Abstract
Purpose
To analyze the Radiation Therapy Oncology Group (RTOG) database of patients with glioblastoma and appraise whether outcome was influenced by time to initiation of radiation therapy (RT).
Patients and Methods
From 1974 through 2003, adult patients with histologically confirmed supratentorial glioblastoma were enrolled onto 16 RTOG studies. Of 3,052 enrolled patients, 197 patients (6%) were either initially rendered ineligible or had insufficient chronologic data, leaving a cohort of 2,855 patients for the present analysis. We selected four patient groups based on the interval from surgery to the start of RT: ≤ 2 weeks, 2 to 3 weeks, 3 to 4 weeks, more than 4 weeks to the protocol eligibility limit of 6 weeks. Survival times were estimated by the Kaplan-Meier method. Multivariate analysis incorporated variables of time interval, recursive partitioning analysis (RPA) class, and treatment regimen.
Results
No decrement in survival could be identified with increasing time to initiation of RT. Among our four temporal groupings, median survival time was unexpectedly and significantly greater in the group with the longest interval (> 4 weeks) than in those with the shortest delay (≤ 2 weeks): respectively, 12.5 months versus 9.2 months (P < .0001). On multivariate analysis, with overall survival as the end point, time interval more than 4 weeks and lower RPA class were both significant predictors of improved outcome. Treatment regimen was not a significant factor.
Conclusion
There is no evident reduction in survival by delaying initiation of RT within the relatively narrow constraint of 6 weeks. An unanticipated yet significantly superior outcome was identified for patients for whom RT was delayed beyond 4 weeks from surgery.
INTRODUCTION
Randomized trials have consistently shown the value of radiotherapy as part of the optimal management of glioblastoma multiforme (GBM).1 Even modern series continue to show a statistically significant advantage in overall survival when radiotherapy alone is compared with best supportive care in various populations.2 With its emergence as a standard of care for GBM, more attention has been devoted toward optimizing the delivery of radiotherapy.3–5
A generally recognized, straightforward means of maximizing the efficacy of cancer treatments is the prompt initiation of such therapies. Indeed, among the first principles of oncology is the expeditious inauguration of cytotoxic therapy.6 Because delay would be expected to have the most detrimental effect on the control of neoplasms with short doubling times,7 patients with rapidly growing tumors such as GBM are theoretically the most vulnerable to negative consequences from delayed initiation of radiotherapy.
The relationship between the delay in radiotherapy and the outcome of radiotherapy has been explored8–13 in several tumor types (eg, breast, head and neck cancer) and less extensively in others (eg, lung, cervix cancer).14,15 To date, only one single-institutional experience has specifically addressed the delayed initiation of irradiation for GBM.16 The current study was undertaken to explore this relationship by analyzing the database of the Radiation Therapy Oncology Group (RTOG), which provides prolonged follow-up from patients treated at multiple centers throughout the United States and Canada.
PATIENTS AND METHODS
Patient Population
Patients entered onto RTOG trials for biopsy-proven GBM constitute the study group for this article. These trials accrued 3,052 patients between 1974 and 2003. Primary treatment outcome reports from these trials have been previously published.3–5,17–21 Eligibility criteria were consistent in all of the studies: histologically confirmed supratentorial GBM; age of at least 18 years; normal hepatic, renal, and bone marrow function; and an interval of 6 weeks or less from surgery to initiation of radiotherapy. Ineligibility criteria included prior malignancies (except skin carcinomas), prior chemotherapy, or head and neck irradiation.
Protocol Summaries
The data for the present secondary analysis were culled from 16 studies. The treatment regimens of the trials included in this analysis are concisely described in Table 1. Additional information is included on the official Web site of the RTOG (www.rtog.org).
Table 1.
RTOG Study Details
| Study No. | Phase | Treatments | Total No. of Patients | No. of Patients With GBM |
|---|---|---|---|---|
| RTOG 7401/ | III | 1. 60 Gy whole-brain RT | 639 | 449 |
| ECOG 1374 | 2. 60 Gy whole-brain RT plus a 10 Gy RT boost dose | |||
| 3. 60 Gy whole-brain RT plus BCNU 80 mg/m2/d × 1 every 8 weeks | ||||
| 4. 60 Gy whole-brain RT plus MeCCNU (125 mg/m2/d × 1 every 8 weeks) and DTIC (150 mg/m2/d × 5 every 4 weeks) | ||||
| RTOG 7918 | III | 1. 60 Gy whole-brain RT and BCNU | 318 | 247 |
| 2. 60 Gy whole-brain RT and BCNU with radiosensitizer misonidazole at a dose of 2.5 mg/m2 before RT each Monday | ||||
| BCNU 80 mg/m2 IV days 3, 4, and 5 of first week of radiotherapy, then BCNU 80 mg/m2 IV × 3 days every 8 weeks beginning day 64 | ||||
| RTOG 8302 | I/II | A dose-escalation trial of hyperfractionated partial-brain RT and accelerated hyperfractionated partial-brain RT with carmustine. Four RT dose levels of hyperfractionated partial brain RT were studied in 1.2 Gy twice-daily fractionation with an interfraction interval of 4to 8 hours. These dose levels were 64.8, 72.0, 76.8, and 81.6 Gy. The final portion of the study was a randomization between the total accelerated hyperfractionated partial-brain RT dose of 48.0 and 54.4 Gy in 1.6 Gy twice-daily fractionation with the same interfraction interval requirements | 786 | 570 |
| RTOG 8409 | I/II | Combined conventional doses of RT with the quinone AZQ (15 mg/m2 once weekly for 4 weeks) | 54 | 46 |
| RTOG 9006 | III | 1. Conventional RT plus BCNU | 712 | 534 |
| 2. Hyperfractionated RT (72 Gy in 1.2 Gy fractions administered twice daily) plus BCNU | ||||
| BCNU 80 mg/m2 IV days 1, 2, and 3 of RT then every 8 weeks for a total of 6 cycles | ||||
| RTOG 9305 | III | 1. Conventional RT plus BCNU | 203 | 203 |
| 2. Conventional RT plus BCNU with upfront radiosurgical boost (tumor size-dependent dosing raging from 15 Gy to 24 Gy) | ||||
| BCNU 80 mg/m2 IV days 1, 2, and 3 of RT then every 8 weeks for a total of 6 cycles | ||||
| RTOG 9411 | II | Accelerated hyperfractionated RT (64.0 or 70.4 Gy) plus BCNU (80 mg/m2 days 1, 2, and 3 of RT and repeated on days 56, 57, and 58 then every 8 weeks for 4 cycles for a total of 6 cycles) | 108 | 108 |
| RTOG 9417 | II | Conventional RT with intravenously administered tirapazamine (159 mg/m2 or 260 mg/m2) | 124 | 124 |
| RTOG 9513 | II | Cranial RT plus topotecan (1.5 mg/m2 per day IV for 3 days/wk every 3 weeks for 3 cycles) | 87 | 87 |
| RTOG 9602 | II | Conventional RT plus weekly paclitaxel (225 mg/m2/3 hours/wk × 6) | 62 | 62 |
| RTOG 9710 | II | Conventional RT followed by recombinant β-interferon (6 million U intramuscularly administered 3 times per week; 3 weeks on drug, 1 week off drug) | 109 | 109 |
| RTOG 9803 | I/II | Conformal RT to doses of 66 Gy, 72 Gy, 78 Gy, or 84 Gy with BCNU (80 mg/m2 days 1, 2, and 3 of RT and repeated on days 56, 57, and 58 then every 8 weeks for 4 cycles for a total of 6 cycles) | 209 | 209 |
| RTOG 9806 | II | Conventional doses of RT with incremental increases of thalidomide starting at 200 mg/d with escalations to a maximal dose of 1,200 mg | 128 | 128 |
| RTOG 0013 | II | Using conventional RT followed by intra-tumoral bleomycin that was delivered with a refillable sustained device | 19 | 19 |
| RTOG 0021 | II | Conventional RT plus high dose tamoxifen (escalated from 20 mg/d to 80 mg/d) | 77 | 77 |
| RTOG 0023 | II | 50 Gy of external-beam RT in 2 Gy fractions followed by a stereotactic RT boost (4 treatments of 5 Gy for tumors > 40 mm or 7 Gy for tumors ≤ 40 mm once per week during weeks 3-6) along with BCNU (80 mg/m2 IV for 3 days, beginning within 1 month after completion of RT then every 8 weeks for a total of 6 cycles) | 80 | 80 |
Abbreviations: RTOG, Radiation Therapy Oncology Group; GBM, glioblastoma multiforme; ECOG, Eastern Cooperative Oncology Group; RT, radiation therapy; BCNU, carmustine; MeCCNU, 1-(2-Chloroethyl)-3-(4-Methylcyclohexyl)-1-Nitrosourea; IV, intravenously; AZQ, diaziquone.
Statistical Methods
The analyses are based on the data used for manuscripts or presentations. Because a new treatment standard for GBM (as manifest by a statistically significant survival advantage attributable to any given therapeutic arm) did not emerge from the respective studies, there was justification in pooling the data. Survival was measured from the date of study registration to the date of death or last follow-up, and survival rates were estimated using the Kaplan-Meier method.22 The log-rank test was used to compare survival between the interval groups.23 Outcome was assessed with the aid of the recursive partitioning technique (a method of building decision trees to model predictors) that was previously published by the RTOG.24 The cutoff points used to define the groups (ie, intervals of ≤ 2 weeks, > 2 to 3 weeks, > 3 to 4 weeks, > 4 weeks) were selected based on the distribution of intervals using percentiles (25%, 50%, 75%). A Cox proportional hazards model was also performed on this database using the variables common to all of the clinical trials that were included in the analysis.25
RESULTS
Patient Characteristics
Within the RTOG database for GBM, 3,052 patients were deemed suitable for the current analysis. Of these, 78 were rendered ineligible because of incomplete outcome data. In 119 cases, the chronology of treatment could not be determined (ie, the date of surgery was unknown for 70 patients; the initiation date of radiotherapy was unknown for 49 patients). Accordingly, 2,855 patients were included in this analysis.
Table 2 lists the pretreatment characteristics for the patients studied as a function of the interval from surgery to the start of radiotherapy. Relationships between pretreatment characteristics (performance status [Karnofsky Score/Zubrod scale], neurologic function, mental status, type of surgical procedure [biopsy, partial resection, total resection], and recursive partitioning analysis [RPA]class), and the time interval were assessed with χ2 tests and revealed statistically significant associations between them (P < .0001).
Table 2.
Pretreatment Characteristics
| Characteristic | Interval From Surgery to Start of Radiation |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 2 Weeks (n = 756) |
> 2 to 3 Weeks(n = 805) |
> 3 to 4 Weeks (n = 757) |
> 4 Weeks (n = 537) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Days from surgery to start of radiation | ||||||||
| Median | 12 | 19 | 26 | 33 | ||||
| Range | 1-14 | 15-21 | 22-28 | 29-73 | ||||
| Age, years | ||||||||
| Median | 57 | 56 | 57 | 55 | ||||
| Range | 14-83 | 19-83 | 18-86 | 17-82 | ||||
| Dose, Gy | ||||||||
| No. of patients with dose | 494 | 676 | 672 | 424 | ||||
| Median | 60 | 60 | 60 | 60 | ||||
| Range | 1.7-82.87 | 6-85.68 | 2-86 | 2-84.32 | ||||
| Performance score* | ||||||||
| Karnofsky | ||||||||
| 20-50 | 77 | 11 | 36 | 4 | 21 | 2 | 4 | < 1 |
| 60 | 82 | 11 | 77 | 10 | 65 | 9 | 30 | 6 |
| 70 | 120 | 16 | 132 | 16 | 97 | 13 | 69 | 13 |
| 80 | 170 | 22 | 185 | 23 | 174 | 23 | 115 | 21 |
| 90 | 176 | 23 | 248 | 31 | 263 | 35 | 189 | 35 |
| 100 | 31 | 4 | 68 | 8 | 72 | 10 | 62 | 12 |
| Zubrod | ||||||||
| 0 | 12 | 2 | 16 | 2 | 35 | 5 | 36 | 7 |
| 1 | 36 | 5 | 29 | 4 | 21 | 3 | 26 | 5 |
| 2 | 31 | 4 | 9 | 1 | 4 | < 1 | 2 | < 1 |
| 3 | 16 | 2 | 5 | 1 | 4 | < 1 | 4 | < 1 |
| 4 | 5 | < 1 | 0 | 0 | 1 | < 1 | 0 | 0 |
| Prior surgery | ||||||||
| Biopsy | 223 | 29 | 178 | 22 | 124 | 16 | 94 | 18 |
| Partial resection | 399 | 53 | 436 | 54 | 426 | 56 | 290 | 54 |
| Total resection | 114 | 15 | 167 | 21 | 190 | 25 | 140 | 26 |
| Other | 15 | 2 | 26 | 2 | 9 | 1 | 10 | 2 |
| Unknown | 5 | < 1 | 8 | 1 | 8 | 1 | 3 | < 1 |
| Neurologic impairment | ||||||||
| None/minor | 290 | 38 | 421 | 52 | 470 | 62 | 375 | 70 |
| Moderate | 351 | 46 | 318 | 40 | 257 | 34 | 151 | 28 |
| Severe | 107 | 14 | 61 | 8 | 29 | 4 | 9 | 2 |
| Unknown/missing | 8 | 1 | 5 | 1 | 1 | 0 | 2 | 0 |
| Mental status | ||||||||
| Normal function | 395 | 52 | 495 | 61 | 486 | 64 | 348 | 65 |
| Minor mental confusion | 294 | 39 | 248 | 31 | 199 | 26 | 125 | 23 |
| Gross confusion | 41 | 5 | 26 | 3 | 21 | 3 | 7 | 1 |
| Rousable with difficulty | 4 | 1 | 2 | < 1 | 0 | 0 | 0 | 0 |
| Unknown/missing | 22 | 3 | 34 | 4 | 51 | 7 | 57 | 11 |
| RPA stage | ||||||||
| III | 83 | 11 | 129 | 16 | 119 | 16 | 113 | 21 |
| IV | 246 | 32 | 317 | 39 | 366 | 48 | 271 | 50 |
| V | 298 | 39 | 274 | 34 | 215 | 28 | 124 | 23 |
| VI | 127 | 17 | 82 | 10 | 53 | 7 | 28 | 5 |
| Unknown | 2 | < 1 | 3 | < 1 | 4 | < 1 | 1 | < 1 |
Abbreviation: RPA, recursive partitioning analysis.
Karnofsky was collected on studies 9806, 9803, 9710, 9602, 9513, 9417, 9411, 9305, 9006, 8409, 8302, and 7918; Zubrod was collected on studies 0023, 0021, and 0013; both Karnofsky and Zubrod were collected for study 7401 (for that study, Karnofsky performance score is reported where available).
Outcome Data
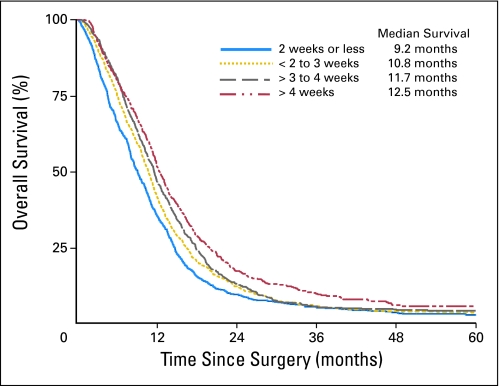
Figure 1 displays the overall survival with regard to the four interval (ie, time between surgery and initiation of radiotherapy) groups. The median survival time of those with the longest interval (ie, > 4 weeks) was significantly greater than the median survival time of the group with the shortest interval (ie, ≤ 2 weeks; 12.5 months v 9.2 months; P < .0001). When this was examined by RPA stage, only RPA V showed the same trend.
Fig 1.
Overall survival by surgery-to-radiation therapy time interval groups.
Twenty-one patients (0.7%) were enrolled at more than 6 weeks from time of surgery (average, 7.2 weeks; range, 6.1 to 10.4 weeks; median, 6.7 weeks). This number was too small to calculate any significant difference or trend as a separate interval group; however, interestingly, the average survival time for this group was higher than expected at 15 months.
Multivariate analysis of overall survival including time intervals (interval of > 4 weeks) and RPA stage showed that both variables are statistically significant factors with respect to overall survival (Table 3). Treatment effects (whole-brain radiation therapy arms v radiation therapy arm and radiation therapy alone arms v radiation therapy plus radiosensitizers or chemotherapy arms), interaction terms with intervals, and RPA classes were also added to the multivariate analysis, and no significant effects were found.
Table 3.
Cox Proportional Hazards Model for Overall Survival
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Time from surgery to start of RT, weeks | |||
| ≤ 2 | RL | — | |
| > 2-3 | 0.97 | 0.87 to 1.07 | .49 |
| > 3-4 | 0.91 | 0.82 to 1.01 | .07 |
| > 4 | 0.84 | 0.75 to 0.95 | .004 |
| RPA | |||
| III | RL | — | |
| IV | 1.72 | 1.53 to 1.93 | < .0001 |
| V | 2.74 | 2.42 to 3.10 | < .0001 |
| VI | 4.39 | 3.74 to 5.14 | < .0001 |
Abbreviations: HR, hazard ratio; RT, radiation therapy; RL, reference level; RPA, recursive partitioning analysis.
Table 4 was constructed to determine whether there was a disproportionate representation of progression during treatment or shortly thereafter among those who initiated radiotherapy within shorter intervals. No statistically significant differences in the rates of progression during treatment or in the month immediately after completion of radiation were observed across the four intervals (≤ 2 weeks, > 2 to 3 weeks, > 3 to 4 weeks, and > 4 weeks) studied.
Table 4.
Progression Period if Progressed
| Progression | Interval From Surgery to Start of Radiation |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 2 Weeks (n = 488) |
> 2 to 3 Weeks (n = 596) |
> 3 to 4 Weeks (n = 587) |
> 4 Weeks (n = 415) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| During radiation | 45 | 9 | 47 | 8 | 35 | 6 | 22 | 5 |
| Within 30 days of radiation end | 82 | 17 | 70 | 12 | 89 | 15 | 63 | 15 |
| After 30 days of radiation end | 361 | 74 | 479 | 80 | 463 | 79 | 330 | 80 |
DISCUSSION
The issue of prolonged waiting time for radiotherapy has been underscored in the literature for nearly two decades. The problem has now reached a state of crisis even in certain modern countries that enjoy a high quality of life by Western standards, such as Canada and Australia.26,27 Longer waiting times are a source of anxiety among patients and health care professionals because of the presumed deleterious effect of delay on tumor control.
The impact of delayed initiation of radiotherapy after surgery has been extensively studied in two settings: carcinoma of the breast as well as head and neck cancer.28 In the case of breast cancer, the most common interval studied is 8 weeks between surgery (usually lumpectomy) and the first administration of radiotherapy. In most of the reported studies,29–33 5-year locoregional recurrence rates in women treated with postoperative irradiation begun more than 8 weeks after surgery (approximately 9%) are significantly higher than those women treated within 8 weeks of surgery (approximately 6%). In several studies,32,33 there also seemed to be an increased rate of distant metastases among women who received postoperative irradiation initiated more than 8 weeks after surgery. Among patients with unresected cancers of the head and neck, 1-month delays in the initiation of radiotherapy tended to increase the risk of local recurrence at 5 years.12,13,34 For head and neck cancers managed with primary surgery followed by postoperative irradiation,35–38 there was a higher probability of locoregional recurrence in patients treated by irradiation begun more than 6 weeks after the operation. In one study,14 actuarial 5-year survival rates were 61%, 46%, and 30% for patients with non–small-cell lung cancer who underwent radiation at 1 to 6 weeks, at 7 to 8 weeks, and at more than 8 weeks after surgery, respectively (P = .046). Although the impact of delayed thoracic radiation has been studied in the context of small-cell and non–small-cell lung cancer, it is difficult to draw conclusions from the literature as a result of the common use of sequential regimens that interpose chemotherapy between surgery and radiotherapy.39
To date, only one report has specifically examined the impact of delayed radiotherapy among patients with high-grade glioma.16 In that study, three variables were identified that had a negative impact on survival: older age, reduced radiation dose, and delayed time from presentation to the radiation department until initiation of radiotherapy, where the hazard of death increased by 2% per day. Several caveats are worth underscoring. First, this report studied grade 3 as well as grade 4 gliomas. Although it was customary in the past to combine these two entities (even in some RTOG trials),3,5,17,19 it is likely that they behave as distinctly separate diseases. Second, the authors did not find a detrimental effect on survival with increased time interval between surgery and commencement of radiation; however, interestingly, they did find a significant effect of decreased survival in patients with longer waiting intervals between presentation to the radiation department and the initiation of radiation treatment. The RTOG database did not track the data point of presentation to the RT department. Third, these observations were made at a single institution. Part of the robustness of the RTOG database is derivative of the vast numbers of patients with retrievable follow-up information and the wide cross-section of medical centers participating, theoretically creating a better reflection of the broader reality. Finally, the RTOG mandated the relatively prompt initiation of treatment as an eligibility criterion for study participation (ie, maximal 6-week wait). The much wider window tolerated by Do et al (range, 1 to 62 days) may have had a negative impact on survival in certain cases.
Notwithstanding, it is noteworthy that our results do not comport with trends recognized in the experience with carcinoma of the breast and head and neck cancer that are outlined above. As well, a recent report that assessed neoadjuvant temozolomide before radiotherapy for newly diagnosed patients with GBM was, by admission of the authors, inferior to standard concomitant radiotherapy plus temozolomide.40 Whether this poor result by Chinot et al reflects a suboptimal radiation–drug interaction, a cohort that had an overrepresentation of patients expressing high levels of methylguanine methyltransferase,41 or simply the consequence of introducing up to a 4-month delay secondary to the induction regimen is unclear.
Although 16 trials comprise the current report, none of these studies were designed a priori to address the specific issue of interval to initiation of radiation therapy after neurosurgical intervention. In fact, the ethical legitimacy of such a protocol design may be dubious at best, and it is unlikely that physicians would marshal the equipoise to conduct such a study. Thus despite the methodologic appeal of designing a prospective randomized trial to directly assess the impact of timing of radiotherapy, it is unlikely that such an effort will ever be mounted. As such, caution must be exercised in assessing whether a true effect of interval prolongation (either beneficial or detrimental) actually exists. Furthermore, although approximately 3,000 patients were entered into the current analysis, it is unclear that the retrospective nature of the review allowed sufficient power to exclude the possibility that delays in initiating radiotherapy may have had small but clinically meaningful deleterious effects.
The danger inherent in the delay of radiotherapy for patients with GBM seemed axiomatic. GBM is known to have a short doubling time.42 In addition, like many tumors, GBM tends to invade locally, and the probability of achieving control is expected to decline as the tumor size increases.7 Accordingly, we were surprised to see that our data did not lend support to this expectation and even yielded a result that was counterintuitive.
It is difficult to propose a plausible mechanism for an association between delayed therapy and improved survival in the treatment of GBM. We explored an alternative explanation, which posits that we were detecting a pragmatic epiphenomenon rather than a true biologic reality. Physician's intuition may have lead to expedited treatment for those patients who looked particularly fragile. If indeed, such patients went on to experience treatment failure quickly then we have simply used exotic statistical techniques to validate the astute judgment of clinicians who selected patients for prompt treatment on the basis of their clinical judgment that therapeutic intervention was required expeditiously. In other words, given the finite resources in many systems, physicians may have chosen to hasten the initiation of treatment for patients with the most advanced tumors when circumstances did not allow all patients to be treated with equal immediacy. As seen in Table 2, there is a larger number of patients with Karnofsky Performance Score of 70 or less/Zubrod score of 3 to 4 in the group radiated earlier; likewise, there is an overrepresentation of patients undergoing biopsy in the groups of patients who underwent radiation earlier.
A surrogate means of checking the hypothesis that physicians treated patients with poorer prognostic factors differently was probed, as displayed in Table 4, by evaluating for early tumor progression as correlating with earlier initiation of radiation therapy. However, in assessing these data, it is impossible to conclude that the more aggressive tumors (as manifest by a propensity to recur during therapy or within a month after completion) were overrepresented in the groups with the shortest intervals between surgery and initiation of radiotherapy.
Finally, there may be a detrimental effect to the injured organ (the brain) when treated with radiation too soon after the primary insult of surgery. Hypoxia from surgical manipulation and edema in the immediate postoperative period may diminish radiosensitivity.
Data from a study using rat models to examine brain surgery followed by radiation at differing onsets (also using controls without radiation) suggests that early initiation of radiation (within 1 to 2 weeks) after surgery, compared with 3 weeks or more, may result in higher levels of tissue damage.43
In summary, within the relatively narrow temporal limits (6 weeks) of initiating radiotherapy after surgery that were permitted by the RTOG in the trials reviewed, we were unable to uncover a disadvantage associated with delayed radiotherapy. However, it is difficult to construct a rationale for the conscious implementation of such delays. Additionally, treatment initiated beyond 6 weeks postoperatively may well be detrimental, but it is beyond the scope of our observations, and it is not feasible to design a prospective trial to test this hypothesis.
Although we are disinclined to recommend deliberately forestalling radiotherapy among patients suffering from GBM, physicians may be able to reassure those patients who are waiting for treatment to commence that cancer control is unlikely to be compromised so long as the guidelines proposed by the RTOG investigators are respected.
Acknowledgment
We thank Diana Nelson, MD, and Steven A. Leibel, MD (deceased), for their contributions to this work.
Footnotes
Supported by Radiation Therapy Oncology Group (D.T.B.).
Presented at the 11th Annual Meeting of the Society of Neuro-Oncology, November 15-19, 2006, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Deborah T. Blumenthal, Schering-Plough (C), Eisai (C) Stock Ownership: None Honoraria: Deborah T. Blumenthal, Schering-Plough Educational Forum Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Deborah T. Blumenthal, Jeff M. Michalski, C. Leland Rogers, Benjamin W. Corn
Financial support: Walter J. Curran
Administrative support: Minesh P. Mehta, Walter J. Curran, Jeff M. Michalski
Provision of study materials or patients: Walter J. Curran, Luis Souhami, Jeff M. Michalski, Benjamin W. Corn
Collection and assembly of data: Minhee Won, Walter J. Curran, Jeff M. Michalski
Data analysis and interpretation: Deborah T. Blumenthal, Minhee Won, Minesh P. Mehta, Jeff M. Michalski, C. Leland Rogers, Benjamin W. Corn
Manuscript writing: Deborah T. Blumenthal, Minhee Won, Minesh P. Mehta, C. Leland Rogers, Benjamin W. Corn
Final approval of manuscript: Deborah T. Blumenthal, Minhee Won, Minesh P. Mehta, Walter J. Curran, Luis Souhami, Jeff M. Michalski, C. Leland Rogers, Benjamin W. Corn
REFERENCES
- 1.Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant gliomas after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 2.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 3.Nelson D, Schoenfeld D, Weinstein A, et al. A randomized comparison of misonidazole sensitized radiotherapy plus BCNU and radiotherapy plus BCNU for treatment of malignant glioma after surgery: Preliminary results of an RTOG study. Int J Radiat Oncol Biol Phys. 1983;9:1143–11151. doi: 10.1016/0360-3016(83)90172-4. [DOI] [PubMed] [Google Scholar]
- 4.Souhami L, Seiferheldl W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of RTOG 93-05. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Werner-Wasik M, Scott C, Nelson D, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiotherapy with carmustine for adults with supratentorial malignant gliomas. Cancer. 1996;77:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Ky GK, Adjei AA. Principles of chemotherapy. In: Chang AE, Ganz PA, Hayes DF, et al., editors. Oncology: An Evidence Based Approach. New York, NY: Springer; 2006. pp. 14–16. [Google Scholar]
- 7.Mackillop WJ, Bates JHT, Withers HR. The effect of delay in treatment on local control by radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:243–250. doi: 10.1016/0360-3016(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 8.Hartsell WF, Recine DC, Griem KL, et al. Delaying the initiation of intact breast irradiation for patients with lymph node positive breast cancer increases the risk of local recurrence. Cancer. 1995;76:2497–2503. doi: 10.1002/1097-0142(19951215)76:12<2497::aid-cncr2820761214>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Recht A, Come SE, Gelman RS, et al. Integration of conservative surgery, radiotherapy, and chemotherapy for the treatment of early stage, node-positive breast cancer: Sequencing, timing and outcome. J Clin Oncol. 1991;9:1662–1667. doi: 10.1200/JCO.1991.9.9.1662. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz TA, Austin-Seymour MM, Moe RE, et al. Effect of delay in radiation in the combined modality treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1993;26:23–35. doi: 10.1016/0360-3016(93)90169-v. [DOI] [PubMed] [Google Scholar]
- 11.Lee AW, Chan DK, Fowler JF, et al. T1 nasopharyngeal carcinoma: The effect of waiting time on tumor control. Int J Radiat Oncol Biol Phys. 1994;30:1111–1117. doi: 10.1016/0360-3016(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 12.Brouha XD, Op DC, Terhaard CH, et al. Does waiting time for radiotherapy affect local control of T1N0M0 glottic laryngeal carcinoma? Clin Otolaryngol. 2000;25:215–218. doi: 10.1046/j.1365-2273.2000.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Barton MB, Morgan G, Smee R, et al. Does waiting time affect the outcome of larynx cancer treated by radiotherapy? Radiother Oncol. 1997;44:137–141. doi: 10.1016/s0167-8140(97)00093-5. [DOI] [PubMed] [Google Scholar]
- 14.Choi N, Baumann M, Flentjie M, et al. Predictive factors in radiotherapy for non-small cell lung cancer. Lung Cancer. 2001;31:43–56. doi: 10.1016/s0169-5002(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CS, Lai CH, Wang CC, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and post-operative radiotherapy. Gynecol Oncol. 1999;75:328–333. doi: 10.1006/gyno.1999.5527. [DOI] [PubMed] [Google Scholar]
- 16.Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57:131–136. doi: 10.1016/s0167-8140(00)00257-7. [DOI] [PubMed] [Google Scholar]
- 17.Chang C, Horton J, Schoenfeld D, et al. Comparison of post-operative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin C, Scott C, Langer C, et al. Phase II, two arm RTOG trial (94-11) of BCNU plus accelerated hyperfractionated radiotherapy based on tumor volume in the treatment of newly diagnosed surgically ineligible GBM patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–1358. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 19.Del Rowe J, Scott C, Werner-Wasik M, et al. A single arm, open label phase II study of intravenously administered Tirapazamine plus radiation therapy for malignant glioma. J Clin Oncol. 2000;18:1254–1259. doi: 10.1200/JCO.2000.18.6.1254. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Won M, MacDonald D, et al. Phase II study of topotecan plus cranial irradiation for glioblastoma multiforme: Results of RTOG 95-13. Int J Radiat Oncol Biol Phys. 2002;53:980–986. doi: 10.1016/s0360-3016(02)02817-1. [DOI] [PubMed] [Google Scholar]
- 21.Langer C, Ruffer J, Rhodes H, et al. Phase II RTOG trial of weekly paclitaxel and conventional external beam radiation therapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:113–119. doi: 10.1016/s0360-3016(01)01597-8. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three RTOG malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 26.Mackillop WJ, Fu H, Quirt CF, et al. Waiting for radiotherapy in Ontario. Int J Radiat Oncol Biol Phys. 1994;30:221–228. doi: 10.1016/0360-3016(94)90538-x. [DOI] [PubMed] [Google Scholar]
- 27.Morgan GW. A synopsis of radiation oncology in Australia, with particular reference to New South Wales. Aust N Z J Surg. 1998;68:225–235. doi: 10.1111/j.1445-2197.1998.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 29.Benk V, Joseph L, Fortin P, et al. Effect of delay in initiating radiotherapy for patients with early stage breast cancer. Clin Oncol. 2004;16:6–11. doi: 10.1016/j.clon.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Clarke DH, Le MG, Sarrazin D, et al. Analysis of local-regional relapses in patients with early breast cancers treated by excision and radiotherapy: Experience of the Institut Gustave Roussy. Int J Radiat Oncol Biol Phys. 1985;11:137–145. doi: 10.1016/0360-3016(85)90372-4. [DOI] [PubMed] [Google Scholar]
- 31.Vujovic O, Perera F, Dar AR, et al. Does delay in breast irradiation following conservative breast surgery in node-negative breast cancer patients have an impact on risk of recurrence? Int J Radiat Oncol Biol Phys. 1998;40:869–874. doi: 10.1016/s0360-3016(97)00922-x. [DOI] [PubMed] [Google Scholar]
- 32.Froud PJ, Mates D, Jackson JS, et al. Effect of time interval between breast conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys. 2000;46:363–372. doi: 10.1016/s0360-3016(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 33.Ampil FL, Burton GV, Li BD, et al. Radiotherapy with and without chemotherapy after breast conservation surgery for early stage breast cancer: A review of timing. Eur J Gynaecol Oncol. 1999;20:254–257. [PubMed] [Google Scholar]
- 34.O'Sullivan B, Mackillop W, Grice B, et al. The influence of delay in the initiation of definitive radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 1998;42(suppl 1):S97. abstr. [Google Scholar]
- 35.Dixit S, Vyas RK, Toparani RB, et al. Surgery versus surgery and post-operative radiotherapy in squamous cell carcinoma of the buccal mucosa: A comparative study. Ann Surg Oncol. 1998;5:502–510. doi: 10.1007/BF02303642. [DOI] [PubMed] [Google Scholar]
- 36.Kajanti M, Holsti LR, Holsti P. Radical surgery and post-operative split course radiotherapy in squamous cell carcinoma of the mobile tongue: Factors influencing local control and the time to recurrence. Radiother Oncol. 1991;22:174–179. doi: 10.1016/0167-8140(91)90021-8. [DOI] [PubMed] [Google Scholar]
- 37.Ampil FL, Buechter KJ, Bairnsfather LE, et al. Timing and dosage of post-operative radiotherapy for squamous cell carcinoma of the upper aerodigestive tract. J Oral Maxillofac Surg. 1993;51:1194–1197. doi: 10.1016/s0278-2391(10)80287-3. [DOI] [PubMed] [Google Scholar]
- 38.Bastit L, Blot E, Debourdeau P, et al. Influence of the delay of adjuvant post-operative radiotherapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49:139–146. doi: 10.1016/s0360-3016(00)01376-6. [DOI] [PubMed] [Google Scholar]
- 39.Work E, Nielsen OS, Bentzen SM, et al. Randomized study of initial versus late chest irradiation combined with chemotherapy in limited stage small cell lung cancer: Aarhus Lung Cancer Group. J Clin Oncol. 1997;15:3030–3037. doi: 10.1200/JCO.1997.15.9.3030. [DOI] [PubMed] [Google Scholar]
- 40.Chinot OL, Barrié M, Fuentes S, et al. Correlation between 0-6-methylguanine DNA methyltransferase and survival in inoperable newly diagnosed GBM patients treated with neoadjuvant Temozolomide. J Clin Oncol. 2007;25:1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]
- 41.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 42.Taghian A, Ramsay J, Allalunis-Turner J, et al. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome in GBM. Int J Radiat Oncol Biol Phys. 1993;25:243–249. doi: 10.1016/0360-3016(93)90345-v. [DOI] [PubMed] [Google Scholar]
- 43.Peker S, Abacioglu U, Sun I, et al. Irradiation after surgically induced brain injury in the rat: Timing in relation to severity of radiation damage. J Neurooncol. 2004;170:17–21. doi: 10.1023/b:neon.0000040820.78643.0a. [DOI] [PubMed] [Google Scholar]