Abstract
Purpose
Recurrence of high-risk neuroblastoma is common despite multimodality therapy. ch14.18, a chimeric human/murine anti-GD2 antibody, lyses neuroblastoma cells. This study determined the maximum tolerable dose (MTD) and toxicity of ch14.18 given in combination with interleukin-2 (IL-2) after high-dose chemotherapy (HDC)/stem-cell rescue (SCR). Biologic correlates including ch14.18 levels, soluble IL-2 receptor levels, and human antichimeric antibody (HACA) activity were evaluated.
Patients and Methods
Patients were given ch14.18 for 4 days at 28-day intervals. Patients received IL-2 during the second and fourth courses of ch14.18 and granulocyte-macrophage colony-stimulating factor (GM-CSF) during the first, third, and fifth courses. The MTD was determined based on toxicities occurring with the second course. After the determination of the MTD, additional patients were treated to confirm the MTD and to clarify appropriate supportive care.
Results
Twenty-five patients were enrolled. The MTD of ch14.18 was determined to be 25 mg/m2/d for 4 days given concurrently with 4.5 × 106 U/m2/d of IL-2 for 4 days. IL-2 was also given at a dose of 3 × 106 U/m2/d for 4 days starting 1 week before ch14.18. Two patients experienced dose-limiting toxicity due to ch14.18 and IL-2. Common toxicities included pain, fever, nausea, emesis, diarrhea, urticaria, mild elevation of hepatic transaminases, capillary leak syndrome, and hypotension. No death attributable to toxicity of therapy occurred. No additional toxicity was seen when cis-retinoic acid (cis-RA) was given between courses of ch14.18. No patient treated at the MTD developed HACA.
Conclusion
ch14.18 in combination with IL-2 was tolerable in the early post-HDC/SCR period. cis-RA can be administered safely between courses of ch14.18 and cytokines.
INTRODUCTION
Neuroblastoma is the most common non-CNS solid tumor of childhood. Although survival has improved with multimodality therapy, relapse is common for patients with high-risk disease.1 Ganglioside GD2 is a glycolipid that is strongly expressed on the surface of neuroblastoma cells. There is little intra- or intertumor heterogeneity of GD2 expression.2 In normal human tissues, GD2 expression is restricted to neurons, melanocytes, and peripheral pain fibers.3 The ch14.18 antibody is a chimeric construct using murine variable region heavy and light chain genes and human constant region genes for heavy chain immunoglobulin G1 and light chain κ.4 ch14.18 binds GD2 and activates complement.5 ch14.18 mediates antibody-dependent cellular cytotoxicity (ADCC) by neutrophils and natural killer (NK) and lymphokine activated killer (LAK) cells.6,7
Granulocyte-macrophage colony-stimulating factor (GM-CSF) enhances ADCC,8 and ch14.18 plus GM-CSF had limited activity in a phase II study for relapsed neuroblastoma.9 Based on the hypothesis that anti-GD2 antibodies would be most useful for the treatment of minimal residual disease (MRD), the Children's Cancer Group evaluated ch14.18 and GM-CSF given in the early post–high-dose chemotherapy (HDC)/stem-cell rescue (SCR) period. Toxicities included neuropathic pain, hypotension, nausea, emesis, mild to moderate capillary leak syndrome, urticaria, and neurotoxicity resulting in diplopia.10
IL-2 augments ADCC by peripheral blood lymphocytes (PBL). Low concentrations of ch14.18 are sufficient for significant ADCC using PBL from patients treated with IL-2.11 Treatment of patients with at least 1 × 106 U/m2/d IL-2 (Hoffman LaRoche IL-2; equivalent to 3 × 106 U/m2/d of Chiron IL-2) for 4 to 7 days generates LAK cells.12 LAK cells are derived primarily from NK cells and are capable of major histocompatibility complex–unrestricted lysis of fresh tumor cells and NK-resistant tumor cell lines.13 Neuroblastoma cells are susceptible to lysis by autologous LAK cells.14,15
We report a study that integrated IL-2 into a regimen of ch14.18 plus GM-CSF after HDC/SCR. The primary aim was to determine the MTD and toxicity of ch14.18 given in combination with IL-2 soon after HDC/SCR. Laboratory studies were performed to assess immune stimulation, ch14.18 levels, and human antichimeric antibody (HACA).
PATIENTS AND METHODS
Patient Enrollment
The study opened in June 1997 and closed to enrollment in February 2002. Patients were enrolled at Children's Oncology Group (COG) phase I study institutions. Patients were younger than 21 years old, had a diagnosis of neuroblastoma (based on tumor histology or bone marrow metastases and elevated urine catecholamine metabolites), and had recently completed HDC followed by autologous SCR (bone marrow or peripheral blood). Patients were enrolled within 8 weeks after the total absolute phagocyte count (WBC × % [segs + bands + monos]) reached more than 1,000/μL after HDC/SCR. Patients had a life expectancy of at least 2 months and adequate renal, liver, cardiac, pulmonary, and CNS function.
Patients previously treated with 14.G2a or ch14.18 antibodies or requiring chronic use of corticosteroids were ineligible. Informed consent was obtained from the patient or guardian in accordance with institutional policies and as approved by the US Department of Health and Human Services.
Study Design
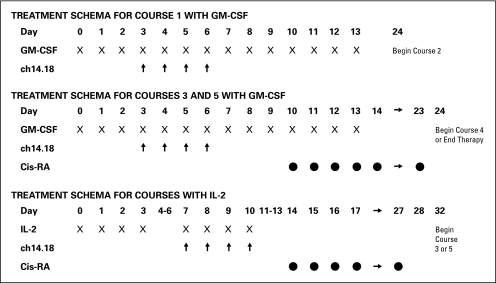
The trial was designed to investigate the tolerability of two dose levels of ch14.18, 20 and 40 mg/m2/d, given in combination with IL-2. This was based on a study of ch14.18 and GM-CSF which escalated the ch14.18 dose from 20 to 50 mg/m2/d and determined the MTD to be 40 mg/m2/d;10 the limited antibody supply available; and the anticipation that a similar antibody dose would be tolerated with IL-2. IL-2 (4.5 × 106 U/m2/d) was given by continuous infusion over 96 hours for 3 weeks. IL-2 was given starting 1 week before the start of ch14.18, during the ch14.18, and starting 1 week after the start of ch14.18 (regimen 1, Table 1). The IL-2 and ch14.18 were given through separate intravenous (IV) lumens because compatibility data were not available. Patients were given 6 courses of ch14.18 at 28-day intervals. The cytokine given with each course of ch14.18 alternated between GM-CSF and IL-2 starting with GM-CSF for the first course. The ch14.18 dose was 40 mg/m2/d when given with GM-CSF, 250 μg/m2/dose, by IV infusion or subcutaneous injection. IL-2 and GM-CSF were not given concurrently due to neurotoxicity noted rarely when given together.16 Patients were premedicated with acetaminophen, hydroxyzine, and morphine before each dose of ch14.18. Ch14.18 was infused over 5 hours each day, but the duration could be extended to as long as 20 hours for anticipated toxicities (pain, fever, tachycardia, tachypnea, hypotension) unresponsive to supportive care measures. Patients were removed from therapy for progressive neuroblastoma after course 2. Standard staging studies (computed tomography and bone scans, bone marrow aspirates, and biopsies) were performed before courses 3 and 5 to assess for progressive disease.
Table 1.
Treatment Regimens Used During the Course of A0935A
| Course | Regimen
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
|||||||
| Ch14.18 | GM-CSF | IL-2 | Ch14.18 | GM-CSF | IL-2 | Ch14.18 | GM-CSF | IL-2 | |
| Time period | July 1997-June 1998 | July 1998-January 1999 | February 1999-February 2002 | ||||||
| 1 | 40 mg/m2 | X | 40 mg/m2 | X | 25 mg/m2 | X | |||
| 2 | 20 mg/m2 | 4.5 × 106 U/m2/d × 4 for the week prior to, the week of, and week after ch14.18 | 20 mg/m2 | 4.5 × 106 U/m2/d × 4 for the week prior to, the week of, and week after ch14.18 | 25 mg/m2 | 3 × 106 U/m2/d × 4 the week prior to and 4.5 × 106 U/m2/d × 4 the week of ch14.18 | |||
| 3 | 40 mg/m2 | X | 40 mg/m2 | X | 25 mg/m2 | X | |||
| 4 | 20 mg/m2 | 4.5 × 106 U/m2/d × 4 for the week prior to, the week of, and week after ch14.18 | 25 mg/m2 | 3 × 106 U/m2/d × 4 the week prior to and 4.5 × 106 U/m2/d × 4 the week of ch14.18 | |||||
| 5 | 40 mg/m2 | X | 25 mg/m2 | X | |||||
| 6 | 20 mg/m2 | 4.5 × 106 U/m2/d × 4 for the week prior to, the week of, and week after ch14.18 | |||||||
NOTE. The ch14.18 dose is the daily dose which was given × 4 days. Ch14.18 courses were given every 28 days for regimens 1 and 3 and every 21 days for regimen 2. The GM-CSF dose was 250 μg/m2/day for all regimens.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin 2.
Dose-limiting toxicity (DLT) for the determination of the MTD was assessed during the second course of therapy (the first course of ch14.18 and IL-2), when the patient was in the early post-HDC/SCR period. Toxicity was predicted to be more likely at this time. Also, it was anticipated that a significant number of patients would be removed from study because of progressive disease, and therefore would not be assessable for course 4. A 3 × 3 design was used. Three patients were enrolled at the 20 mg/m2/d ch14.18 dose level. If none had DLT, 40 mg/m2/d was used for the next three patients. If one of three patients had DLT, then another three patients were enrolled at the same dose. The MTD would be the dose at which fewer than two of six patients had DLT.
The study design was amended when cis-retinoic acid (cis-RA) was shown to improve survival.1 cis-RA started a median of 97 days after HDC/SCR in this report. The number of ch14.18 courses was decreased to three and the interval between courses was shortened from 28 days to 21 days to complete immunotherapy before starting cis-RA at a similar time point (regimen 2, Table 1). This schedule accomplished dose intensification by interval compression. It was not tolerated and the study was amended again.
Several changes were made in anticipation of a groupwide phase III trial of this therapy. In the prior study, ch14.18 40 mg/m2/d for 4 days was tolerated with GM-CSF.10 However, in this study, two of six assessable patients had toxicity that met DLT criteria (used for the IL-2 cycle) with this ch14.18 dose. Because of this experience, the very good tolerability of 20 mg/m2/d for 5 days of ch14.18 (100 mg/m2/course; R. Handgrettinger, personal communication, October 1998), and a desire to explore a dose that would be feasible for a groupwide phase III trial, a ch14.18 dose of 25 mg/m2/d for 4 days was chosen for all courses. The dose escalation of ch14.18 with IL-2 was changed to 25 mg/m2/d instead of 40 mg/m2/d. The interval between ch14.18 courses returned to every 28 days. Five courses were given based on a study which showed that patients who received ≥ 4 cycles of another anti-GD2 antibody, 3F8, had significantly better survival than those who received fewer than 4 cycles.17 The IL-2 dose was reduced to decrease toxicity and to improve the likelihood that the higher daily dose of ch14.18 would be tolerated. IL-2 3 × 106 U/m2/d for 96 hours was given during the week before ch14.18 and 4.5 × 106 U/m2/d for 96 hours given during the ch14.18 (regimen 3, Table 1).
Another study aim was added to determine the feasibility of interdigitating cis-RA between antibody courses. cis-RA was given for 2 weeks with a 2-week break between courses. Patients received a total of 6 courses of cis-RA, with 3 given between courses 2 to 5 of antibody therapy (Fig 1). Because of prior experience with some patients not being assessable, 10 patients were enrolled to ensure that six would be assessable. After the MTD was established, six more patients were enrolled to confirm the MTD and to further define the supportive care required during this treatment before opening the phase III study. For determination of the MTD, the regimen was considered tolerable if fewer than four of 12 assessable patients on regimen 3 experienced DLT during the second course of therapy.
Fig 1.
Treatment schema of Children's Oncology Group study A0935A. The schema reflects the final treatment regimen (regimen 3 in Table 1). GM-CSF, granulocyte-macrophage colony-stimulating factor; Cis-RA, cis-retinoic acid; IL-2, interleukin 2.
Dose-Limiting Toxicity and Dose Escalation
The primary aim was to determine the MTD of ch14.18 plus IL-2 soon after HDC/SCR. A patient was assessable for toxicity after starting the second course of therapy. DLT was defined as any National Cancer Institute Common Toxicity Criteria version 2 grade 3 or 4 toxicity attributable to combination therapy with the exception of anticipated toxicities (Table 2).
Table 2.
Anticipated Toxicities That Were Exceptions to DLT
| Grade | Toxicity |
|---|---|
| 4 | Pain |
| 3 | Fever |
| 3 | Hypotension |
| 4 | Hypotension that could be readily controlled with supportive care measures such as fluid boluses or slowing the rate of ch14.18 infusion |
| 3 or 4 | Allergic reactions controlled with supportive care measures |
| 3 | Capillary leak syndrome that did not recur when the dose of IL-2 was reduced |
| 3 | Skin toxicity that improved with treatment (including holding the ch14.18 infusion) within 24 hours |
| 3 | Neurotoxicity that reversed within 2 days of stopping the scheduled infusion of ch14.18 |
| 3 | Nausea |
| 3 | Vomiting |
| 3 | Electrolyte disturbances |
| 3 | Hypertension |
| 3 | Hepatic toxicity that returned to normal prior to the next scheduled dose of ch14.18 |
| 4 | Hematologic toxicity that improved to grade 2 or baseline after completion of the IL-2 infusion |
| Karnofsky or Lansky performance status of 30-50 |
NOTE. DLT was defined as any National Cancer Institute Common Toxicity Criteria version 2 code III or IV toxicity attributable to combination therapy with the exception of the toxicities in the Table.
Abbreviations: DLT, dose-limiting toxicity; IL-2, interleukin 2.
ch14.18 levels in patients’ sera were measured by enzyme-linked immunosorbent assay (ELISA) as previously described.10,18 HACA levels were estimated by the ELISA bridging assay as published previously.10,19
Soluble IL-2 receptor alpha subunit (sIL-2Rα)was measured in serum using a commercially available double monoclonal antibody ELISA kit (IM10559; Beckman/Coulter, Fullerton, CA) according to manufacturer's specifications.20
Survival
Overall survival was calculated as the time from study enrollment to death date. The survivor function was estimated by the Kaplan-Meier method.21 The SAS statistical package (SAS Institute, Cary, NC) and EPILOG statistical package (Epicenter Software, Pasadena, CA) were used for data management and analysis.
RESULTS
Twenty-five patients were enrolled (Table 3). All patients had stage IV neuroblastoma and 64% has measurable disease at enrollment. Two patients never received protocol therapy (one withdrawn at parental request; one due to study suspension). These patients were excluded from all analyses. Four patients received treatment but were not assessable for the assessment of DLT for ch14.18 and IL-2. These four patients are included in all other analyses.
Table 3.
Characteristics of Children With Neuroblastoma Enrolled on A0935A Study (N = 25)
| Characteristic | No. | % |
|---|---|---|
| Median age at diagnosis, years | 2 | |
| Range | 0-13 | |
| Median age at study entry, years | 4 | |
| Range | 1-14 | |
| Sex | ||
| Male | 15 | 60 |
| Female | 10 | 40 |
| Race | ||
| White | 18 | 72 |
| Hispanic | 2 | 8 |
| Black | 1 | 4 |
| Asian | 2 | 8 |
| Filipino | 1 | 4 |
| Other | 1 | 4 |
| Disease stage at diagnosis | ||
| IV | 25 | 100 |
| Other | 0 | |
| Measurable disease at study entry | ||
| Yes | 16 | 64 |
| No | 9 | 36 |
| Prior radiation | ||
| Yes | 20 | 80 |
| No | 5 | 20 |
| Bone marrow metastases at study entry | ||
| Yes | 6 | 24 |
| No | 19 | 76 |
| Median time from stem-cell rescue to starting protocol therapy, days | 64 | |
| Range | 23-77 | |
Study Progress and MTD
Four patients were enrolled on regimen 1 and two assessable patients did not have DLT. One patient was not assessable for DLT because he did not receive ch14.18 plus IL-2 due to anaphylaxis with ch14.18 plus GM-CSF and removal from protocol therapy. The other patient did not receive protocol therapy because of study suspension.
Five patients were enrolled on regimen 2. Of the four assessable patients, two had DLT. One DLT was hypotension and decreased cardiac ejection fraction with IL-2 alone. Another DLT was gram-negative sepsis and grade 4 hyperbilirubinemia during course 2 (ch14.18 plus IL-2). Of note, this patient also had bacteremia with different organisms during course 1 (ch14.18 plus GM-CSF). One patient was not assessable for DLT because he did not receive course 2. He was removed from study because of H. flu pneumonia (grade 4 infection, grade 4 hyperbilirubinemia, grade 3 pulmonary) during course 1.
Sixteen patients were enrolled on regimen 3. Eight of the first 10 patients enrolled on regimen 3 were assessable, and none had DLT. Two patients were not assessable for DLT (one withdrawn before protocol therapy and one with progressive neuroblastoma after course 1). An additional six patients were enrolled to optimize the supportive care for therapy-related capillary leak syndrome. Five patients were assessable (one not assessable for DLT because he was taken off study at parent request after course 1) and none had DLT in course 2. Regimen 3 was the MTD because none of 13 assessable patients had DLT (Table 4).
Table 4.
DLT During the Second Course at Each Dose Level of ch14.18 (19 assessable)
| Regimen | No. Enrolled | No. Assessable | No. of Patients With DLT During Course 2 | Type and Grade of DLT Observed | Attribution |
|---|---|---|---|---|---|
| 1 | 4 | 2 | 0 | — | — |
| 2 | 5 | 4 | 2 | Grade 4 hypotension and grade 2 cardiac (decreased cardiac output) | Probably due to IL-2 week 1 (no ch14.18) |
| Grade 3 infection (sepsis) and grade 4 | Possibly related | ||||
| 3 | 16 | 13 | 0 | — | — |
| Total | 25 | 19 | 2 |
Abbreviations: DLT, dose-limiting toxicity; IL-2, interleukin 2.
The toxicity of ch14.18 and IL-2 was significant but controllable (Table 5). Grade 3 pain requiring morphine was very common. Fever and hypotension were also common. The following toxicity data are for the 26 courses of ch14.18 and IL-2 received by 13 patients on regimen 3, the MTD. Twelve patients had fever, but only five had grade 3 fever. Six patients had grade 3 or 4 hypotension. The hypotension was usually not clinically significant and responded to fluid boluses. Decreasing the rate of infusion of ch14.18 also helped. Grade 3 capillary leak occurred in three patients, two of whom required a 50% reduction of the IL-2 dose due to capillary leak (one in course 2 and one in course 4). The latter also required a 50% reduction in the ch14.18 dose due to pleural effusions and hypoxia in course 4. Grade 3 hypoxia (requiring supplemental oxygen) occurred in two patients. The etiology was unclear but could have been due to sedation and hypoventilation from morphine. Neither of them had capillary leak or pulmonary edema. Five patients had grade 3 nausea. Also of note is that three patients developed an allergic reaction (erythema, itching) at the GM-CSF injection site during course 5 and GM-CSF was discontinued.
Table 5.
Number and Percentage of Courses With Grade III or IV Toxicities by Dose Level for Courses of ch14.18 Plus Interleukin-2
| Toxicity | ch14.18 Dose Level
|
|||
|---|---|---|---|---|
| 20 mg/m2/dose (23 courses)
|
25 mg/m2/dose (60 courses)
|
|||
| No. | % | No. | % | |
| Neuropathic pain | 7 | 35.0 | 55 | 87.3 |
| Fever without infection | 4 | 20.0 | 8 | 12.7 |
| Renal | ||||
| Low systolic BP | 10 | 15.9 | ||
| Low diastolic BP | 1 | 5.0 | 14 | 22.2 |
| Cardiac | ||||
| Hypertension | 2 | 3.2 | ||
| Hypotension | 3 | 15.0 | 2 | 3.2 |
| Peripheral capillary leak | 1 | 5.0 | 6 | 9.5 |
| Diarrhea | 1 | 1.6 | ||
| Nausea | 3 | 15.0 | 13 | 20.6 |
| Vomiting | 1 | 5.0 | 4 | 6.3 |
| Hypoxia | 1 | 5.0 | 3 | 4.8 |
| CNS cortical | 2 | 10.0 | 2 | 3.2 |
| Prolonged PTT | 1 | 5.0 | 4 | 6.3 |
| Hypokalemia | 1 | 5.0 | 2 | 3.2 |
| Infection | 4 | 20.0 | 2 | 3.2 |
| Decreased performance status | 3 | 4.8 | ||
| Leukopenia | 5 | 25.0 | 1 | 1.6 |
| Neutropenia | 6 | 30.0 | 9 | 14.3 |
| Thrombocytopenia | 7 | 35.0 | 12 | 19.0 |
| Anemia | 5 | 25.0 | 8 | 12.7 |
| Lymphopenia | 1 | 5.0 | 16 | 25.4 |
| Elevated | ||||
| AST | 2 | 10.0 | 1 | 1.6 |
| ALT | 3 | 15.0 | 1 | 1.6 |
| Alkaline phosphatase | 1 | 5.0 | ||
| Bilirubin | 2 | 10.0 | ||
Abbreviations: BP, blood pressure; CNS, cortical, included somnolence, agitation, confusion, and hallucinations; PTT, partial thromboplastin time.
Immunological Monitoring Results (serum ch14.18 peak level, HACA, and sIL-2Rα)
Serum samples were obtained from patients before starting the administration of ch14.18 antibody, after the ch14.18 infusion on the last day of ch14.18, and on days 4, 8, and 11 after antibody treatment (depending on the course; Fig 1). Specimens were available from two patients on regimen 1, four patients on regimen 2, and 12 patients on regimen 3. For patients treated on regimen 3, the range of peak serum ch14.18 levels were similar for courses 1 to 5. The mean peak levels for courses 4 and 5 were slightly lower than for courses 1 to 3 (P = .002 by Wilcoxon signed rank test; Appendix Table A1, online only).
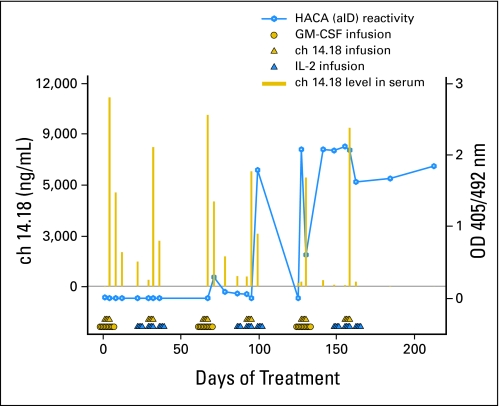
None of the samples from 12 patients on regimen 3 treatment showed HACA. HACA was detectible in two of 18 patients, both on regimen 1. Time points of samples use the notation of CnDn, referring to the course number (Cn) and the day of the course (Dn). Patient 2 had HACA starting from C3D10, peaking at C4D14, and continuing through C6D64 (Appendix Fig A1, online only). Patient QQ-5 showed HACA at C5D17 (not shown). The peak serum levels of ch14.18 for these two patients, during courses where HACA was observed, were similar to peak levels before development of HACA (Appendix Figure A1 for patient 2; not shown for patient QQ-5).
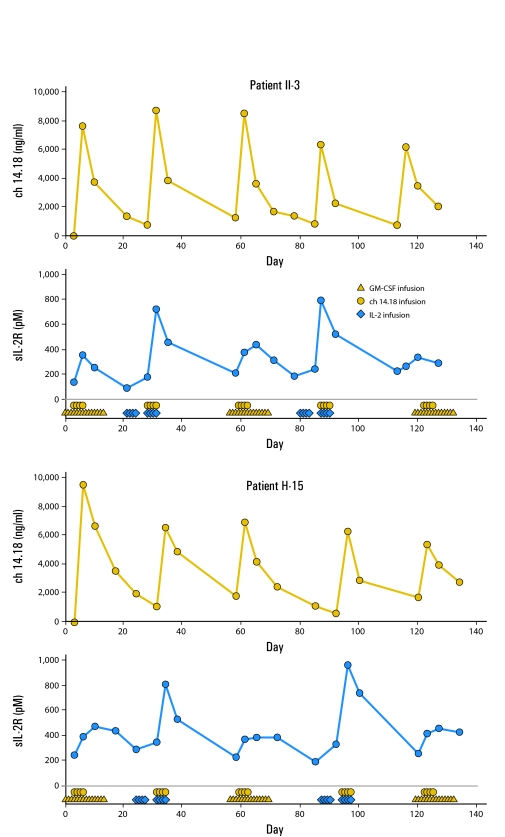
Serum from 10 patients was tested for sIL-2Rα. These data (Appendix Table A2, online only) show higher sIL-2Rα in samples collected after IL-2 administration compared with samples collected after GM-CSF treatment. This was statistically significant by one-sided paired t-test (P = .033). Appendix Figure A2 (online only) shows representative data for ch14.18 levels and sIL-2Rα levels from two patients treated on regimen 3.
Survival
Four patients died during or after protocol therapy from progressive neuroblastoma. The estimated 3-year survival probability was 78% (95% CI, 45% to 93%). The median follow-up time for those alive at last contact was 494 days (range, 0 to 1,772 days).
DISCUSSION
This study was designed to determine the MTD of ch14.18 when given with IL-2. The study built on a prior study that used ch14.18 and GM-CSF soon after HDC/SCR. The goal was to incorporate IL-2 to provide additional effector cells (NK cells) for ADCC and to generate LAK cells as another way of eradicating residual neuroblastoma cells. During the study, results from another study became available and showed an improved event-free survival for patients receiving cis-RA.1 The protocol therapy was therefore modified to accommodate this new standard of care. The study demonstrated the feasibility of alternating cis-RA with antibody and cytokines. Finally, additional patients were enrolled to confirm the MTD and to optimize the supportive care required before opening a phase III trial of this therapy.
The toxicity of ch14.18 given with IL-2 was considerable but manageable and reversible. Common toxicities included pain, fever, hypotension, and capillary leak. The pain was probably a result of binding of ch14.18 to peripheral nerves and of complement activation. It was controllable with narcotics, and usually resolved within hours of stopping the antibody. Fever, hypotension, and capillary leak syndrome were slightly more severe in courses with IL-2 than in courses with GM-CSF (data not shown). This is not surprising in light of the fact that IL-2 alone can cause these adverse effects. Capillary leak was usually not clinically significant and could be managed by diuresis during the part of the day when the patient was not receiving ch14.18. It was anticipated that course 4 would be tolerated better than course 2 because it was more removed from the toxicities of HDC/SCR, and this was the case.
Immune activation induced by IL-2 in courses 2 and 4 was confirmed by sIL-2Rα levels, a marker of in vivo response to IL-2.20 Serum ch14.18 levels showed concentrations of ch14.18 that readily induce ADCC when NK cells from IL-2–treated neuroblastoma patients are used as effectors.11,22 These data confirmed the desired biologic activity at the doses used. The IL-2 therapy was given during the week before ch14.18 and during the week of ch14.18. The ability of NK cells to mediate ADCC is augmented by repeated exposure to IL-2.22 Giving IL-2 before ch14.18 was also associated with a lower frequency of HACA in patients with melanoma than when IL-2 was begun concurrently with ch14.18.23
We identified only two patients (both on regimen 1) with HACA. This frequency of HACA is far less than that seen in adult patients with melanoma,23 and may reflect the very immunosuppressive “ablative” therapy these patients with neuroblastoma received shortly before starting ch14.18 treatment. In contrast, most patients with melanoma received little or no immunosuppressive chemotherapy before receiving ch14.18. Furthermore, and in contrast to that seen in some patients with melanoma, the HACA detected in the two patients in this study did not affect the peak serum level of ch14.18 in those treatment courses where HACA was observed (Appendix Figure A1, online only).
The study demonstrated the feasibility of incorporating IL-2 into a regimen of ch14.18 and GM-CSF. A dose of ch14.18 which could be tolerated with IL-2 was established. The study did not attempt to identify maximum doses of cytokine that could be used. For biotherapy, evidence of biologic activity is often the goal. The immunological monitoring documented the intended immune stimulation with the doses used. Although the survival data are interesting, the patient cohort was heterogeneous and poststudy therapy was not controlled. An appropriate group for comparison is therefore hard to identify. A randomized trial will be necessary to determine the benefit of this therapy. The regimen established in this study is currently being tested in a randomized phase III COG study to test the hypothesis that ch14.18, GM-CSF, and IL-2 will prevent relapse in the setting of MRD after HDC/SCR.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew L. Gilman, M Fevzi Ozkaynak, Katherine K. Matthay, Mark Krailo, Alice L. Yu, Jacquelyn A. Hank, Gregory H. Reaman, Paul M. Sondel
Administrative support: M Fevzi Ozkaynak
Provision of study materials or patients: Katherine K. Matthay, Paul M. Sondel
Collection and assembly of data: Jacek Gan, Adam Sternberg, Jacquelyn A. Hank, Robert Seeger, Paul M. Sondel
Data analysis and interpretation: Andrew L. Gilman, Katherine K. Matthay, Mark Krailo, Jacek Gan, Adam Sternberg, Jacquelyn A. Hank, Gregory H. Reaman, Paul M. Sondel
Manuscript writing: Andrew L. Gilman, Mark Krailo, Alice L. Yu, Jacek Gan, Gregory H. Reaman, Paul M. Sondel
Final approval of manuscript: M Fevzi Ozkaynak, Katherine K. Matthay, Jacquelyn A. Hank, Robert Seeger, Gregory H. Reaman, Paul M. Sondel
Appendix
Fig A1.
Serum ch14.18 level (gold bars) and human antichimeric antibody (HACA) reactivity (blue open circles, and sometimes referred to as “anti-idiotypic” antibody [aID]) detected in specimens collected from patient who received six courses of treatment (regimen 1). This was the only patient showing strong HACA reactivity starting after fourth infusion of ch14.18 antibody. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2 interleukin 2; OD, optical density.
Fig A2.
Soluble interleukin 2 receptor alpha subunit (sIL-2Rα; blue circles) and ch14.18 (gold circles) level in serum in two representative patients who received five courses of treatment (on regimen 3). GM-CSF, granulocyte-macrophage colony-stimulating factor.
Table A1.
ch14.18 Peak Level in Serum (ng/mL)
| Time Point Course and Day
|
No. of Patients | Mean Concentration (ng/mL) | Standard Deviation | Range | |
|---|---|---|---|---|---|
| Course | Day | ||||
| 1 | 6 | 11 | 13,082 | 2,999 | 7,620-17,165 |
| 2 | 10 | 11 | 11,216 | 3,885 | 6,574-18,816 |
| 3 | 6 | 12 | 12,654 | 4,225 | 6,953-20,707 |
| 4 | 10 | 9 | 9,489 | 3,543 | 6,157-15,849 |
| 5 | 6 | 7 | 8,831 | 3,250 | 5,405-14,038 |
NOTE. Patients on regimen 3. Serum samples obtained at the indicated times (ie, course 1, day 6) were assayed for ch14.18 levels.
Table A2.
Soluble Interleukin-2 Receptor α Subunit Peak Level in Serum: Patients on Regimen 3
| Time Point
|
No. of Patients | Mean Concentrations (pM) | Standard Deviation | Range | |
|---|---|---|---|---|---|
| Course | Day | ||||
| 1 | 10 | 4 | 377.7 | 101.9 | 252.7-474.8 |
| 2 | 10 | 4 | 753.3 | 40.6 | 718.1-810.7 |
| 3 | 10 | 4 | 360.0 | 78.3 | 250.8-435.5 |
| 4 | 10 | 4 | 863.8 | 195.5 | 629.2-1,073.4 |
| 5 | 10 | 4 | 382.2 | 86.0 | 285.1-458.7 |
published online ahead of print at www.jco.org on December 1, 2008.
Supported by COG Grant No. CA 98543. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Matthay KK, Villablanca JG, Seeger RC, et al: Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med 341:1165-1173, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Schulz G, Cheresh DA, Varki NM, et al: Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res 44:5914-5920, 1984 [PubMed] [Google Scholar]
- 3.Svennerholm L, Bostrom K, Fredman P, et al: Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biochim Biophys Acta 1214:115-123, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Gillies SD, Lo K-M, Wesolowski J: High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods 125:191-202, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Fest S, Kunert R, et al: Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol 42:1311-1319, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Barker E, Mueller BM, Handgretinger R, et al: Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res 51:144-149, 1991 [PubMed] [Google Scholar]
- 7.Sabzevari H, Gillies SD, Mueller BM, et al: A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A 91:9626-9630, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kushner BH, Cheung NKV: GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 73:1936-1941, 1989 [PubMed] [Google Scholar]
- 9.Yu AL, Batova A, Alvarado C, et al: Usefulness of a chimeric anti-GD2 (ch14.18) and GM-CSF for refractory neuroblastoma: A POG phase II study. Proc Am Soc Oncol 16:416a, 1997. (abstr 1846) [Google Scholar]
- 10.Ozkaynak MF, Sondel PM, Krailo M, et al: A phase I study of ch14.18 with GM-CSF in children with neuroblastoma immediately after hematopietic stem cell transplantation: Children's Cancer Group Study. J Clin Oncol 18:4077-4085, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Hank JA, Robinson RR, Surfus J, et al: Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res 50:5234-5239, 1990 [PubMed] [Google Scholar]
- 12.Hank JA, Kohler PC, Weil-Hillman G, et al: In vivo induction of the lymphokine-activated killer phenomenon: Interleukin-2-dependent human non-major histocompatibility complex-restricted cytotoxicity generated in vivo during administration of human recombinant interleukin-2. Cancer Res 48:1965-1971, 1988 [PubMed] [Google Scholar]
- 13.Lotze MT, Grimm EA, Mazumder A, et al: Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T cell growth factor. Cancer Res 41:4420-4425, 1981 [PubMed] [Google Scholar]
- 14.Grimm EA, Mazumder A, Zhang HZ, et al: The lymphokine activated killer cell phenomenon: Lysis of NK resistant fresh solid tumor cells by IL-2 activated autologous human peripheral blood lymphocytes. J Exp Med 155:1823-1841, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ades EW, Peacocke N, Sabio H: Lymphokine-activated killer cell lysis of human neuroblastoma cells: A model for purging tumor cells from bone marrow. Clin Immunol Immunopathol 46:150-156, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Schiller JH, Hank JA, Khorsand M, et al: Clinical and immunological effects of granulocyte-macrophage colony-stimulating factor coadministered with interleukin 2: A phase IB study. Clin Cancer Res 2:319-330, 1996 [PubMed] [Google Scholar]
- 17.Cheung NK, Kushner BH, Cheung IY, et al: Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol 16:3053-3060, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gan J, Kendra K, Ricci M, et al: Specific ELISAs for quantitation of antibody-cytokine fusion proteins. Clin Diagn Lab Immunol 6:236-242, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albertini M, Hank JA, Schiller JH, et al: Phase IB trial of chimeric anti-disialoganglioside antibody plus IL-2 for melanoma patients. Clin Cancer Res 3:1277-1288, 1997 [PubMed] [Google Scholar]
- 20.King DM, Albertini MR, Schalch H, et al: Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol 22:4463-4473, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:451-481, 1958 [Google Scholar]
- 22.Hank J, Surfus J, Gan J, et al: Treatment of neuroblastoma patients with antiganglioside GD2 antibody plus Interleukin-2 induces antibody dependent cellular cytotoxicity against neuroblastoma detected in vitro. J Immunother 15:29-37, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Albertini MR, Gan J, Jaeger P, et al: Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol 19:278-295, 1996 [DOI] [PubMed] [Google Scholar]