Abstract
Purpose
The PI3K/Akt/mTOR pathway is activated in the majority of pancreatic cancers, and inhibition of this pathway has antitumor effects in preclinical studies. We performed a multi-institutional, single-arm, phase II study of RAD001(everolimus), an oral inhibitor of mTOR, in patients who experienced treatment failure on first-line therapy with gemcitabine.
Patients and Methods
Thirty-three patients with gemcitabine-refractory, metastatic pancreatic cancer were treated continuously with RAD001 at 10 mg daily. Prior treatment with fluorouracil in the perioperative setting was allowed. Patients were observed for toxicity, treatment response, and survival.
Results
Treatment with single-agent RAD001 was well-tolerated; the most common adverse events were mild hyperglycemia and thrombocytopenia. No patients were removed from the study because of drug-related adverse events. No complete or partial treatment responses were noted, and only seven patients (21%) had stable disease at the first restaging scans performed at 2 months. Median progression-free survival and overall survival were 1.8 months and 4.5 months, respectively. One patient (3%) had a biochemical response, defined as ≥ 50% reduction in serum CA19-9.
Conclusion
Although well-tolerated, RAD001 administered as a single-agent had minimal clinical activity in patients with gemcitabine-refractory, metastatic pancreatic cancer. Future studies in metastatic pancreatic cancer should assess the combination of mTOR inhibitors with other agents and/or examine inhibitors of other components of the PI3K/Akt/mTOR pathway.
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States.1 More than 95% of patients with pancreatic cancer will ultimately develop metastatic disease, yet traditional cytotoxic agents have little therapeutic efficacy. Initial treatment with gemcitabine has demonstrated modest improvements in cancer-related symptoms and survival.2 Multiple other chemotherapeutic agents have been added to gemcitabine, without clear therapeutic benefit.3-9 Recently, the addition of erlotinib, an inhibitor of the epidermal growth factor receptor, to gemcitabine led to a statistically significant improvement in overall survival, yet median survival remained approximately 6 months.10 After treatment failure of a gemcitabine-containing regimen, the utility of second-line therapy is unclear, with no generally accepted standard of care.11
A strong need exists to investigate novel therapeutics that exploit the molecular basis of pancreatic cancer. The vast majority of pancreatic ductal adenocarcinomas harbor activating mutations in K-RAS, which promote cellular proliferation and survival through engagement of several downstream effector pathways, including the PI3K/Akt/mTOR pathway.12,13 Increased activation of the PI3K/Akt/mTOR pathway has been noted in approximately half of pancreatic cancers14-19 and has been associated with a poorer prognosis.14,16 In preclinical models of pancreatic cancer, inhibition of this pathway has demonstrated antitumor activity.20-25
To evaluate whether downstream inhibition of the PI3K/Akt/mTOR pathway is safe and effective in patients with pancreatic cancer, we initiated a multi-institutional, nonrandomized, phase II study of RAD001 (everolimus), an oral small-molecule inhibitor of mTOR, in patients with gemcitabine-refractory, metastatic pancreatic cancer.
PATIENTS AND METHODS
Patient Population
The study population consisted of patients with histologically confirmed, metastatic pancreatic ductal adenocarcinoma who had experienced treatment failure with prior gemcitabine-based chemotherapy. Patients may have received prior fluorouracil-based perioperative therapy with or without external-beam radiotherapy. Patients were excluded if they received more than one prior chemotherapy regimen for the treatment of metastatic disease. Participating centers included Brigham and Women's Hospital, Dana-Farber Cancer Institute, and Massachusetts General Hospital (all in Boston, MA). The study was approved by the institutional review boards of the participating institutions, and all patients provided informed consent.
Patients were further required to have measurable disease (by Response Evaluation Criteria in Solid Tumors [RECIST]); Eastern Cooperative Oncology Group performance status of 1 or better; life expectancy of at least 12 weeks; and adequate renal function (serum creatinine ≤ 1.5), hepatic function (serum bilirubin ≤ 1.5× the upper limit of normal [ULN] and AST/ALT ≤ 2.5× the ULN or ≤ 5× the ULN if there was evidence of liver metastases), bone marrow function (absolute neutrophil count ≥ 1,500 μL; platelets ≥ 100,000 μL; hemoglobin ≥ 9.0 g/dL), and coagulation parameters (International Normalized Ratio ≤ 1.3). If the marker lesion was previously irradiated, evidence of progression after radiation was required.
Patients were excluded if they had another malignancy (other than basal cell or squamous cell cancer of the skin), uncontrolled CNS metastases or carcinomatous meningitis, uncontrolled concomitant medical illnesses (eg, diabetes mellitus, hypertension, severe infection, congestive heart failure, ventricular arrhythmia, symptomatic coronary artery disease, or myocardial infarction within the last 6 months), or any of the following within 2 weeks of enrollment: major surgery, radiotherapy, or systemic anticancer treatment. Patients who were pregnant or lactating, chronically receiving immunosuppressant therapy, receiving treatment doses of a vitamin K antagonist, or had a history of HIV were excluded from study entry.
Treatment Program
RAD001 was administered continuously at a dose of 10 mg daily by mouth until disease progression, unacceptable toxicity, or withdrawal of consent. Four weeks of study drug was considered to be one cycle of treatment. For grade 3 to 4 hematologic or grade 3 nonhematologic toxicity, RAD001 was interrupted and supportive management was instituted. RAD001 was reinitiated with a 50% dose reduction if resolution of toxicity to less than grade 2 occurred within 14 days; otherwise, treatment was discontinued. Treatment was also discontinued for grade 4 nonhematology toxicity, grade 2 or higher pneumonitis, or continued toxicity after reinstitution of RAD001 at 2.5 mg daily.
On-study evaluations included toxicity assessments and measurement of peripheral-blood counts and a full chemistry panel every other week. Lipid panel and serum CA19-9 were measured monthly. Patients were evaluated with computed tomography every 8 weeks; response and progression were evaluated using RECIST by independent radiologic review.
Statistical Methods
The study was designed with a primary end point of progression-free survival (PFS), defined as the time from study entry to documentation of progressive disease or death from any cause. On the basis of prior studies of second-line treatment in metastatic pancreatic cancer, we estimated that such treatment has been associated with a median PFS of 2 months. Our study design used a one-stage design with a target accrual of 35 eligible patients, with the assumption that an improvement in PFS at 2 months from 50% to 71% would warrant further study in this patient population. The secondary objectives of the study were to assess toxicity, tumor response rate, biochemical response rate (defined as ≥ 50% reduction in serum CA19-9), and overall survival. Overall survival was defined as the time from study entry until death from any cause.
RESULTS
A total of 33 eligible patients were enrolled between January 2007 and March 2008. All patients received at least 1 week of study drug and are included in our toxicity and efficacy analyses. Baseline characteristics of the study population are listed in Table 1. The median age of this patient population was 61 years; 55% were male and 45% were female. As anticipated in a second-line study, most patients were symptomatic from their disease; only 24% had an ECOG performance status of 0, and 76% had a performance status of 1. Four patients (12%) had undergone surgery, and three of these patients had received subsequent adjuvant chemoradiotherapy. One patient received neoadjuvant chemoradiotherapy, but was not able to undergo subsequent resection. All 33 patients had received prior therapy with a gemcitabine-containing regimen for metastatic disease.
Table 1.
Baseline Patient Characteristics (N = 33)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 61 | |
| Range | 39-78 | |
| Sex | ||
| Male | 18 | 55 |
| Female | 15 | 45 |
| ECOG performance status | ||
| 0 | 8 | 24 |
| 1 | 25 | 76 |
| Location of metastases* | ||
| Lymph nodes | 12 | 36 |
| Liver | 32 | 97 |
| Lungs | 14 | 24 |
| Prior surgical resection | ||
| Whipple procedure | 3 | 9 |
| Distal pancreatectomy | 1 | 3 |
| Prior perioperative chemotherapy† | 4 | 12 |
| Prior perioperative radiotherapy† | 4 | 12 |
| Prior treatment regimens for metastatic disease | ||
| Gemcitabine | 15 | 45 |
| Gemcitabine/erlotinib | 8 | 24 |
| Gemcitabine/oxaliplatin | 2 | 6 |
| Gemcitabine/sunitinib‡ | 1 | 3 |
| Gemcitabine/erlotinib/bevacizumab‡ | 6 | 18 |
| Gemcitabine/erlotinib/cisplatin | 1 | 3 |
| Serum CA19-9, U/mL | ||
| Median | 4,299 | |
| Range | 3-149,096 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Does not sum to 100%, as patients may have metastases to more than one location.
One patient received preoperative chemoradiotherapy, but was not able to undergo subsequent tumor resection. Three patients received adjuvant chemoradiotherapy.
Received first-line therapy on a clinical trial.
Patient compliance with self-administration of the study drug was good; only two patients reported missing more than one dose of RAD001 while on study. One patient reported missing one dose during cycle 1 and three doses during cycle 2, whereas a second patient reported missing four doses during cycle 1 and one dose during cycle 2.
Treatment-related adverse events are listed in Table 2. Overall, treatment with RAD001 was well-tolerated. Thrombocytopenia and hyperglycemia were the most common grade 3 or 4 treatment-related toxicities, observed in 12% and 18% of patients, respectively. Patients were not required to fast before blood draws; the average peak glucose in those patients with grade 3 hyperglycemia was 297 mg/dL (range, 256 to 413 mg/dL). Other adverse events included anemia, neutropenia, fatigue, oral mucositis/stomatitis, and nausea. Lymphopenia was the only grade 4 adverse event noted and occurred in a single patient.
Table 2.
Treatment-Related Adverse Events
| Adverse Event | Maximum Grade
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic | ||||||||
| Lymphocytes | 4 | 12 | 4 | 12 | 1 | 3 | 1 | 3 |
| Neutrophils | 5 | 15 | — | 3 | 9 | — | ||
| Hemoglobin | 9 | 27 | 8 | 24 | — | — | ||
| Platelets | 8 | 24 | 6 | 18 | 4 | 12 | — | |
| Nonhematologic | ||||||||
| Hypokalemia | 7 | 21 | — | 2 | 6 | — | ||
| Hyperglycemia* | 5 | 15 | 11 | 33 | 6 | 18 | — | |
| ALT | 3 | 9 | — | 1 | 3 | — | ||
| AST | 9 | 27 | 1 | — | — | |||
| Hypercholesterolemia | 6 | 18 | — | 1 | 3 | — | ||
| Other | ||||||||
| Fatigue | 10 | 30 | 6 | 18 | 3 | 9 | — | |
| Nausea | 14 | 42 | — | 1 | 3 | — | ||
| Vomiting | 3 | 9 | 1 | 3 | 1 | 3 | — | |
| Oral mucositis/stomatitis | 8 | 24 | 1 | 3 | 1 | 3 | — | |
| Anorexia | 3 | 9 | 2 | 6 | — | — | ||
| Diarrhea | 6 | 18 | 2 | 6 | — | — | ||
| Constipation | 2 | 6 | 3 | 9 | — | — | ||
Serum glucose was not required to be measured after an overnight fast.
Eleven patients (33%) required delays in treatment of 7 to 14 days, but were able to restart therapy with RAD001 at a reduced dose. The reasons for treatment delays were hyperglycemia (four patients), thrombocytopenia (three patients), neutropenia (two patients), and nausea or vomiting (two patients). Three patients (9%) had treatment with RAD001 held because of grade 3 oral mucositis, neutropenia, or thrombocytopenia, but did not restart treatment as a result of progressive disease.
The majority of patients (67%) were removed from study because of progressive disease, documented by imaging studies performed at or before the 2-month evaluation. Three patients were removed from study before the 2-month follow-up because of withdrawal of consent without documented progression by RECIST guidelines. One patient was removed after only 8 days of treatment as a result of worsening of a perineal abscess requiring surgical intervention. No patient was removed from the study because of a treatment-related adverse event.
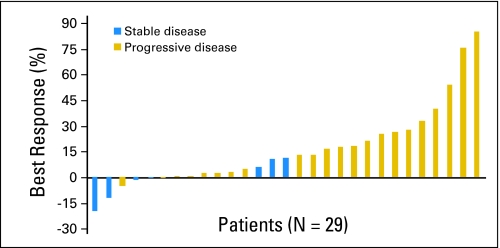
No complete or partial responses by RECIST guidelines were noted (Table 3). Seven patients (21%) had stable disease at the 2-month follow-up imaging study, of which only one patient (3%) continued to have stable disease at 4 months. One patient with stable disease also had a reduction in serum CA19-9 by greater than 50%. Best overall percentage change in target lesion measurement from baseline was available for 29 patients (Fig 1). Only two patients (6%) had evidence of meaningful tumor regression (20% and 12% reduction from baseline, respectively), yet both patients demonstrated progressive disease by the 4-month evaluation. Among all 33 patients, the median PFS time was 1.8 months. To date, 29 patients (88%) have died, and median overall survival time for the entire study population was 4.5 months.
Table 3.
Tumor Response Among Patients Receiving RAD001
| Disease Response | No. | % |
|---|---|---|
| Complete or partial response | 0 | 0 |
| Stable disease, months | 7 | 21 |
| > 2 | 6 | 18 |
| > 4 | 1 | 3 |
| Progressive disease | 22 | 67 |
| Not assessable* | 4 | 12 |
Three patients were removed from study because of withdrawal of consent without documented progressive disease by Response Evaluation Criteria in Solid Tumors guidelines. One patient was removed from study after 8 days of treatment because of worsening of a preexisting perirectal fistula, requiring surgical intervention.
Fig 1.
Best overall percentage change from baseline in target lesion measurement by Response Evaluation Criteria in Solid Tumors guidelines. Note: Eleven patients had progressive disease as a result of the development of new lesions, rather than growth of the target lesions by ≥ 20%.
DISCUSSION
Considerable evidence supports an important role for the PI3K/Akt/mTOR pathway in pancreatic cancer biology.13 Point mutations in K-RAS are an early molecular event in the progression of normal pancreatic ducts to ductal adenocarcinoma.12,26 These mutations lead to constitutive activation of the K-RAS protein, and subsequently, the activation of several downstream intracellular pathways, including the RAF/MAPK, PI3K/Akt/mTOR, and Ral GDS pathways.13 In addition, excess energy balance, as noted with obesity and a sedentary lifestyle, increases pancreatic cancer risk27,28 and leads to activation of the PI3K/Akt/mTOR pathway upstream through the insulin and insulin-like growth factor receptors29 and at the level of mTOR by energy and nutrient availability.30 When activated by these mechanisms, the PI3K/Akt/mTOR pathway provides important downstream signaling that promotes cellular proliferation, survival, and neoangiogenesis.31 In preclinical studies, inhibitors of PI3K, Akt, and mTOR have demonstrated antitumor activity in pancreatic cancer cells, both alone and in combination with other agents, suggesting their possible utility in patients with pancreatic cancer.20-25 Therefore, there is a strong rationale to examine inhibitors of mTOR in patients with pancreatic cancer.
In this multi-institutional, single-arm phase II study, the oral mTOR inhibitor RAD001 was successfully administered to patients with gemcitabine-refractory, metastatic pancreatic cancer with modest toxicity. When necessary, treatment delays and dose reductions were due primarily to resultant grade 3 hyperglycemia and thrombocytopenia. Nonetheless, RAD001 as a single agent failed to demonstrate meaningful clinical activity in this patient population, with no objective treatment responses and relatively brief median PFS and overall survival times.
Traditional chemotherapeutic agents have limited efficacy in patients with metastatic pancreatic cancer.2,10 After these patients experience progressive disease on a gemcitabine-containing regimen, appropriate second-line therapy is poorly defined.11 Several second-line studies of cytotoxic agents have demonstrated median survival times of 3 to 7 months.32-38 Recently, we reported that the combination of capecitabine and erlotinib in patients with gemcitabine-refractory disease had an overall response rate of 10%, a median PFS of 3.4 months, and median survival time of 6.5 months.39 In contrast, in the current study of RAD001 conducted at the same institutions and for the same indication, we observed no objective responses, a median PFS of 1.8 months, and median overall survival of 4.5 months.
In phase I studies, 10 mg of daily RAD001 has demonstrated the ability to inhibit mTOR activity in peripheral mononuclear cells, skin cells, and tumors, as measured by abrogated phosphorylation of downstream target proteins.40-42 In addition, these studies have suggested that once-daily dosing may result in more profound and persistent inhibition of mTOR activity than other schedules of administration. In the current study, patient compliance with oral RAD001 was good, with only two of 33 patients reporting missing more than a single dose of the drug. Therefore, inconsistent administration of drug or lack of target inhibition seems to be less likely reasons for the ineffectiveness of RAD001 in this patient population.
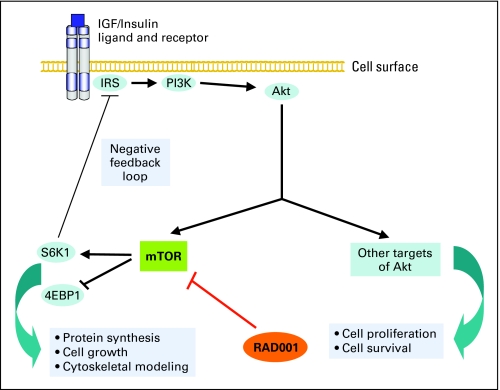
In recent years, the complexity of the PI3K/Akt/mTOR pathway has become increasingly apparent, particularly in relation to its potential as a therapeutic target in cancer.43,44 Recent data suggest the presence of a negative feedback loop, whereby increased activation of mTOR leads to a physiologic brake on further stimulation of this pathway (Fig 2).45-48 In some tumor types, mTOR inhibitors may interfere with this inhibitory feedback, resulting in a paradoxical increase in signaling by PI3K and increased activation of other Akt-target proteins that promote cell survival. Although the loss of this negative feedback from mTOR inhibition may limit the efficacy of single-agent mTOR inhibitors in these tumor types, it also supports the investigation of treatment regimens that combine mTOR inhibitors with other agents, such as inhibitors of PI3K and upstream receptor tyrosine kinases.
Fig 2.
Schematic representation of the PI3K/Akt/mTOR pathway. Binding of insulin or IGF to transmembrane receptors leads to activation mTOR via PI3K and Akt. Activation of mTOR promotes protein synthesis, cell growth, and cytoskeletal modeling, important factors stimulating malignant progression and spread. A possible mechanism of tumor cell resistance to mTOR inhibition in some tumor types may be the abrogation of negative feedback on the PI3K/Akt/mTOR pathway by S6K1, a target protein of mTOR. IGF, insulin-like growth factor; IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase; Akt, also known as protein kinase B; mTOR, mammalian target of rapamycin; S6K1, ribosomal S6 kinase 1; 4EBP1, eukaryotic translation initiation factor 4E binding protein.
In conclusion, daily RAD001 administered as a single agent had minimal clinical activity in patients with gemcitabine-refractory, metastatic pancreatic cancer. Nonetheless, given substantial preclinical data implicating activation of the PI3K/Akt/mTOR pathway in pancreatic cancer, this pathway remains an interesting target in the treatment of patients with this disease. To realize the potential of this strategy, future studies of mTOR inhibitors will likely need to assess the combination of these agents with drugs that inhibit upstream components of the PI3K/Akt/mTOR pathway. Concurrent work will be necessary to verify target inhibition and investigate potential mechanisms of resistance in patients with this difficult to treat disease.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Charles S. Fuchs, Genentech, AstraZeneca, Roche Research Funding: Jennifer A. Chan, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Brian M. Wolpin, Aram F. Hezel, Charles S. Fuchs
Financial support: Brian M. Wolpin, Charles S. Fuchs
Administrative support: Brian M. Wolpin, Brittany Allen, Charles S. Fuchs
Provision of study materials or patients: Brian M. Wolpin, Aram F. Hezel, Thomas Abrams, Lawrence S. Blaszkowsky, Jeffrey A. Meyerhardt, Jennifer A. Chan, Peter C. Enzinger, Jeffrey W. Clark, David P. Ryan, Charles S. Fuchs
Collection and assembly of data: Brian M. Wolpin, Brittany Allen, Charles S. Fuchs
Data analysis and interpretation: Brian M. Wolpin, Aram F. Hezel, Charles S. Fuchs
Manuscript writing: Brian M. Wolpin, Charles S. Fuchs
Final approval of manuscript: Brian M. Wolpin, Aram F. Hezel, Thomas Abrams, Lawrence S. Blaszkowsky, Jeffrey A. Meyerhardt, Jennifer A. Chan, Peter C. Enzinger, Brittany Allen, Jeffrey W. Clark, David P. Ryan, Charles S. Fuchs
published online ahead of print at www.jco.org on December 1, 2008
Supported by Novartis Pharmaceuticals and Grants No. P01 CA87969, P01 CA55075, P50 CA127003, R01 CA124908, and T32 CA09001 from the National Cancer Institute, National Institutes of Health.
Previously presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00409292
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58:71-96, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Burris HA 3rd, Moore MJ, Andersen J, et al: Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 15:2403-2413, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Berlin JD, Catalano P, Thomas JP, et al: Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270-3275, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Heineman V, Quietzsh D, Gieseler F, et al: A phase III trial comparing gemcitabine plus cisplatin vs. gemcitabine alone in advanced pancreatic carcinoma. Proc Am Soc Clin Oncol 22:251, 2003. (abstr 1003) [Google Scholar]
- 5.Rocha Lima CM, Green MR, Rotche R, et al: Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 22:3776-3783, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Louvet C, Labianca R, Hammel P, et al: Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509-3516, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Herrmann R, Bodoky G, Ruhstaller T, et al: Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Res and the Central European Cooperative Oncology Group. J Clin Oncol 25:2212-2217, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kindler H, Niedzwiecki D, Hollis D, et al: A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB). J Clin Oncol 25:199s, 2007. (suppl; abstr 4508) [Google Scholar]
- 9.Philip PA, Benedetti J, Fenoglio-Preiser C, et al: Phase III study of gemcitabine [G] plus cetuximab [C] versus gemcitabine in patients [pts] with locally advanced or metastatic pancreatic adenocarcinoma [PC]: SWOG S0205 study. J Clin Oncol 25:199s, 2007. (suppl; abstr LBA4509) [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al: Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960-1966, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Boeck S, Heinemann V: Second-line therapy in gemcitabine-pretreated patients with advanced pancreatic cancer. J Clin Oncol 26:1178-1179; author reply 1179, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Almoguera C, Shibata D, Forrester K, et al: Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53:549-554, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Hezel AF, Kimmelman AC, Stanger BZ, et al: Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20:1218-1249, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Schlieman MG, Fahy BN, Ramsamooj R, et al: Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer 89:2110-2115, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altomare DA, Tanno S, De Rienzo A, et al: Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem 87:470-476, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Tomita Y, Hoshida Y, et al: Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res 10:2846-2850, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Asano T, Yao Y, Zhu J, et al: The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene 23:8571-8580, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Velculescu VE: Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 3:1221-1224, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Maitra A, Hruban RH: A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell 8:171-172, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Cheng JQ, Ruggeri B, Klein WM, et al: Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A 93:3636-3641, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perugini RA, McDade TP, Vittimberga FJ Jr, et al: Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res 90:39-44, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Bondar VM, Sweeney-Gotsch B, Andreeff M, et al: Inhibition of the phosphatidylinositol 3`-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther 1:989-997, 2002 [PubMed] [Google Scholar]
- 23.Bruns CJ, Koehl GE, Guba M, et al: Rapamycin-induced endothelial cell death and tumor vessel thrombosis potentiate cytotoxic therapy against pancreatic cancer. Clin Cancer Res 10:2109-2119, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Asano T, Yao Y, Zhu J, et al: The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun 331:295-302, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Ito D, Fujimoto K, Mori T, et al: In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer 118:2337-2343, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Moskaluk CA, Hruban RH, Kern SE: P16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 57:2140-2143, 1997 [PubMed] [Google Scholar]
- 27.Michaud DS, Giovannucci E, Willett WC, et al: Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 286:921-929, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al: Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 294:2872-2878, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Pollak MN, Schernhammer ES, Hankinson SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505-518, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Reiling JH, Sabatini DM: Stress and mTORture signaling. Oncogene 25:6373-6383, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Manning BD, Cantley LC: Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 4:257-262, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Oettle H, Arnold D, Esser M, et al: Paclitaxel as weekly second-line therapy in patients with advanced pancreatic carcinoma. Anticancer Drugs 11:635-638, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Ulrich-Pur H, Raderer M, Verena Kornek G, et al: Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer 88:1180-1184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantore M, Rabbi C, Fiorentini G, et al: Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology 67:93-97, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Tsavaris N, Kosmas C, Skopelitis H, et al: Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs 23:369-375, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Reni M, Pasetto L, Aprile G, et al: Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer. Br J Cancer 94:785-791, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demols A, Peeters M, Polus M, et al: Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: A phase II study. Br J Cancer 94:481-485, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boeck S, Weigang-Kohler K, Fuchs M, et al: Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: A multicenter phase II trial. Ann Oncol 18:745-751, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Kulke MH, Blaszkowsky LS, Ryan DP, et al: Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 25:4787-4792, 2007 [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell A, Faivre S, Burris HA 3rd, et al: Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588-1595, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Tabernero J, Rojo F, Calvo E, et al: Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603-1610, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Tanaka C, O'Reilly T, Kovarik JM, et al: Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 26:1596-1602, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Corradetti MN, Guan KL: Upstream of the mammalian target of rapamycin: Do all roads pass through mTOR? Oncogene 25:6347-6360, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Sabatini DM: MTOR and cancer: Insights into a complex relationship. Nat Rev Cancer 6:729-734, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Yan H, Frost P, et al: Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4:1533-1540, 2005 [DOI] [PubMed] [Google Scholar]
- 46.O'Reilly KE, Rojo F, She QB, et al: MTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500-1508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Cosimo S, Scaltriti D, Val D, et al: The PI3-K/AKT/mTOR pathway as a target for breast cancer therapy. J Clin Oncol 25:140s, 2007. (abstr 3511) [Google Scholar]
- 48.Sun SY, Rosenberg LM, Wang X, et al: Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65:7052-7058, 2005 [DOI] [PubMed] [Google Scholar]