg/kg, sc; Fig. 5 and 6). This discrepancy may be due to differences in type of lesion (intra-striatal lesions in the current study versus medial forebrain bundle lesions) and consequent DA depletion (55% versus over 98%), or the type and dose of D1R agonist (SKF81297 (0.8 mg/kg) used in the current study versus SKF38393 (2.0 mg/kg). Surprisingly, in the current study, disability was almost completely eliminated upon treatment with systemic ±8-OH-DPAT alone regardless of dose (Fig. 5). Systemic ±8-OH-DPAT in combination with SKF81297 did not differ from ±8-OH-DPAT alone, likely reflecting a ceiling effect. Interestingly, similar motor-enhancing effects were observed upon intrastriatal administration of ±8-OH-DPAT (15 μg) alone and together with SKF81297 (0.8 mg/kg, sc) administration (Fig. 6). In these experiments, ±8-OH-DPAT administration augmented stepping in both the lesioned and intact forepaw (Table 1), corroborating previous research showing an increase in movement from 5-HT1AR stimulation (Matsubara et al., 2006; Mignon & Wolf, 2007). The current study’s unique findings are the first to show that 5-HT1AR stimulation can reverse motor impairments associated with PD as demonstrated by the FAS test and suggest an important role for the striatum in these effects.
The current study is not the first to describe extra-raphe, forebrain regions involved in the beneficial effects of 5-HT1AR agonists. Multiple lines of research report that the motor cortex is an integral site for the expression of LID (Antonelli et al., 2005; Mignon & Wolf, 2005; Delfino et al., 2007). As such, the anti-parkinsonian and anti-dyskinetic effects of 5-HT1AR have been attributed in part to post-synaptic 5-HT1AR in the cortex whose stimulation blunts corticostriatal glutamate release (Mignon & Wolf, 2005; Saigal et al., 2006; Mignon & Wolf, 2007). The present study, by employing site-specific microinfusions, bypassed this purported cortical mechanism, supporting a unique role for the striatum in the anti-parkinsonian and anti-dyskinetic effects of 5-HT1AR agonists. While the functional implications of striatal 5-HT1AR stimulation are presently demonstrated, how this influences D1R-mediated behaviors and improves motor disability remains an important question. Based on mRNA expression, binding studies, and protein levels, striatal 5-HT1AR appear to act as presynaptic heteroreceptors on glutamatergic corticostriatal terminals (Pasqualetti et al., 1996; Frechilla et al., 2001; Bezard et al., 2006). As such, intrinsic 5-HT1AR stimulation may act to normalize excessive glutamate levels. Because interactions between striatal glutamate, DA, and their respective N-methyl D-aspartate receptors (NMDAR) and D1R play a central role in synaptic plasticity (Calabresi et al., 2000), 5-HT1AR stimulation may correct the aberrant motor learning that portends PD symptoms and LID (Chase & Oh, 2000; Kreipke & Walker, 2004; Cenci, 2007). In recent support of this, we found that coincident 5-HT1AR stimulation and NMDAR blockade produces synergistic anti-dyskinetic and pro-rotational effects on L-DOPA-induced behaviors (Dupre et al., 2008). While it is also possible that striatal 5-HT1AR may also influence the formation and function of D1R-NMDAR complexes, which would significantly impact the trafficking, signaling, and desensitization of both receptors (Fiorentini et al., 2003), additional research is required to support this hypothesis.
In conclusion, the current study revealed that direct striatal 5-HT1AR stimulation diminished D1R-mediated dyskinesia and alleviated motor disability in DA-depleted rats. These results indicate a novel interaction between striatal 5-HT1AR and D1R that could be utilized to advance the understanding and treatment of PD and LID. By extension, these findings also support the use of 5-HT1AR agonists as adjuncts to DA agonist monotherapy by improving efficacy, decreasing dyskinetic liability, and delaying L-DOPA therapy.
Acknowledgments
This work was supported by funds from the American Parkinson Disease Association (C.B.), the Center for Development and Behavioral Neuroscience at Binghamton University (C.B.), and NIH NS059600 (C.B.).
Abbreviations
- AIMs
Abnormal Involuntary Movements
- ALO
Axial, Llimb and Orolingual
- Benserazide
DL-Serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride
- D1R
D1 receptor
- D2R
D2 receptor
- DA
Dopamine
- DMSO
Dimethyl Sulfoxide
- DOPAC
3,4-dihydroxyphenylacetic acid
- FAS
Forepaw Adjusting Steps
- HPLC-EC
high performance liquid chromatography coupled to electrochemical detection
- ±8-OH-DPAT
(±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide
- 6-OHDA
6-hydroxydopamine hydrobromide
- 5-HIAA
5-hydroxyindole-3-acetic acid
- PPD
preprodynorphin
- 5-HT
Serotonin
- 5-HT1AR
Serotonin 1A receptor
- L-DOPA
L-3,4-dihydroxyphenylalanine methyl ester
- LID
L-DOPA-induced dyskinesia
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NE
Norepinephrine
- PD
Parkinson’s disease
- SKF81297
R(+)-SKF-81297 hydrobromide
- VEH
Vehicle
- WAY100635
N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–32. [PubMed] [Google Scholar]
- Anderson KD, Reiner A. Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: An evolutionarily conserved feature of basal ganglia organization. J Comp Neurol. 1990;295:339–369. doi: 10.1002/cne.902950302. [DOI] [PubMed] [Google Scholar]
- Antonelli T, Fuxe K, Tomasini MC, Bartoszyk GD, Seyfried CA, Tanganelli S, Ferraro L. Effects of sarizotan on the corticostriatal glutamate pathways. Synapse. 2005;58:193–199. doi: 10.1002/syn.20195. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Ba M, Kong M, Ma G, Yang H, Lu G, Chen S, Liu Z. Cellular and behavioral effects of 5-HT1A receptor agonist 8-OH-DPAT in a rat model of levodopa-induced motor complications. Brain Res. 2006;1127:177–184. doi: 10.1016/j.brainres.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, et al. Effects of serotonin 5-HT1A agonist in advanced Parkinson’s disease. Mov Disord. 2005;20:932–936. doi: 10.1002/mds.20370. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gerlach I, Moratalla R, Gross CE, Jork R. 5-HT1A receptor agonist-mediated protection from MPTP toxicity in mouse and macaque models of Parkinson’s disease. Neurobiol Dis. 2006;23:77–86. doi: 10.1016/j.nbd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Bishop C, Walker PD. Combined intrastriatal dopamine D1 and serotonin 5-HT2 receptor stimulation reveals a mechanism for hyperlocomotion in 6-hydroxydopamine-lesioned rats. Neuroscience. 2003;121:649–657. doi: 10.1016/s0306-4522(03)00516-5. [DOI] [PubMed] [Google Scholar]
- Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci. 2006;23:2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- Cai G, Wang HY, Friedman E. Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J Pharmacol Exp Ther. 2002;302:1105–1112. doi: 10.1124/jpet.102.036673. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Giacornini P, Centonze D, Bernardi G. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol. 2000;47 (Suppl 1):S60–S68. [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–33. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–43. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–28. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23:S86–S91. doi: 10.1016/s1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- Cho YH, Kim DS, Kim PG, Hwang YS, Cho MS, Moon SY, et al. Dopamine neurons derived from embryonic stem cells efficiently induce behavioral recovery in a Parkinsonian rat model. Biochem Biophys Res Comm. 2006;341:6–12. doi: 10.1016/j.bbrc.2005.12.140. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: Further validation of the rat dyskinesia model. Beh Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini IR, et al. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharm. 2007;32:1911–21. doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT1A receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res. 2007;1158:135–43. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Steiniger A, Klioueva A, Negron G, Lormand L, et al. Effects of coincident 5-HT1A receptor stimulation and NMDA receptor antagonism on L-DOPA-induced dyskinesia and rotational behaviors in the hemi-parkinsonian rat. Psychopharm (Berl) 2008 doi: 10.1007/s00213-008-1135-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT1A agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharm Biochem Behav. 2007;87:306–14. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Frechilla D, Cobreros A, Saldise L, Moratalla R, Insausti R, Luquin M, et al. Serotonin 5-HT(1A) receptor expression is selectively enhanced in the striosomal compartment of chronic parkinsonian monkeys. Synapse. 2001;39:288–296. doi: 10.1002/1098-2396(20010315)39:4<288::AID-SYN1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E. Altered D1 dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Disease. 2007;26:452–463. doi: 10.1016/j.nbd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist(R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with increased motor disability. J Pharmacol Exp Ther. 2006;319:1225–1234. doi: 10.1124/jpet.106.110429. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord. 2005;20(Suppl 11):S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- Kannari K, Kurahashi K, Tomiyama M, Maeda T, Arai A, Baba M, et al. Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease. No To Shinkei. 2002;54:133–137. [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–76. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kreipke CW, Walker PD. NMDA receptor blockade attenuates locomotion elicited by intrastriatal dopamine D1-receptor stimulation. Synapse. 2004;53:28–35. doi: 10.1002/syn.20035. [DOI] [PubMed] [Google Scholar]
- Lane EL, Cheetham SC, Jenner P. Does contraversive circling in the 6-OHDA-lesioned rat indicate an ability to induce motor complications as well as therapeutic effects in Parkinson’s disease? Exp Neurol. 2005;197:284–90. doi: 10.1016/j.expneurol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Anderson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kannari K, Shen H, Arai A, Tomiyama M, Matsunaga M, et al. Rapid induction of serotonergic hyperinnervation in the adult rat striatum with extensive dopaminergic denervation. Neuroscience Letters. 2003;343:17–20. doi: 10.1016/s0304-3940(03)00295-7. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Marracci S, Palego L, Rotondo A, Mazzanti C, Nardi I, et al. Localization and gene expression of serotonin 1A (5HT1A) receptors in human brain postmortem. Brain Res. 1994;658:55–9. doi: 10.1016/s0006-8993(09)90010-5. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Shimizu K, Suno M, Ogawa K, Awaya T, Yamada T, et al. Tandospirone, a 5-HT1A agonist, ameliorates movement disorder via non-dopaminergic systems in rats with unilateral 6-hydroxydopamine-generated lesions. Brain Res. 2006;1112:126–133. doi: 10.1016/j.brainres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates L-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Mignon LJ, Wolf WA. 8-Hydroxy-2-(di-n-propylamino)tetralin reduces striatal glutamate in an animal model of Parkinson’s disease. Neuroreport. 2005;16:699–703. doi: 10.1097/00001756-200505120-00009. [DOI] [PubMed] [Google Scholar]
- Mignon L, Wolf WA. Postsynaptic 5-HT(1A) receptor stimulation increases motor activity in the 6-hydroxydopamine-lesioned rat: implications for Parkinson’s disease. Psychopharmacology (Berl) 2007;192:49–59. doi: 10.1007/s00213-006-0680-0. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB. Validation of the L-DOPA-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Research Bulletin. 2005;68:16–23. doi: 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–75. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano GB. Comparative anatomical distribution of serotonin 1A, 1Da and 2A receptor mRNAs in human brain postmortem. Mol Brain Res. 1996;39:223–233. doi: 10.1016/0169-328x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease. Neurology. 2006;67:1612–17. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pollack AE, Yates TM. Prior dopamine D1 receptor stimulation is required to prime D2-mediated striatal fos expression in 6-hydroxydopamine-lesioned rats. Neuroscience. 1999;94:505–514. doi: 10.1016/s0306-4522(99)00338-3. [DOI] [PubMed] [Google Scholar]
- Queiroz CMT, Alcantara FB, Yague AML, Bibancos T, Frussa-Filho R. Acute buspirone abolishes the expression of behavioral dopaminergic supersensitivity in mice. Braz J Med Biol Res. 2002;35:237–242. doi: 10.1590/s0100-879x2002000200013. [DOI] [PubMed] [Google Scholar]
- Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, et al. Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson disease. Arch Neurol. 2001;58:249–254. doi: 10.1001/archneur.58.2.249. [DOI] [PubMed] [Google Scholar]
- Saigal N, Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J Nuclear Medicine. 2006;47:1697–1706. [PubMed] [Google Scholar]
- Santiago M, Matarredona ER, Machado M, Cano J. Influence of serotoninergic drugs on in vivo dopamine extracellular output in rat striatum. J Neurosci Res. 1998;52:591–8. doi: 10.1002/(SICI)1097-4547(19980601)52:5<591::AID-JNR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kannari T, Maeda M, Tomiyama T, Suda M, Matsunaga M. Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport. 1999;10:631–634. doi: 10.1097/00001756-199902250-00034. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacol Biochem Behav. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD. Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not L-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacology. 2006;50:761–768. doi: 10.1016/j.neuropharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Kimura T, Maeda T, Kannari K, Matsunaga M, Baba M. A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson’s disease. Neurosci Res. 2005;52:185–194. doi: 10.1016/j.neures.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of l-DOPA–induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]