Abstract
Purpose
To determine the prevalence of adenomatous and hyperplastic polyps in a large cohort of individuals with a germline mutation in a mismatch repair (MMR) gene, the major genetic determinant of hereditary nonpolyposis colorectal cancer (HNPCC). These prevalences have been estimated previously in smaller studies, and the results have been found to be variable.
Patients and Methods
Colorectal Adenoma/Carcinoma Prevention Programme 2 trial is a chemoprevention trial in people classified as having HNPCC. The 695 patients with a proven germline MMR mutation and documented screening history before the chemoprevention study were the focus of this study. The number, histology, size, and location of polyps found at the participants' first ever colonoscopy were analyzed in a cross-sectional study.
Results
Seventy-four patients (10.6%) were found to have at least one adenoma at first colonoscopy, whereas 37 (5.3%) had at least one hyperplastic polyp. The frequency of an adenoma at first colonoscopy increased from 5.0% (95% CI, 2.8% to 8.3%) in patients younger than 35 years old to 18.9% (95% CI, 9.4% to 32.0%) in patients age at least 55 years (P = .0001 for trend). No such trend was observed for hyperplastic polyps. No sex differences were found for either type of polyp. A marginal association was found between the co-occurrence of adenomas and hyperplastic polyps. Adenomas tended to be more proximally distributed through the colon, whereas hyperplastic polyps tended to be located in the distal colon.
Conclusion
Adenoma prevalence increases with age among MMR mutation carriers, whereas hyperplastic polyp prevalence is consistent. No sex differences were observed for either type of lesion.
INTRODUCTION
In families with hereditary nonpolyposis colorectal cancer (HNPCC), a germline mutation in a mismatch repair (MMR) gene (MLH11 or MSH22 or, more rarely, MSH63) is found to segregate with disease, predominantly colorectal cancer and endometrial cancer. Surveillance programs including colonoscopy and polypectomy in nonrandomized controlled trials suggest reduction in the incidence and mortality of colorectal cancer among susceptible individuals.4 Adenomas are a well-recognized precursor of colorectal cancer development, and some studies suggest that HNPCC patients have accelerated progression of the adenoma-carcinoma sequence.5 The prevalence of adenomas in HNPCC patients is reported to lie within a range of 17% to 49%6–15 depending on age, but sample sizes and study design limit the precision and interpretability of these estimates. For instance, many studies have observed patients with a personal and family history consistent with HNPCC, and within such studies, it is difficult to estimate the neoplasia burden of the average gene carrier. This study focuses entirely on gene carriers, which removes many of the difficulties.
It is still uncertain whether HNPCC individuals have a higher prevalence of adenomas than the general population. In one study, HNPCC individuals were found to have more adenomas than a reference population.13 The significance of the hyperplastic polyp (metaplastic polyp) in HNPCC is also still unknown. In one study, the prevalence of hyperplastic polyps in HNPCC during follow-up was 23%, and a correlation between the occurrence of adenomas and hyperplastic polyps was found.14
In a randomized phase III trial (the Colorectal Adenoma/Carcinoma Prevention Programme 2 [CAPP2] chemoprevention trial; results not yet published), the target was to recruit 1,000 proven MMR carriers, although some persons were included on the basis of their family history and personal medical history being consistent with HNPCC. The analysis presented here is based on those individuals with a proven germline MMR mutation. Information obtained from all of the recruits' first ever colonoscopy was reviewed with the aim of describing the prevalence of adenomas and hyperplastic polyps across age groups and to evaluate the association between these two types of polyps.
PATIENTS AND METHODS
Study Design
CAPP2 is a multicenter chemoprevention trial attempting to reduce the incidence of neoplasia in persons with HNPCC (participants are randomly assigned in a 2 × 2 factorial design to 600 mg aspirin and/or 30 g of resistant starch with placebo controls for a 2- to 4-year treatment period). Recruitment is restricted to individuals classified as HNPCC, which is defined as either a detected pathologic mutation in one of the MMR genes (MSH2, MLH1, or MSH6) or an HNPCC-related neoplasm in a patient whose family fulfills either the Amsterdam I or the modified Amsterdam II Criteria.16 All trial participants are at least 25 years old and have an essentially intact colon. The exclusion criteria include pregnancy, contraindications for aspirin, already taking nonsteroidal anti-inflammatory drugs or corticosteroids, or severe comorbid disease including HIV. We use the term study to indicate the cross-sectional analysis reported here and the term trial to indicate CAPP2.
This study is based on persons randomly assigned within the CAPP2 trial with a proven germline mutation; participants in the trial with a personal and family history consistent with HNPCC but without a proven mutation are excluded from this study because one of their trial inclusion criteria (having an adenoma) is the end point of this study. The records of the first ever colonoscopy of the persons with a proven germline MMR mutation have been examined to identify the prevalence of lesions in this cohort.
Patients
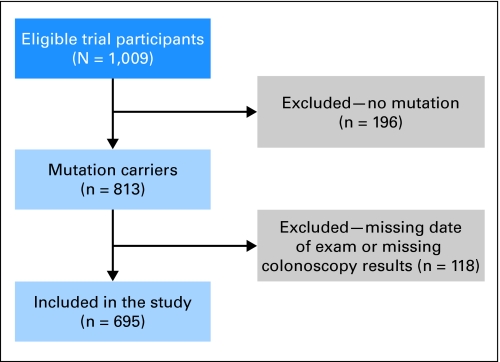
Enrollment for the CAPP2 trial started in 1998 and ended in 2006. Thirty centers from 15 countries recruited 1,009 participants, of whom 813 had a proven germline MMR mutation (in MSH2, MLH1, or MSH6) at recruitment. At recruitment, various baseline questions were asked including a question regarding previous bowel screening. This question asked about the date of the first colonoscopic screening, the clinical facility in which the procedure was performed, and the outcome of that procedure including the presence or absence of polyps. For many individuals, the clinical facility performing this endoscopic examination was different than that at which the recruitment to CAPP2 took place, so that efforts were made to contact the initial facility and retrieve the results of the endoscopic examination. Information about the number, size, and location of adenomas and hyperplastic polyps found at recruits' first ever colonoscopy were abstracted and analyzed as a cross-sectional study. Those persons with complete information are the focus of this study. Furthermore, because we could not retrieve records for all participants, we also performed a subset analysis involving the persons whose endoscopic examination took place in the same facility as the CAPP2 recruitment. For these individuals, we were confident that all relevant details of the examination had been identified. This analysis addressed the concern that we might be more likely to retrieve records for persons with bowel pathology than for persons without. Such a bias would have resulted in inaccurate estimates of prevalence. Figure 1 shows a flow diagram of the inclusion/exclusion criteria.
Fig 1.
Flow diagram of inclusion/exclusion criteria.
Colonoscopy
For the purpose of the present study, information about the recruits' first ever colonoscopy results was retrieved from medical records. For some individuals, this colonoscopy was the entry examination for the CAPP2 trial, whereas for others, the examination occurred before consideration of involvement in CAPP2. Polyp characteristics were taken from the medical records of the reporting department. These characteristics included histology, anatomic location, and size as reported in the clinical and pathologic notes. In the description of the location of the adenomas and hyperplastic polyps, the different parts of colon were defined as follows: proximal colon included the cecum, the ascending colon, the hepatic flexure, the transverse colon, and the splenic flexure; and the distal colon included the colon distal to the splenic flexure and to the end of the rectum.
Statistical Methods
Score tests were used to test for the trend of increasing odds of an adenoma and a hyperplastic polyp with increasing age at first colonoscopy (age considered as a four-category factor). The difference in mean age at first colonoscopy between mutation types (MLH1, MSH2, or MSH6) was analyzed by analysis of variance. The difference in mean age at first colonoscopy between adenoma (yes or no) and hyperplastic polyp (yes or no) was analyzed by the t test. The variable for age at first colonoscopy was normally distributed. The associations between type of mutation and adenoma prevalence and between type of mutation and hyperplastic polyp prevalence were analyzed by the χ2 test. Univariate and multivariate logistic regression was used to estimate crude and adjusted odds ratios, including 95% CIs, for the binary outcomes of adenoma present (yes or no) and hyperplastic polyp present (yes or no). Potential confounders were age and sex. The association analyses of the adenomas and hyperplastic polyps and the relationship between size of adenomas and hyperplastic polyps were analyzed by χ2 tests. All tests were two-sided, and a P < .05 was considered to indicate statistical significance. Calculations were performed with the statistical package STATA 8.0 (STATA Corp, College Station, TX). For the purpose of examining the prevalence of lesions across the large number of participating centers, tests of homogeneity were conducted taking the centers contributing more than 30 participants separately from all other centers and then grouping all other centers within a continent into a further group.
Ethics
All participating centers received approval from their regional/national ethical research committees, and signed consent was obtained from all recruits.
RESULTS
The CAPP2 Trial recruited 813 persons known to have a germline MMR mutation at that time; complete information on the date of each person's first colonoscopy and the results of that colonoscopy were retrieved. Failure to retrieve complete information excluded that person from this analysis, except that persons were included if the medical record indicated that pathologic review had not been possible or if only the size or location of the lesion was not recorded. Persons with colorectal cancer were predominantly (but not entirely) excluded from participation in the CAPP2 trial and thus are not included in this study. Basic demographics for the study population by age group are listed in Table 1 and demonstrate that there are more females (n = 374) than males (n = 321) in this study group. Table 1 includes classification by age at first colonoscopic examination; although participants in the CAPP2 trial were at least 25 years of age, their first colonoscopy could have occurred before this age. The mean age at first colonoscopy was 38.9 years of age (standard deviation [SD], 10.3 years). Pathologic information was available on 94 lesions classified as adenomatous polyps and 53 lesions classified as hyperplastic polyps. Fourteen lesions were of unknown histology predominantly because the specimen was too small for pathologic examination; these lesions are not included in the analyses. There were more persons with an MLH1 mutation (n = 433) than with an MSH2 mutation (n = 245; Table 2
Table 1.
Prevalence of Adenomas and Hyperplastic Polyps in Different Age Groups
| Characteristic | Whole Mutation-Positive Study Population* (N = 695) |
Age Group |
P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16–34 Years (n = 278) |
35–44 Years (n = 243) |
45–54 Years (n = 121) |
55–75 Years (n = 53) |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Sex | |||||||||||
| Male | 321 | 139 | 108 | 49 | 25 | ||||||
| Female | 374 | 139 | 135 | 72 | 28 | ||||||
| Age at first colonoscopy, years | |||||||||||
| Male | |||||||||||
| Mean | 38.2 | ||||||||||
| SD | 10.1 | ||||||||||
| Female | |||||||||||
| Mean | 39.4 | ||||||||||
| SD | 10.5 | ||||||||||
| No. of adenomas | 94 | 17 | 37 | 28 | 12 | ||||||
| Persons with at least one adenoma | 74 | 14 | 31 | 19 | 10 | .0001† | |||||
| Prevalence of persons with at least one adenoma, % | 10.6 | 5.0 | 12.8 | 15.7 | 18.9 | ||||||
| 95% CI | 8.5 to 13.2 | 2.8 to 8.3 | 8.8 to 17.6 | 9.7 to 23.4 | 9.4 to 32.0 | ||||||
| No. of adenomas | |||||||||||
| 0 | 621 | 89.4 | 264 | 95.0 | 212 | 87.2 | 102 | 84.3 | 43 | 81.1 | |
| 1 | 61 | 8.8 | 12 | 4.3 | 27 | 11.1 | 14 | 11.6 | 8 | 15.1 | |
| 2 or more | 13 | 1.9 | 2 | 0.7 | 4 | 1.6 | 5 | 4.1 | 2 | 3.8 | |
| No. of hyperplastic polyps | 53 | 13 | 23 | 12 | 5 | ||||||
| Persons with at least one hyperplastic polyp | 37 | 10 | 15 | 8 | 4 | .1† | |||||
| Prevalence of persons with at least one hyperplastic polyp, % | 5.3 | 3.6 | 6.2 | 6.6 | 7.5 | ||||||
| 95% CI | 3.8 to 7.3 | 1.7 to 6.5 | 3.5 to 10.0 | 2.9 to 12.6 | 2.1 to 18.2 | ||||||
| No. of hyperplastic polyps | |||||||||||
| 0 | 658 | 94.7 | 268 | 96.4 | 228 | 93.8 | 113 | 93.4 | 48 | 92.5 | |
| 1 | 28 | 4.0 | 9 | 3.2 | 11 | 4.5 | 5 | 4.1 | 3 | 5.7 | |
| 2 or more | 9 | 1.3 | 1 | 0.4 | 4 | 1.6 | 3 | 2.5 | 1 | 1.8 | |
Abbreviation: SD, standard deviation.
Age range: 16.4 to 75.4 years.
Score test for trend.
Table 2.
Prevalence of Adenomas and Hyperplastic Polyps in MHL1, MSH2, and MSH6 Mutation Carriers
| Characteristic |
MLH1 (n = 433) |
MSH2 (n = 245) |
MSH6 (n = 17) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Sex | |||||||
| Male | 188 | 123 | 10 | ||||
| Female | 245 | 122 | 7 | ||||
| Age at first colonoscopy, years | .5* | ||||||
| Mean | 38.9 | 38.7 | 41.9 | ||||
| SD | 10.7 | 9.8 | 9.2 | ||||
| No. of adenomas | 59 | 32 | 3 | ||||
| Persons with at least one adenoma | 47 | 24 | 3 | .6† | |||
| Prevalence of persons with at least one adenoma, % | 10.9 | 9.8 | 17.6 | ||||
| 95% CI | 8.1 to 14.2 | 6.4 to 14.2 | 3.8 to 43.4 | ||||
| No. of adenomas | |||||||
| 0 | 386 | 89.2 | 221 | 90.2 | 14 | 82.4 | |
| 1 | 40 | 9.2 | 18 | 17.3 | 3 | 17.6 | |
| 2 or more | 7 | 1.6 | 6 | 2.4 | 0 | 0 | |
| No. of hyperplastic polyps | 22 | 24 | 7 | ||||
| Persons with at least one hyperplastic polyp | 16 | 16 | 5 | .001† | |||
| Prevalence of persons with at least one hyperplastic polyp, % | 3.7 | 6.5 | 29.4 | ||||
| 95% CI | 2.1 to 5.9 | 3.8 to 10.4 | 10.3 to 56.0 | ||||
| No. of hyperplastic polyps | |||||||
| 0 | 417 | 96.3 | 229 | 93.5 | 12 | 70.6 | |
| 1 | 13 | 3.0 | 11 | 4.5 | 4 | 23.5 | |
| 2 or more | 3 | 0.7 | 5 | 2.0 | 1 | 5.9 | |
Abbreviation: SD, standard deviation.
One-way analysis of variance.
χ2 test with 2 df.
All analyses have been repeated for the persons whose first endoscopic examination occurred in the same facility as the CAPP2 trial recruitment. Because point estimates vary little from those of the total group, we restrict the presentation to the total group of 695 persons.
Prevalence of Adenomas
There were no differences in the prevalence of adenomas by recruitment center; thus for all analyses, data were pooled across all sites (P = .16 for homogeneity; data not shown). In the whole study population, the mean age of persons with adenomas was 43.9 years (SD, 9.1 years), whereas a lower mean age of 38.3 years (SD, 10.3 years) was found in persons without adenomas (t test, P < .0001). The prevalence of having an adenoma at first colonoscopy was 10.6% (74 of 695 persons; 95% CI, 8.5% to 13.2%; Table 1). Thirteen (17.6%) of the 74 persons had multiple adenomas. Increasing prevalence of adenomas was found by increasing age (P = .0001; Table 1), with the prevalence ranging from 5.0% (95% CI, 2.8% to 8.3%) among persons age 16 to 34 years at first colonoscopy to 18.9% (95% CI, 9.4% to 32.0%) among persons age 55 to 75 years. There were no differences regarding prevalence of adenomas between persons harboring mutations in MLH1, MSH2, or MSH6 (P = .6; Table 2).
Prevalence of Hyperplastic Polyps
There were no differences in the prevalence of hyperplastic polyps by recruitment center, so analyses were pooled across all centers (P = .8 for homogeneity; data not shown). The mean age of individuals with and without hyperplastic polyps was 41.3 years (SD, 10.5 years) and 38.8 years (SD, 10.3 years), respectively (t test, P = .14). The prevalence of having a hyperplastic polyp was 5.3% (n = 37) in the whole study population (95% CI, 3.8% to 7.3%); nine of the 37 persons had multiple hyperplastic polyps. No increased prevalence of hyperplastic polyps was found by increasing age (P = .1; Table 1). More persons with an MSH6 mutation had a hyperplastic polyp than MSH2 or MLH1 carriers (P = .001; Table 2).
Predictors for the Presence of an Adenoma Among Mutation Carriers
We investigated the independent predictors of having one or more adenomas at first colonoscopy. In univariate and multivariate regression models, age, sex, and the presence of at least one hyperplastic polyp were tested (Table 3). Age at first colonoscopy was associated in both the univariate and multivariate analyses; sex was not associated in either analysis; and the presence of a hyperplastic polyp had an effect in univariate analysis, but the adjustment for age removed some of this effect.
Table 3.
Univariate and Multivariate Analyses Regarding Adenomas and Hyperplastic Polyps at First Colonoscopy
| Factor | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P | |
| Adenoma | ||||||
| Age* | 1.05 | 1.03 to 1.07 | < .001 | 1.05 | 1.03 to 1.07 | < .001 |
| Sex | 1.26 | 0.78 to 2.04 | .35 | 1.32 | 0.81 to 2.16 | .27 |
| Hyperplastic polyp: yes v no | 2.47 | 1.09 to 5.64 | .03 | 2.22 | 0.95 to 5.13 | .07 |
| Hyperplastic polyp | ||||||
| Age* | 1.02 | 0.99 to 1.05 | .15 | 1.02 | 0.99 to 1.05 | .25 |
| Sex | 1.24 | 0.64 to 2.41 | .52 | 1.24 | 0.64 to 2.42 | .53 |
| Adenoma: yes v no | 2.47 | 1.09 to 5.64 | .03 | 2.22 | 0.96 to 5.14 | .06 |
Age at first colonoscopy by year.
Predictors for the Presence of Hyperplastic Polyps
The presence of an adenoma was significantly associated with having a hyperplastic polyp at first colonoscopy in univariate analysis, but neither age nor sex modified this effect (Table 3). Adjustment for age in the multivariate analysis again reduced some of the effect of having an adenoma.
Association Between Adenomas and Hyperplastic Polyps
Table 4 lists the actual distribution of persons by presence/absence of an adenoma and/or hyperplastic polyp among tested positive mutation carriers (P = .03). This association was marginally significant in the multivariate regression analysis in which the presence of a hyperplastic polyp was a predictor for the detection of an adenoma after adjusting for age and sex (P = .07; Table 3).
Table 4.
Association of Adenoma and Hyperplastic Polyp at First Colonoscopy Among Mutation Carriers
| Adenoma at First Colonoscopy | Hyperplastic Polyp at First Colonoscopy | χ2 | P | ||
|---|---|---|---|---|---|
| No (No.) | Yes (No.) | % | |||
| No | 592 | 29 | 4.7 | 4.9 | .03 |
| Yes | 66 | 8 | 10.8 | ||
Distribution and Size
In the whole study population, size data were available for 77 of 94 adenomas and 46 of 53 hyperplastic polyps. Forty-five of the adenomas (58.4%) and 14 of the hyperplastic polyps (30.4%) were at least 5 mm in maximum dimension (P = .003 for equal distribution). Location data were available for 87 of 94 adenomas and 48 of 53 hyperplastic polyps. For adenomas, 41% were in the proximal colon, 33% were in the distal colon, and 25% were in the rectum. For hyperplastic polyps, 29% were in the proximal colon, 33% were in the distal colon, and 38% were in the rectum. There was a marginal nonsignificant excess of adenomas in the proximal bowel (41%) compared with hyperplastic polyps (29%), which were evenly distributed across the proximal bowel, distal bowel, and rectum.
DISCUSSION
To our knowledge, this is the largest sample of HNPCC individuals that has been evaluated regarding the prevalence of adenomas and hyperplastic polyps. We found that the most important predictive factor for the discovery of an adenoma in such individuals is age, which is in accordance with earlier studies.8–10,14,17–19
There are a number of features to this study that complement the existing literature. First, this is the largest study; and second, to our knowledge, this is the first study to be entirely based on proven gene mutation carriers. Previous studies have had to rely on personal medical history of those with significant family histories, and this process is likely to have significant misclassification because persons with suspected HNPCC may not have a germline MMR mutation or persons with a germline mutation may not be identified by family history. By dealing only with persons with a positive genetic test, individuals can be identified asymptomatically and indeed before any visible neoplasia development, giving a clearer indication of HNPCC natural history.
There are major challenges in estimating the prevalence of neoplasia in mutation carriers. First, persons attending screening clinics who have been identified clinically may well have more neoplasia than the average mutation carrier because it is the occurrence of neoplasia in their relatives that suggests clinical referral. Second, attendance at screening clinics and also willingness to participate in the CAPP2 trial will each have inherent biases that are impossible to quantify. Are persons with an adenoma more or less likely to participate in the CAPP2 trial? However, although some issues are difficult to address because of the potential biases, others are less of an issue, such as the prevalence of hyperplastic polyps.
There are only a few reports about the prevalence of hyperplastic polyps in HNPCC. The finding of 5.3% prevalence of hyperplastic polyps in carriers is in accordance with a study14 in which the prevalence was 9.3% in 108 individuals (known and unknown mutation status). The significance of the hyperplastic polyp as a marker for increased risk for colorectal cancer is still uncertain, but with the small number of hyperplastic polyps reported in this population, the predictive effect is limited. Right-sided large hyperplastic polyps could develop into sporadic microsatellite instability–positive cancer.20–22 The marginal results of an association (P < .07) between adenomas and hyperplastic polyps shown in Tables 3 and 4 is in accordance with another study.14 However, in another study, no correlation was found between the presence or location of hyperplastic polyps and adenomas in HNPCC (n = 50 mutation carriers),23 but this study sample included a significantly smaller number of mutation carriers than our study.
One of the most interesting biologic questions regarding the natural history of HNPCC is the number of polyps in mutation carriers compared with noncarriers. However, persons with proven or suspected HNPCC are given colonoscopies at ages at which the general population are not offered routine surveillance using colonoscopy, which implies that there are few population-based data to compare with this study. Thus, one of the few opportunities is to compare the incidence of polyps found in autopsy studies. Of course, the comparison of the prevalence of adenomas and hyperplastic polyps from this study with autopsy studies17,24,25 needs to be interpreted with extreme caution. The detection rate of polyps in autopsy studies is optimized by time-independent careful inspection of all mucosal surfaces,26 and the miss rate of small adenomas in colonoscopy, through back-to-back and other study methodologies, is reported to be 15% to 27%.27,28 Accordingly, the prevalence of adenomas detected by colonoscopy may be lower than the prevalence detected in the same patients if autopsy is performed because the measurement techniques are not equivalent. Comparing the adenoma prevalence in this study with three autopsy studies17,24,25 suggests that adenoma prevalence is consistent with the adenoma prevalence in the general population. If we attempt to match the participants in this study with those reported in the three reference autopsy populations, we find approximately 30% lower rates among the CAPP participants. Hyperplastic polyps were noticeably less common in this study compared with the autopsy studies. There are several possible explanations for a lower reported prevalence of hyperplastic polyps in the present study compared with the three reference studies17,24,25 but a similar prevalence to an HNPCC patient population.14 The information about the hyperplastic polyps was collected retrospectively. In addition, some colonoscopists may not report small, distal, flat hyperplastic polyps, which may well have been recorded in the autopsy studies, and/or these small lesions may simply have been missed.
Two weaknesses of our study deserve discussion. First, the CAPP2 trial could not collect baseline lifestyles and dietary characteristics of participants for logistical reasons. However, two of the major interests, aspirin and resistant starch, are the focus of the CAPP2 trial; results will be available soon. Second, a pathologic review of all polyps was not feasible. Evidence from the intervention phase of the CAPP2 trial suggests that there is considerable uniformity in the pathologic classification because review by a single pathologist (J.J.) agreed more than 95% with the local pathologists (data not shown).
In conclusion, the wide range of prevalence of adenomas was primarily associated with age at first colonoscopy. Future follow-up results from the CAPP2 cohort will provide information on the incidence of new colonic polyps.
Acknowledgment
CAPP2 is hosted by the University of Newcastle upon Tyne, 1 Park Terrace, Newcastle upon Tyne, NE1 7RU United Kingdom.
Footnotes
Supported by European Union European Commission Directorate General XII Science, Research and Development–Joint Research Centre Life Sciences Technologies Medical Research, Brussels, Belgium; Cancer Research UK, London, United Kingdom; Medical Research Council, London, United Kingdom; Bayer AG GB Consumer Care Medizin Gebäude, Leverkusen, Germany; National Starch and Chemical Company Natural Polymer Research, Bridgewater, NJ; Cancer Council Victoria, Carlton, Victoria, Australia; Swiss Institute for Applied Cancer Research, Coordinating Centre, Bern, Switzerland; Newcastle upon Tyne Hospitals, National Health Services Trust Special Trustees, Freeman Hospital, Newcastle upon Tyne, United Kingdom; Nilsson-Ehle Foundation in Sweden; and Finnish Cancer Foundation and Sigrid Juselius Foundation in Finland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Annelie Liljegren, Bo Nilsson, John Mathers, John Burn, D. Timothy Bishop
Administrative support: Gail Barker
Provision of study materials or patients: Annelie Liljegren, Lucio Bertario, Marie Luise Bisgaard, Diana Eccles, Gareth Evans, Finlay Macrae, Eamonn Maher, Annika Lindblom, Jukka-Pekka Mecklin, Gabriela Möslein, Jeremy Jass, Riccardo Fodde, John Burn
Collection and assembly of data: Gail Barker, John Burn, D. Timothy Bishop
Data analysis and interpretation: Annelie Liljegren, Faye Elliott, Samuel Rotstein, Bo Nilsson, John Mathers, John Burn, D. Timothy Bishop
Manuscript writing: Annelie Liljegren, John Burn, D. Timothy Bishop
Final approval of manuscript: Annelie Liljegren, John Burn, D. Timothy Bishop
REFERENCES
- 1.Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 2.Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 3.Miyaki M, Konishi M, Tanaka K, et al. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 5.Lynch HT, Smyrk T, Jass JR. Hereditary nonpolyposis colorectal cancer and colonic adenomas: Aggressive adenomas? Semin Surg Oncol. 1995;11:406–410. doi: 10.1002/ssu.2980110607. [DOI] [PubMed] [Google Scholar]
- 6.Love RR, Morrissey JF. Colonoscopy in asymptomatic individuals with a family history of colorectal cancer. Arch Intern Med. 1984;144:2209–2211. [PubMed] [Google Scholar]
- 7.Mecklin JP, Jarvinen HJ, Aukee S, et al. Screening for colorectal carcinoma in cancer family syndrome kindreds. Scand J Gastroenterol. 1987;22:449–453. doi: 10.3109/00365528708991489. [DOI] [PubMed] [Google Scholar]
- 8.Houlston RS, Murday V, Harocopos C, et al. Screening and genetic counselling for relatives of patients with colorectal cancer in a family cancer clinic. BMJ. 1990;301:366–368. doi: 10.1136/bmj.301.6748.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanspa SJ, Lynch HT, Smyrk TC, et al. Colorectal adenomas in the Lynch syndromes: Results of a colonoscopy screening program. Gastroenterology. 1990;98:1117–1122. doi: 10.1016/0016-5085(90)90323-s. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson SV, Harocopos C, Gaglia P. Screening results in a family cancer clinic: Five years experience. Anticancer Res. 1993;13:2581–2585. [PubMed] [Google Scholar]
- 11.Jass JR, Stewart SM, Stewart J, et al. Hereditary non-polyposis colorectal cancer: Morphologies, genes and mutations. Mutat Res. 1994;310:125–133. doi: 10.1016/0027-5107(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF, Taal BG, Nagengast FM, et al. Hereditary nonpolyposis colorectal cancer: Results of long-term surveillance in 50 families. Eur J Cancer. 1995;31A:1145–1148. doi: 10.1016/0959-8049(95)00249-i. [DOI] [PubMed] [Google Scholar]
- 13.Lindgren G, Liljegren A, Jaramillo E, et al. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut. 2002;50:228–234. doi: 10.1136/gut.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liljegren A, Lindblom A, Rotstein S, et al. Prevalence and incidence of hyperplastic polyps and adenomas in familial colorectal cancer: Correlation between the two types of colon polyps. Gut. 2003;52:1140–1147. doi: 10.1136/gut.52.8.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dove-Edwin I, de Jong AE, Adams J, et al. Prospective results of surveillance colonoscopy in dominant familial colorectal cancer with and without Lynch syndrome. Gastroenterology. 2006;130:1995–2000. doi: 10.1053/j.gastro.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas: A necropsy study in New Zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaglia P, Atkin WS, Whitelaw S, et al. Variables associated with the risk of colorectal adenomas in asymptomatic patients with a family history of colorectal cancer. Gut. 1995;36:385–390. doi: 10.1136/gut.36.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponz de Leon M, Della Casa G, Benatti P, et al. Frequency and type of colorectal tumors in asymptomatic high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:639–641. [PubMed] [Google Scholar]
- 20.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–1313. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 21.Jass JR, Iino H, Ruszkiewicz A, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43–49. doi: 10.1136/gut.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid A, Houlihan PS, Booker S, et al. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology. 2000;119:323–332. doi: 10.1053/gast.2000.9361. [DOI] [PubMed] [Google Scholar]
- 23.Rijcken FE, van der Sluis T, Hollema H, et al. Hyperplastic polyps in hereditary nonpolyposis colorectal cancer. Am J Gastroenterol. 2003;98:2306–2311. doi: 10.1111/j.1572-0241.2003.07629.x. [DOI] [PubMed] [Google Scholar]
- 24.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 25.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: A necropsy study in Liverpool. Gut. 1982;23:835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd RL, Boggs HW, Jr, Slagle GW, et al. Reliability of colonoscopy. Dis Colon Rectum. 1989;32:1023–1025. doi: 10.1007/BF02553873. [DOI] [PubMed] [Google Scholar]
- 27.Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990;82:1769–1772. doi: 10.1093/jnci/82.22.1769. [DOI] [PubMed] [Google Scholar]
- 28.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]