Abstract
Purpose
To evaluate single-agent activity of bevacizumab in patients with recurrent glioblastoma.
Patients and Methods
Patients with recurrent glioblastoma were treated with bevacizumab 10 mg/kg every 2 weeks. After tumor progression, patients were immediately treated with bevacizumab in combination with irinotecan 340 mg/m2 or 125 mg/m2 every 2 weeks, depending on use of enzyme-inducing antiepileptic drugs. Complete patient evaluations were repeated every 4 weeks.
Results
Forty-eight heavily pretreated patients were accrued to this study. Thromboembolic events (12.5%), hypertension (12.5%), hypophosphatemia (6%), and thrombocytopenia (6%) were the most common drug-associated adverse events. Six patients (12.5%) were removed from study for drug-associated toxicity (five thromboembolic events, one bowel perforation). Thirty-four patients (71%) and 17 patients (35%) achieved radiographic response based on Levin and Macdonald criteria, respectively. Median progression-free survival (PFS) was 16 weeks (95% CI, 12 to 26 weeks). The 6-month PFS was 29% (95% CI, 18% to 48%). The 6-month overall survival was 57% (95% CI, 44% to 75%). Median overall survival was 31 weeks (95% CI, 21 to 54 weeks). Early magnetic resonance imaging response (first 96 hours and 4 weeks) was predictive of long-term PFS, with the Levin criteria being more predictive than Macdonald criteria. Of 19 patients treated with bevacizumab plus irinotecan at progression, there were no objective radiographic responses. Eighteen patients (95%) experienced disease progression by the second cycle, and the median PFS was 30 days.
Conclusion
We conclude that single-agent bevacizumab has significant biologic and antiglioma activity in patients with recurrent glioblastoma.
INTRODUCTION
Despite modest improvements in the multimodality therapy of malignant gliomas, the overall prognosis of patients with glioblastoma remains poor, with median survival rates of little more than 14 months and few long-term survivors.1 New therapeutic approaches are clearly needed.
Antiangiogenic strategies are a promising approach for malignant gliomas secondary to the highly vascular nature of these tumors, and preclinical data have demonstrated the dependence of glioma growth on generation of tumor-associated blood vessels.2,3 Glioblastoma cells express high levels of vascular endothelial growth factor (VEGF) in situ, and inhibition of VEGF signaling impedes growth of glioma xenografts in immunodeficient mice.4 Bevacizumab is a humanized monoclonal antibody that targets VEGF and has demonstrated significant clinical activity in a number of human tumors, including colorectal cancer and non–small-cell lung cancer.5,6 Although bevacizumab seemed to have single-agent activity in these tumors, optimal clinical activity was seen when bevacizumab was given in combination with cytotoxic agents standard for those cancers.
Despite initial reluctance to evaluate bevacizumab in patients with brain tumors for fear of inducing intracerebral hemorrhage, a phase I study suggested that bevacizumab in combination with irinotecan can be safely administered to patients with malignant gliomas.7 Twenty-three patients in this study were included in the later report of a phase II trial by Vrendenburgh et al,8 evaluating the efficacy of bevacizumab in combination with irinotecan in 35 patients with recurrent glioblastoma. Significant antitumor activity was observed in comparison to published historical controls. The findings, though promising, raise the question of irinotecan's contribution to the combination. In two large multi-institutional trials of single-agent irinotecan for recurrent glioma, radiographic response rates were 6% and 2.5%, with no obvious prolongation of progression-free survival (PFS).9,10 We therefore conducted a phase II trial of single-agent bevacizumab in patients with recurrent glioblastoma. A companion trial evaluated the efficacy of adding irinotecan immediately after tumor progression on bevacizumab.
PATIENTS AND METHODS
Eligibility Criteria
Patients ≥ 18 years of age with histologically confirmed glioblastoma, recurrent after standard external-beam fractionated radiotherapy and temozolomide chemotherapy, were eligible. Patients were required to have a Karnofsky performance status (KPS) of ≥ 60%, normal metabolic and end-organ function, and an estimated survival of at least 2 months. Competent patients or their Designated Power of Attorney/Health Care Proxy were required to sign informed consent of for this National Cancer Institute institutional review board–approved trial. There were no limits on the number of prior therapies, although patients who received prior irinotecan were not eligible for treatment with irinotecan plus bevacizumab. Patients had to be on a stable dose of corticosteroids for at least 5 days before obtaining their baseline magnetic resonance imaging (MRI) scan. Patients with acute intracranial hemorrhage determined by non–contrast-enhanced computed tomography scan were ineligible, as were patients receiving anticoagulation therapy.
Treatment
Patients were treated with bevacizumab 10 mg/kg every 14 days on a 28-day cycle. Dose delays were allowed for reversible and preventable toxicity. Patients with progressive tumor growth on bevacizumab were asked to participate in a companion trial where their next scheduled dose of bevacizumab would be given in conjunction with irinotecan (340 mg/m2 or 125 mg/m2 depending on use of enzyme-inducing antiepileptic drugs) administered every 2 weeks along with bevacizumab on a 4-week cycle. Dose reductions were allowed for significant drug-associated toxicities.
Patient Assessment
All patients underwent a perfusion MRI scan at baseline, within 96 hours of the first bevacizumab infusion, and then at 4-week intervals. A fluorodeoxyglucose (FDG) positron emission tomography (PET) scan was performed at baseline and at the end of the first 4-week cycle. Blood counts were obtained every 2 weeks. A full metabolic screen, history, physical, and neurologic examination were performed before each cycle. MRI scans were assessed using both the more historical subjective Levin criteria and the newer objective Macdonald criteria.11,12 The Levin criteria consider extent of gadolinium enhancement, edema, and mass effect in a global assessment of the scan compared with baseline. Response is scored as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). In contrast, the Macdonald criteria use linear measurements of target lesion cross-sectional diameters to define response. We modified both criteria by requiring that patients be treated with a stable or decreasing dose of corticosteroids and that the amount of T2 and fluid-attenuated inversion recovery abnormality be stable or improved for a scan to be scored PR or SD. A finding of disease progression by either Levin or Macdonald criteria was sufficient to terminate treatment.
Study Design and Statistical Analysis
PFS at 6 months (PFS6) was the primary end point of this study. Using Kaplan-Meier methodology, we estimated PFS6 and associated 95% CIs, censoring patients who were removed from study for toxicity at the last evaluation before removal. Target accrual was 32 patients with glioblastoma to distinguish between a 15% and 35% PFS6, with less than 0.10 probability of concluding the agent is effective if PFS6 is less than 15% and ineffective if PFS6 is more than 35%. At the time of study design, the historical control benchmark was an older single-institution study by Wong et al.13 Subsequent historical control benchmarks are derived from more contemporary National Cancer Institute–sponsored clinical consortia/group studies by Lamborn et al and Ballmann et al, both showing PFS6 of 9%.14,15 These later studies have a similar patient population, follow-up schedule, and method for censoring data compared with this trial. In the midst of conducting the single-agent study, a companion trial was designed to investigate whether patients who experienced disease progression would benefit from the addition of irinotecan, where 20 patients who experienced treatment failure with bevacizumab would be eligible for the combination. If three or more of the 20 patients responded after 4 weeks of combination therapy, then the addition of irinotecan would have been considered potentially effective. With this design, we had less than a 0.10 probability of concluding that the combination was effective if the response rate is less than 5% or concluding that the combination is ineffective if the response rate is more than 25%. A total of 48 patients were accrued to the trial (original 32 patients for the single-agent trial and an 16 additional patients to complete the companion study).
Secondary end points were MRI and PET response rates and exploratory analyses of how those responses are correlated with PFS. Two group comparisons in PFS were done with two-sided log-rank tests, where a P value less than .05 was considered statistically significant.
RESULTS
Patient Characteristics
Patients were accrued and treated at the Clinical Center of the National Institutes of Health between July 2006 and November 2007. Forty-eight patients (20 women and 28 men) were enrolled, and all were assessable for toxicity and response (Table 1). All patients were previously treated with radiation and temozolomide. The median age was 53 years (range, 21 to 69 years), and median KPS was 90% (range, 60% to 100%). Twenty-three patients (48%) received three or more prior chemotherapy regimens not including initial adjuvant temozolomide therapy (median, two regimens; range, one to seven regimens).
Table 1.
Patient Characteristics (n = 48)
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Male | 28 | 58 |
| Female | 20 | 42 |
| Age, years | ||
| Median | 53 | |
| Range | 21-69 | |
| Prior radiation therapy | 48 | 100 |
| Prior chemotherapy regimens | ||
| Median | 2 | |
| Range | 1-7 | |
| 1-3 | 25 | 52 |
| ≥ 3 | 23 | 48 |
| Corticosteroid use | ||
| Patients receiving corticosteroids at study entry | 26 | 54 |
| Patients able to reduce dose of corticosteroids while on study | 15/26 | 58 |
| Average reduction in corticosteroid dose | 59 | |
Toxicity
Overall, patients tolerated treatment well. As seen in Table 2, thromboembolic events occurring in six patients (12.5%) were the most frequently observed severe adverse event possibly or probably related to bevacizumab. Three of these events were pulmonary emboli and one was a cerebral vascular event. The second most frequent drug-related adverse event was hypertension. All cases were ultimately controlled with antihypertensive medication, and no patient required removal from study for this toxicity. Overall, six patients (12.5%) were removed from study for drug-associated toxicity (five thromboembolic events, one bowel perforation). Two additional patients were removed for non–bevacizumab-related adverse events (one seizure, one opportunistic infection). No patients had intracranial hemorrhage.
Table 2.
Bevacizumab-Related Adverse Events
| Toxicity | No. of Events |
|||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Thromboembolic event | 2 | 4 | ||
| Hypertension | 4 | 2 | ||
| Hypophosphatemia | 1 | 2 | ||
| Thrombocytopenia | 2 | 1 | ||
| Hepatic dysfunction | 1 | |||
| Proteinuria | 1 | |||
| Bowel perforation | 1 | |||
Response
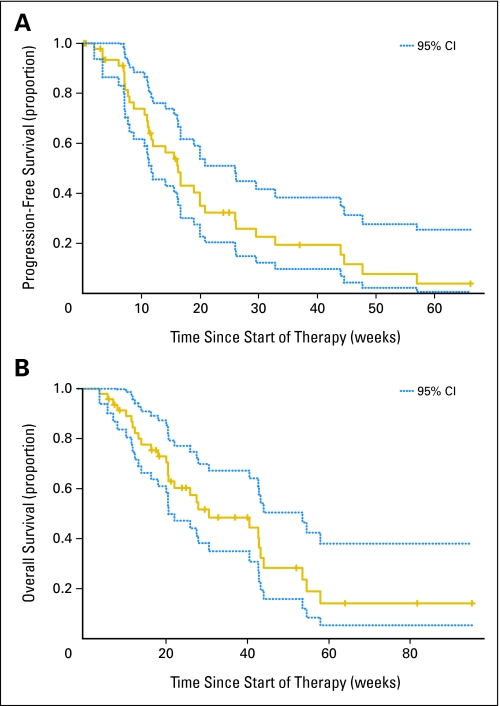
The median PFS is 16 weeks (95% CI, 12 to 26 weeks). The PFS6 is 29% (95% CI, 18% to 48%; Fig 1A). The 95% CI excludes the 9% null rate described in North Central Cancer Treatment Group studies of ineffective therapies for recurrent malignant glioma.14 Further, the lower limit of the 95% CI excludes the upper limit of the interval presented in Lamborn et al (PFS6, 9%; 95% CI, 6% to 13%).15 In a more conservative analysis, we treated the six patients removed from the study for treatment-related toxicity as failures rather than censored. The PFS6 estimated in this analysis was 24% (95% CI, 14% to 41%), where the exclusive CI also indicates significant improvement relative to historical control data.
Fig 1.
(A) Progression-free survival (PFS) with point-wise 95% CIs (dotted lines). The 9-week PFS is 74% (95% CI, 62% to 89%). The 6-month PFS is 29% (95% CI, 18% to 48%). The median PFS is 16 weeks (95% CI, 12 to 26 weeks). (B) Overall survival with point-wise 95% CIs (dotted lines). The 6-month OS is 57% (95% CI, 44% to 75%). The median OS is 31 weeks (95% CI, 21 to 54 weeks).
The 6-month survival rate is 57% (95% CI, 44% to 75%). The median overall survival is 31 weeks (95% CI, 21 to 54 weeks; Fig 1B).
In addition to an apparent prolongation of PFS, clinical benefit was evident by decreased cerebral edema in 24 patients (50%); 15 (58%) of 26 patients receiving corticosteroids at the start of treatment were able to decrease their requirement for corticosteroids by an average dose reduction of 59%, and 25 patients (52%) had improved neurologic symptoms, including a number who did not meet the PFS6 landmark.
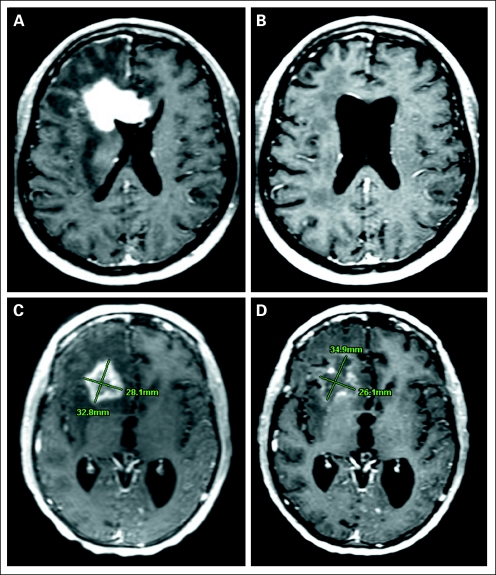
The overall radiographic response rate based on the Levin criteria was 71% (34 PRs), whereas the response rate based on Macdonald criteria was 35% (one CR, 16 PRs). After the first 4 weeks of therapy, 49% of patients demonstrated diminished FDG uptake by PET scan, 37% demonstrated equivalent uptake, and 14% demonstrated increased uptake compared with baseline. Typical bevacizumab-mediated radiographic response is shown in Figures 2A and 2B.
Fig 2.
Magnetic resonance imaging (MRI)–documented response to bevacizumab. (A) Pretreatment MRI scan for patient 1. (B) Post-treatment MRI scan for patient 1 after 3 months of bevacizumab without concomitant corticosteroid therapy. (C) Pretreatment MRI scan for patient 2. (D) Post-treatment MRI scan for patient 2 showing a partial response by Levin criteria versus stable disease by cross-sectional diameters (Macdonald criteria).
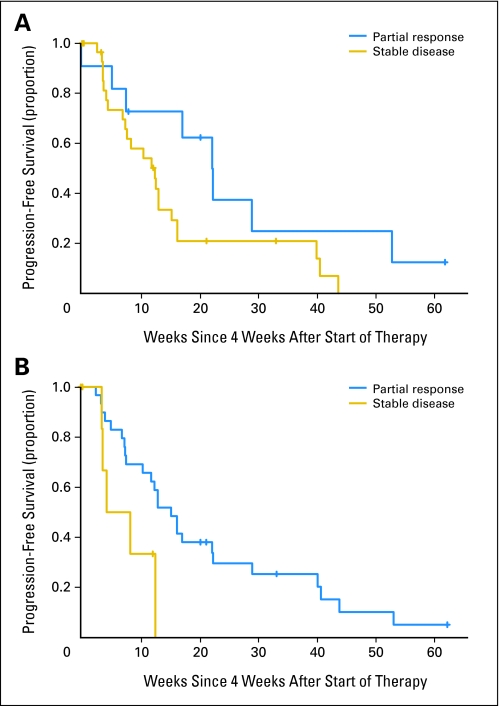
We asked whether early MRI responses (at 4 weeks after the first infusion of bevacizumab) were predictive of clinical outcome as measured by PFS. Patients who had PR by Macdonald criteria showed a trend toward improved PFS compared with those with SD. A two-sided log-rank test was not statistically significant (P = .07; Fig 3A). Patients who had PR by Levin criteria had statistically significant improvement in PFS compared with patients with SD (log-rank test P = .03; Fig 3B). In fact, there were no patients with PFS greater than 3 months who did not have at least a PR by Levin criteria after 4 weeks of treatment.
Fig 3.
(A) Progression-free survival based on 4-week magnetic resonance imaging (MRI) response (Macdonald criteria). Patients with partial response (PR; top curve) versus stable disease (SD; bottom curve). Log-rank test (two-sided) P = .07. (B) Progression-free survival based on 4-week MRI response (Levin criteria). Patients with PR (top curve) versus SD (bottom curve). Log-rank test (two-sided) P = .03.
We next evaluated whether MRI scans within 96 hours of the first dose of bevacizumab were predictive of long-term outcome. Interestingly, the response rate by Macdonald criteria was only 6% (three PRs, 42 SDs, one PD), whereas the response rate by the Levin criteria was 87% (42 PRs, four SDs, one PD). There were too few PRs at 96 hours by Macdonald criteria to allow a statistical analysis. Patients with PR at 96 hours by Levin criteria had a highly significant improvement in PFS compared with those with SD (log-rank test P < .001); PFS6 was 32% and 0% for PR and SD, respectively. Given the small number of patients with SD in this analysis, this finding should be interpreted cautiously.
In contrast to the MRI findings, there was no significant difference in PFS for patients who had diminished FDG uptake on their week 4 PET scan compared with those with no significant change.
We evaluated other variables that might have an effect on clinical outcome. KPS at the time of enrollment and the number of prior chemotherapy regimens had no effect on PFS. Age had a major effect, although opposite to what has been generally reported in glioma trials.16,17 The median PFS for patients with a median age of 53 years or older was 30 weeks versus a median PFS of 11 weeks for younger patients (log-rank test P < .001).
Irinotecan and Avastin
In the second part of the study evaluating combination therapy, 19 patients were enrolled when accrual was terminated after it became clear that we could not reach our efficacy rule of three or more of 20 patients responding. Irinotecan was well tolerated with hypophosphatemia (four grade 2, one grade 3), neutropenia (one grade 2), and lymphopenia (three grade 2, one grade 3) being the most commonly observed drug-associated toxicities. A limited number of irinotecan cycles were administered, potentially minimizing observed drug-related toxicity. Twelve (71%) of 17 assessable patients demonstrated tumor progression after just one cycle of bevacizumab and irinotecan, and only one patient received more than three cycles of the combination in the setting of SD. There were no radiographic responses per Macdonald criteria and only one PR based on Levin criteria. The median time to tumor progression was 30 days.
DISCUSSION
Our data demonstrate that single-agent bevacizumab is well tolerated and has biologic activity in patients with recurrent glioblastoma as demonstrated by significant reductions in gadolinium enhancement, T2 abnormalities, and diminished uptake of FDG on PET scans. The observed PFS6 of 29% compares favorably with historical controls of ineffective regimens, including those from previous National Institutes of Health phase I and phase II trials of patients with recurrent glioblastoma (data not shown). A significant number of patients derived clinical benefit from treatment, manifested as decreased cerebral edema, improved neurologic symptoms, and decreased requirement for corticosteroids.
Results from this trial are consistent with data presented by Vrendenburgh et al.8 Not surprisingly, there seemed to be less overall toxicity when bevacizumab was used alone. No patients withdrew from bevacizumab monotherapy in this study secondary to fatigue, in contrast to four patients treated with combination therapy in the prior study. Response rates in our patients treated with monotherapy were lower compared with those treated with combination therapy in the Vrendenburgh study (PFS6, 29% v 46%; and response rate by Macdonald criteria, 35% v 57%). These data are consistent with a recently completed company-sponsored randomized phase II trial of bevacizumab alone versus bevacizumab in combination with irinotecan, in which the PFS6 for bevacizumab alone was 36% compared with 51% for the combination.18 The difference between the two treatment groups was not, however, statistically significant, and thus it remains unclear whether irinotecan adds significant antiglioma activity to bevacizumab. Our data clearly demonstrate the futility of adding irinotecan to bevacizumab after tumor progression during bevacizumab treatment. Thus in contrast to colorectal and lung cancer, where bevacizumab is an effective adjunct to principally effective cytotoxic standard therapy, bevacizumab seems to contribute a significant amount of the activity in treating gliomas. We cannot rule out a contribution by irinotecan if both agents are used simultaneously as part of initial treatment. Demonstrating this likely small benefit will require a relatively large phase III randomized trial to establish whether the added toxicity of irinotecan is warranted.
Much has been written regarding the clinical relevance of standard MRI response criteria in the setting of VEGF inhibition. Neutralization of VEGF-induced vascular permeability stabilizes the blood-brain barrier, with subsequent decreased extravasation of gadolinium and intravascular fluid into the surrounding brain parenchyma. Therefore, decreased gadolinium enhancement cannot be considered an accurate marker of tumor mass in the setting of a VEGF inhibition. Our data are consistent with this model, where we observed profound reductions in enhancement on MRI scans obtained as soon as 24 hours after the first dose of bevacizumab, clearly too early for an equally profound antitumor effect. The antivascular permeability effect of VEGF inhibition almost certainly accounts for the high initial radiographic response rate. An accompanying lack of effect on tumor cell biology likely explains the rapid subsequent progression in nearly half of these initial responders. Nevertheless, a bevacizumab-mediated antitumor effect likely occurs in a subpopulation of patients, because nearly half of the initial radiographic responders were progression-free for more than 6 months.
Despite the caveats of MRI interpretation in the setting of bevacizumab treatment, our data suggest that vascular permeability (ie, gadolinium extravasation) may be a useful biomarker for drug activity in situ. Patients who did not have PR on early MRI scans (4 and 28 days after first infusion of bevacizumab) had a significantly shorter PFS. If no change in vascular permeability is seen on an early MRI scan, one may suppose that VEGF inhibition is not being achieved with this regimen, conferring a low probability of long-term tumor control with bevacizumab.
The importance of MRI interpretation begs consensus on criteria for evaluating scans in the setting of antiangiogenic therapy. In this study, the predictive value of the more subjective Levin criteria seemed greater than the objective Macdonald criteria. The two methods may measure different biologic processes, explaining the discrepancy. Gadolinium enhancement is a direct and proportional measure of tumor mass for discrete intracerebral lesions, such as brain metastases and systemic solid tumors. Circumferential decrease in the size of the mass after treatment with standard cytotoxic therapies is therefore a reasonable objective radiographic criterion for measuring therapeutic response (ie, Response Evaluation Criteria in Solid Tumors).19,20 In contrast, gliomas consist of asymmetrically infiltrating tumor cells causing variable blood-brain barrier disruption. Spatial measurements of enhancement are a problematic surrogate marker for glioma mass at baseline, becoming even more questionable in the setting of VEGF inhibition. Indeed, the majority of early MRI changes we saw were substantial decreases in the intensity and extent of enhancement, edema, and mass effect rather than reduction in diameter of the enhancing volume. These qualitative changes are factored into the Levin criteria but not the Macdonald criteria, accounting for the much higher response rate. For example, in Figures 2C and 2D, measurement of cross-sectional diameters of the tumor results in SD by Macdonald criteria rather than a clear PR by Levin criteria. The Levin criteria may provide a more accurate method of assessing VEGF inhibition in situ and explain why early MRI responses were statistically more predictive of longer PFS than when determined by Macdonald criteria.
Finally, it is of interest that older patients in our study had a significantly longer PFS than younger patients, in contrast to the observation in most trials of cytotoxic agents. These findings may reflect statistical variance secondary to small patient numbers. Alternatively, bevacizumab may potentially have greater activity in the biologic subtype of gliomas more commonly found in older patients.21 Similarly, PFS was not influenced by the number of prior treatment regimens, as is typical of most trials for recurrent disease. Prior treatment with standard cytotoxic agents may not be a relevant exclusion criterion in the design of trials evaluating antiangiogenic and possibly other molecularly targeted agents.
In conclusion, single-agent bevacizumab seems to have significant effects on vascular permeability and cerebral edema, as well as significant antiglioma activity in patients with recurrent glioblastoma. A randomized phase III trial will be required to determine whether irinotecan adds any clinical benefit to treatment with bevacizumab. Future trials should focus on the role of bevacizumab in the initial treatment of glioblastoma, novel combinations of bevacizumab with other cytotoxic and molecularly targeted agents, and the identification of accurate radiographic and biologic markers for predicting long-term benefit from bevacizumab therapy.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Paul S. Albert, Howard A. Fine
Administrative support: Cheryl Royce
Provision of study materials or patients: Lyndon Kim, Kraig Moore, Kevin Camphausen, John Park, Howard A. Fine
Collection and assembly of data: Teri N. Kreisl, Lyndon Kim, Kraig Moore, Paul Duic, Cheryl Royce, Irene Stroud, Nancy Garren, Megan Mackey, John A. Butman, John Park, Howard A. Fine
Data analysis and interpretation: Teri N. Kreisl, John A. Butman, Kevin Camphausen, Paul S. Albert, Howard A. Fine
Manuscript writing: Teri N. Kreisl, Kevin Camphausen, Paul S. Albert, Howard A. Fine
Final approval of manuscript: Teri N. Kreisl, Lyndon Kim, Kraig Moore, Paul Duic, Cheryl Royce, Irene Stroud, Nancy Garren, Megan Mackey, John A. Butman, Kevin Camphausen, John Park, Paul S. Albert, Howard A. Fine
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi JA, Fukumoto M, Igarashi K, et al. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J Neurosurg. 1992;76:792–798. doi: 10.3171/jns.1992.76.5.0792. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell M, Naber SP, Wolfe HJ, et al. Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res. 1991;51:1345–1351. [PubMed] [Google Scholar]
- 4.Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55:91–100. doi: 10.1023/a:1013329832067. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Kerr C. Bevacizumab and chemotherapy improves survival in NSCLC. Lancet Oncol. 2005;6:266. doi: 10.1016/s1470-2045(05)70155-8. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 8.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: Final report of NABTT 97-11. Neuro Oncol. 2004;6:21–27. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin VA, Crafts DC, Norman DM, et al. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 13.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 14.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Genentech. Avastin shows encouraging results in phase II study in patients with the most aggressive form of brain cancer. http://www.gene.com/gene/news/press-releases/display.do?method=detail&id=10887.
- 19.Therasse P, Le Cesne A, Van Glabbeke M, et al. RECIST vs. WHO: Prospective comparison of response criteria in an EORTC phase II clinical trial investigating ET-743 in advanced soft tissue sarcoma. Eur J Cancer. 2005;41:1426–1430. doi: 10.1016/j.ejca.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Park JO, Lee SI, Song SY, et al. Measuring response in solid tumors: Comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–537. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 21.Laigle-Donadey F, Delattre JY. Glioma in the elderly. Curr Opin Oncol. 2006;18:644–647. doi: 10.1097/01.cco.0000245324.19411.19. [DOI] [PubMed] [Google Scholar]