Abstract
Purpose
Prophylactic cranial irradiation (PCI) in patients with extensive-disease small-cell lung cancer (ED-SCLC) leads to significantly fewer symptomatic brain metastases and improved survival. Detailed effects of PCI on health-related quality of life (HRQOL) are reported here.
Patients and Methods
Patients (age, 18 to 75 years; WHO ≤ 2) with ED-SCLC, and any response to chemotherapy, were randomly assigned to either observation or PCI. Health-related quality of life (HRQOL) and patient-reported symptoms were secondary end points. The European Organisation for the Research and Treatment of Cancer core HRQOL tool (Quality of Life Questionnaire C30) and brain module (Quality of Life Questionnaire Brain Cancer Module) were used to collect self-reported patient data. Six HRQOL scales were selected as primary HRQOL end points: global health status; hair loss; fatigue; and role, cognitive and emotional functioning. Assessments were performed at random assignment, 6 weeks, 3 months, and then 3-monthly up to 1 year and 6-monthly thereafter.
Results
Compliance with the HRQOL assessment was 93.7% at baseline and dropped to 60% at 6 weeks. Short-term results up to 3 months showed that there was a negative impact of PCI on selected HRQOL scales. The largest mean difference between the two arms was observed for fatigue and hair loss. The impact of PCI on global health status as well as on functioning scores was more limited. For global health status, the observed mean difference was eight points on a scale 0 to 100 at 6 weeks (P = .018) and 3 months (P = .055).
Conclusion
PCI should be offered to all responding ED SCLC patients. Patients should be informed of the potential adverse effects from PCI. Clinicians should be alert to these; monitor their patients; and offer appropriate support, clinical, and psychosocial care.
INTRODUCTION
Small-cell lung cancer (SCLC) represents approximately 15% of newly diagnosed lung cancers.1 Most patients present with extensive disease (ED-SCLC). Brain metastases are common and response to treatment is poor.2-4
We recently studied the role of prophylactic cranial irradiation (PCI) in patients with ED-SCLC disease.5 Complete response is uncommon in extensive disease, so patients with any response to chemotherapy were included.5 We showed that PCI significantly decreases the risk of symptomatic brain metastases (from 40.4% to 14.6% at 1 year) and increases significantly overall survival (mortality hazard ratio of 0.68; 95% CI, 0.52 to 0.88). Median overall survival was 6.74 months in the PCI arm, compared with 5.42 months in the control arm. Survival at 1 year was 27.1% in the PCI arm, compared with 13.3% in the control arm.5
While PCI may lead to improved functioning by preventing or delaying the occurrence of symptomatic brain metastases, it can also have adverse effects. Therefore, an evaluation of the influence of PCI on health-related quality of life (HRQOL) and patient-reported symptoms was undertaken as part of this study and the detailed results are reported here.
PATIENTS AND METHODS
Study Design
This was an international multicenter phase III randomized controlled trial (RCT). Patients with ED-SCLC, who responded to chemotherapy, were randomly assigned between PCI or no further treatment. Patients were stratified for institution and performance status. The primary study end point was time to development of symptomatic brain metastases. Details on trial conduct and clinical outcome were reported previously.5 The protocol was reviewed by the European Organisation for Research and Treatment of Cancer (EORTC) protocol review committee and approved by the ethics committee in each institution. All patients provided written informed consent and the study was conducted in accordance with the Helsinki principles.
HRQOL and patient-reported symptoms were secondary study end points. Two HRQOL measures were selected: the EORTC Quality of Life Questionnaire C30 (EORTC-QLQ-C30, version 3)6 and the EORTC QLQ Brain Cancer Module (EORTC-QLQ-BN20).7 Both tools have robust psychometric properties resulting from rigorous testing, development, and external validity, and in the case of the core tool from their use in hundreds of cancer RCTs.8 The EORTC QLQ-C30 is a core measure designed to be supplemented with disease-specific questionnaires, in this case EORTC QLQ-BN20. This module has just undergone the final phase of validation.9 Both instruments were available in the language of all participating countries.10
The EORTC-QLQ-C30 measure comprises of five functioning scales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, nausea/vomiting, and pain), six single-item scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial impact), and the overall HRQOL-scale. The EORTC-QLQ-BN20 is designed for use with patients undergoing chemotherapy or radiotherapy, and is composed of 20 questions assessing visual disorders, motor dysfunction, communication deficit, various disease symptoms (eg, headaches and seizures), treatment toxicities (eg, hair loss), and future uncertainty.
The questions on both measures were scaled and scored using the recommended EORTC procedures.11 Raw scores were transformed to a linear scale ranging from 0 to 100, with a higher score representing a higher level of functioning or higher level of symptoms. Provided at least half of the items in the scale were completed, the scale score was calculated using only those items for which values existed.
The results of this study are presented in accordance with recent guidelines for reporting HRQOL RCTs.12 Differences of at least 10 points (on a 0 to 100 scale) were classified as the minimum clinically meaningful change in the mean value of a HRQOL parameter.13 A mean increase or decrease by ≥ 10 points would mean a moderate improvement or worsening. Mean changes of fewer than 10 effect points were considered as not to be clinically significant. Mean changes of more than 20 points were classed as large effects.
Before consent, patients were informed that their HRQOL would be assessed regularly. Patients completed the EORTC-QLQ-C30 and EORTC-QLQ-BN20 questionnaires at random assignment, at 6 weeks and at 3, 6, 9, and 12 months after random assignment and thereafter every 6 months until 3 years or death. Although we recognized the high attrition rate in this disease, we wanted to obtain data on quality of life in the longer-term survivors. The questionnaires were given to the patients by the investigator or a study nurse and completed before clinical evaluation.
Compliance levels were calculated using a standard EORTC procedure (number of forms received divided by number expected) at each assessment point. Time windows for acceptable HRQOL forms were defined as follows: at baseline fewer than 2 weeks before or 3 weeks after randomization, and before the start of PCI; thereafter, adjacent time windows were used to gather the maximum of information available (< 3 weeks before and after for assessments at 6 weeks, < 3 weeks before and < 6 weeks after at 3 months, < 6 weeks before and after every 3 months and < 3 months before and after for assessments collected every 6 months).
Statistical Analysis
During the design of the study, in order to reduce type I errors from multiple testing of HRQOL scales, six key HRQOL and symptom scales were preselected based on discussions with clinicians, which informed the protocol. The level of statistical significance was initially set at P = .01 to account for multiple comparisons (several HRQOL scales and different time points). The main objective of the HRQOL assessment was to determine the impact of PCI on the global health status scale and additionally to study the expected adverse effects of PCI: hair loss, fatigue, and restrictions in daily activities (role functioning). An improvement was expected after treatment. Moreover, as PCI leads to a significantly lower rate of symptomatic brain metastases and improved survival, an early and more pronounced deterioration of global health status, cognitive and emotional functioning was expected in the non-PCI group. Other HRQOL scales were analyzed on an exploratory basis.
All data analyses were performed using the Statistical Analysis Software version 9 (SAS, Cary, NC). A mixed-model with an undefined covariance structure was fitted to the longitudinal HRQOL data of each selected score to test differences between the two treatment groups. HRQOL scores were analyzed as normal continuous data and data summarized in terms of mean scores and its evolution over time. All patients with at least one valid HRQOL form were included in the analysis (n = 280). As missing HRQOL data is a common problem, particularly in patients with a poor prognosis such as ED-SCLC, the mechanism of missing data was investigated to check the reliability of using a mixed model.
To analyze HRQOL scores as continuous can be criticized especially for scores with only a few number of categories. Therefore, the percentages of patients who experienced more than a 20-point worsening from baseline up to 3 months after random assignment in each selected scale were also reported. The percentages were computed on the total number of patients with a baseline HRQOL assessment and with at least an additional follow-up HRQOL form at 6 weeks or 3 months.
RESULTS
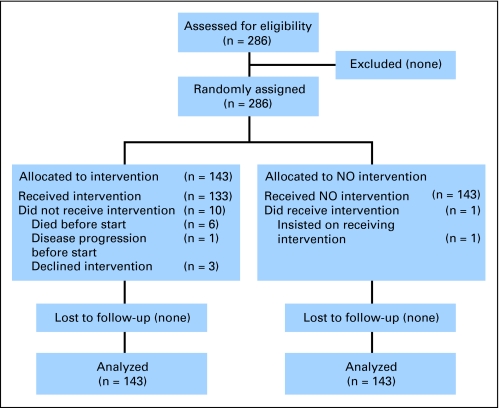
Between February 2001 and March 2006, 286 patients were recruited (143 in each arm) from 40 centers. A total of 280 patients had at least one valid HRQOL form and 268 (93.7%) had a baseline assessment. Per study design, it was expected that the number of patients involved in the study at 3 years would be 120. The HRQOL assessments were to be performed until 3 years after random assignment or death. However, the duration of survival was shorter than expected, with median survival times around 6 months. In addition, compliance with the HRQOL assessment dropped to 60.0% at 6 weeks, 54.5% at 3 months, 60.8% at 6 months, 46.3% at 9 months, and 48.9% at 1 year (Table 1). Therefore, only data obtained up to 9 months were included in the analysis because of the small number of patients’ data at 1 year (only six patients in the non-PCI arm).
Baseline clinical characteristics of patients with a baseline HRQOL assessment were very similar to those without it. Baseline HRQOL scores were similar in both arms, and Table 2 presents a comparison with normative data and other reference data in ED-SCLC.14 As can be expected by the inclusion of only responding patients in our study, our sample had less symptoms and HRQOL problems at baseline on a number of HRQOL and symptom scales.
Fisher tests revealed no significant difference in compliance between the two arms at any time point. Reason for noncompletion of HRQOL forms was reported for 302 forms. Most common reasons were administrative failure (40.1%) and the patient being too ill to complete the questionnaire (23.8%). In the graphical investigation for drop-out mechanisms, no systematic sign of an informative drop-out was observed (data not shown). However, the mean scores for role and cognitive functioning showed a sharp decrease before drop-out at 6 months. The rapid deterioration of the patients could have led to an informative drop-out for some of the HRQOL scores. Therefore, a sensitivity analysis with HRQOL data cutoff at 3 months was performed. This period was considered long enough to observe any impairment of HRQOL due to PCI during and shortly after the treatment period.
The mean global health status score was eight points higher in the PCI group at 6 weeks (P = .018) and 3 months (P = .056; Table 3 and Fig 2). These observed differences were below the cutoff of 10-point difference for clinical significance. None of the P values were below .01 but the P value at 6 weeks was close to .01. The results of the sensitivity analysis did not differ (Appendix Table A1, online only). In terms of proportions, there was 12.5% more patients in the PCI arm compared with the control arm experiencing a worsening ≥ 20 points (large effects) in global health status from baseline up to 3 months (Table 4).
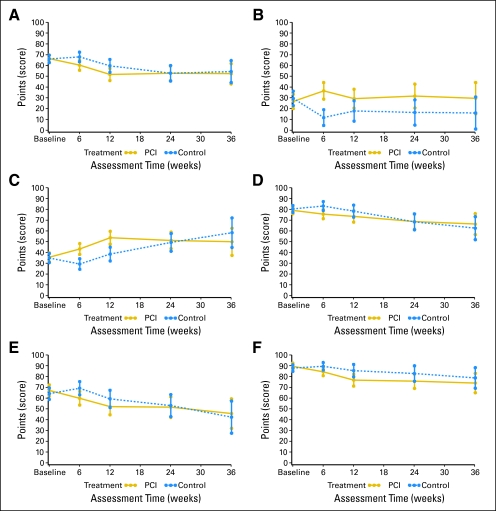
Fig 2.
Scales for (A) global health status, (B) hair loss, (C) fatigue, (D) role functioning, (E) cognitive functioning, and (F) emotional functioning. PCI, prophylactic cranial irradiation.
As expected, there were some treatment-related effects and mean scores for both hair loss and fatigue were significantly higher in the PCI arm (overall test, P < .001; Fig 2). The difference in mean scores for hair loss between the two arms exceeded 10 points at all time points after baseline, attained statistical significance (P < .001) at 6 weeks, with a mean score of 36.5 for the PCI group and 11.7 for controls. For fatigue, the mean difference was statistically and clinically significant at 6 weeks (43.2 for PCI and 29.3 for control), and at 3 months (53.6 for PCI arm and 38.5 for control). The impact of PCI was limited for role, emotional, and cognitive functioning. There was a maximum mean difference of 9.4 at week 6 for role functioning, 8.8 at month 3 for cognitive functioning, and 7.4 at week 6 for emotional functioning, with all favoring the control arm. None of the P values were below .01 nor was the 10-point clinical significant difference reached at any time point. These results were confirmed by the sensitivity analysis. The analysis of the proportions of patients experiencing a worsening from baseline up to 3 months confirmed that the larger impact of PCI was on fatigue. The impact of PCI on functioning scales was similar to the one on global health status—more limited (Table 5). For hair loss, the difference between the two arms was larger, as there was an improvement in the control arm (Fig 1). No assessment of the long-term impact or benefit of PCI on HRQOL was possible due to the small number of patients with data at later time points.
Fig 1.
CONSORT diagram. Other selected health-related quality-of-life scales.
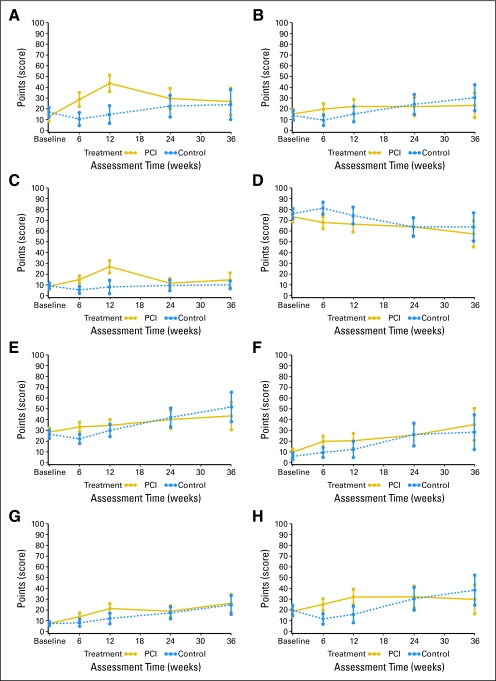
Our exploratory analysis of the remaining HRQOL and symptom scales (ie, not preselected for the analysis) showed statistically significant (P < .01) and clinically significant (or near to) mean differences between the two arms for appetite loss, constipation, nausea/vomiting, social functioning, future uncertainty, headaches, motor dysfunction, and weakness of the legs at 6 weeks and/or at 3 months (Fig 2). Clearly, while these are only exploratory analyses, they do indicate a trend toward worsening of HRQOL on some scales in the PCI arm. The lack of data does not allow us to report reliably on more long-term results.
DISCUSSION
In the 1990s, a number of randomized trials unequivocally showed that PCI reduces the incidence of brain metastases in patients with limited disease–SCLC, without increasing toxicity if not given concurrently with chemotherapy.15-19 Meta-analyses revealed a survival benefit for PCI,18,19 with a 3-year survival rate of 21% in patients who received PCI, versus 15% in those who did not.18 Our recent study in patients with ED-SCLC showed that PCI resulted in a significantly improved survival and a significant reduction in the rate of brain metastases.5 Due to the poor survival in patients with ED-SCLC, inclusion in this study was limited to patients with a response after chemotherapy. In addition, short and relatively low-dose fractionation schemes were used. We demonstrated that the reduction in hazard rate for symptomatic brain metastases was around 75%. PCI also significantly prolonged overall survival (P = .0033).10
This study reported here gives further insights into the effect of PCI on the patient. As expected, short-term results showed that PCI had a negative impact on selected HRQOL scales. Among the selected HRQOL scales, the larger difference between the two arms was observed on hair loss and fatigue at 6 weeks and 3 months in favor of the control arm. For hair loss, the difference was larger as there was an improvement in the control arm. The impact of PCI on functioning scales was limited. The key HRQOL results showed an eight-point difference in observed mean scores in global health status in favor of the control arm at 6 weeks and 3 months after random assignment (below the minimum clinical difference of 10 points). The P value was close to .01 at 6 weeks. There were 12.5% more patients in the PCI arm who experienced severe worsening (> 20 points) in global health status from baseline up to 3 months. Short-term results were quite similar for role, cognitive, and emotional functioning.
No reliable assessment of a more long-term impact and expected benefit of PCI on HRQOL was possible due to the small number of patients with data at later time points. In addition, there was the suspicion of data not missing at random. Reason for noncompletion of HRQOL forms was reported for 302 forms and 23.8% were not filled in because the patient was or felt too ill to complete the questionnaire. Further analysis revealed that, although there was no statistically significant difference in compliance between the two arms, the return rate of HRQOL forms for patients with brain metastases at 3 months was 31.8% compared with 56.8% in patients without brain metastases. Therefore, the occurrence of brain metastases even at these early time points, could have resulted in a lack of reporting rather than in a reporting of a deterioration, not favoring the PCI arm.
For cognitive functioning, the results were opposite to the anticipated effects, as scores after 3 months were 8.8 points higher in the control arm. This could be due to the lower return rate for patients with brain metastases, but we cannot exclude the possibility of a real difference in favor of the non-PCI group in the self-report of cognitive functioning. In particular, the PCI group reported more fatigue at 3 months, which can also adversely affect cognitive functioning. Finally, it is known that the self-report of cognitive functioning and clinical assessment of cognitive functioning may be poorly correlated.
The remaining HRQOL and symptom scores were analyzed on an exploratory basis. PCI clearly does have an adverse impact on appetite loss, constipation, nausea/vomiting, social functioning, future uncertainty, headaches, motor dysfunction, and weakness of legs at 6 weeks and/or at 3 months. These are important issues for patients and treating clinicians to be aware of.
This study had a number of limitations and weaknesses. The significant challenge was the small number of patients for whom data were still available at 1 year (only six patients in the non-PCI arm); therefore, only data up to 9 months were analyzed. The compliance with measures at follow-up assessments was lower than expected, although it was almost 50% at 1 year. The major causes were administrative failure or patients being too sick to complete the measures. However, this is often the case in patients with advanced cancer, as recently noted in a systematic review.20 In this study, survival was shorter than expected, thereby limiting power estimates considerably. This is a well-known problem when trials are powered for other clinical end points and not HRQOL end points.13 With few patients, the lack of statistical significance should not be necessarily interpreted as a proof of no difference.
This study clearly demonstrates the significant challenge of effective data collecting in palliative care patients. Although we undertook an extensive statistical analysis to evaluate most sources of bias, this must be noted as a major caution in interpreting the results. Patient HRQOL and symptom results are best interpreted on the data collected early in the study.
In the majority of cases, missing data was due to administrative failure and this must be addressed in future trials, with different approaches (eg, by providing extra funding for and education and training in HRQOL assessment, ensuring better monitoring of HRQOL data, using the newly validated shortened EORTC palliative care module [a tool not available at the start of this study], and even ultimately considering selecting only good recruiting centers to participate in HRQOL studies when studies involve patients in the palliative setting). Hence, while this trial has limitations in terms of HRQOL compliance and symptom-reported data, it has helped direct increased attention to the future design of studies with palliative patients.
In summary, this study has shown that PCI improves survival and reduces the incidence of brain metastases in patients who have shown a response to chemotherapy for their ED-SCLC. There is a cost in terms of more prolonged hair loss and increased fatigue, both expected treatment-related adverse effects. There was a negative impact of PCI on functioning scales but limited. Therefore, PCI should be offered to all patients with ED-SCLC who respond to initial chemotherapy. Patients should be told of the benefits of PCI and of its possible negative impact on QOL empowering them with relevant information to allow informed, individualized treatment choices.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Berend J. Slotman, Murielle E. Mauer, Andrew Bottomley, Corinne Faivre-Finn, Gijs WPM Kramer, Elaine M. Rankin, Pieter E. Postmus, Laurence Collette, Suresh Senan
Administrative support: Murielle E. Mauer, Laurence Collette
Provision of study materials or patients: Berend J. Slotman, Corinne Faivre-Finn, Elaine M. Rankin, Michael Snee, Matthew Hatton, Pieter E. Postmus, Suresh Senan
Collection and assembly of data: Berend J. Slotman, Murielle E. Mauer, Andrew Bottomley, Gijs WPM Kramer, Laurence Collette
Data analysis and interpretation: Berend J. Slotman, Murielle E. Mauer, Andrew Bottomley, Laurence Collette, Suresh Senan
Manuscript writing: Berend J. Slotman, Murielle E. Mauer, Andrew Bottomley, Corinne Faivre-Finn, Elaine M. Rankin, Michael Snee, Matthew Hatton, Pieter E. Postmus, Suresh Senan
Final approval of manuscript: Berend J. Slotman, Murielle E. Mauer, Andrew Bottomley, Corinne Faivre-Finn, Elaine M. Rankin, Michael Snee, Matthew Hatton, Pieter E. Postmus, Suresh Senan
Supplementary Material
Acknowledgments
We thank the clinicians in the following centers for their participation in this study: C. Faivre-Finn, Christie Hospital Manchester, United Kingdom (UK); G. Kramer (deceased), Arnhem's Radiotherapeutisch Instituut, the Netherlands; E. Rankin, Ninewells Hospital Dundee, UK; M. Snee, Cookridge Hospital Leeds, UK; M. Hatton, Weston Park Hospital Sheffield, UK; R. Gafaar, National Cancer Institute, Egypt; J. Bussink, UMC St Radboud Nijmegen, the Netherlands; A. Armour, N. Mohammed, Western Infirmary Glasgow, UK; S. Senan, J. Van Meerbeeck, P. Levendag, M. Van Mierlo, Universitair Ziekenhuis. Rotterdam, the Netherlands; A. Price, Western General Hospital, UK; J. Christian, S. Morgan, Nottingham General/City Hospital, UK; C. Ottensmeier, Southampton General Hospital, UK; P. Rodrigus, M. Van De Pol, B. Verbeeten Instituut, Tilburg, the Netherlands; R. Dziadziuszko, Gdansk-Medical University, Poland; W. Smit, RIF Leeuwarden, the Netherlands; J. Immerzeel, RISO, Deventer, the Netherlands; A. Van der Leest, UMC Groningen, the Netherlands; F. Macbeth, Velindre Hospital, UK; P. Mulvenna, Newcastle General Hospital, UK; J. Stigt, Sophia Ziekenhuis, Zwolle, the Netherlands; J. Van Meerbeeck, Universiteit Gent, Belgium; L. Uitterhoeve, AMC, Amsterdam, the Netherlands; S. Falk, Bristol Oncology Center, UK; L. Bosquee, CHR La Citadelle, Liege, Belgium; C. Goor, AZ Middelheim, Antwerp, Belgium; D. Papamichael, Bank of Cyprus Oncology Center, Cyprus; E. Marshall, Clatterbridge Centre, UK; M. Abacioglu, Marmara University Hospital, Turley; M OBrien, Royal Marsden Hospital, UK; T. Scolaro, Universita Genova, Italy; B. Slotman, VUmc, Amsterdam, the Netherlands; R. Cooper, Dokuz Eylul University, Turkey; N. Shah, Mount Vernon Northwood, UK; J. Lester, Nevill Hall Hospital, Abergavenny, UK; M. Lind, Princess Royal Hospital Hull, UK; D. Whillis, Raigmore Inverness, UK; T. Tzuk-Shina, Rambam Medical Center, Israel; G. Numico, Santa Croce Cuneo, Italy; L. Willems, UMC Leiden, the Netherlands; and K. Hideghety, University of Kaposvar, Hungary.
Appendix
Table A1.
Sensitivity Analysis Results of the Global Quality-of-Life Scale With Data Cut-Off at 3 Months
| Assessment Time | PCI
|
Control
|
P for Treatment Difference | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Overall* | .0372 | ||||
| Baseline | 66.5 | 1.68 | 66.1 | 1.72 | .8672 |
| At 6 weeks | 60.2 | 2.35 | 67.9 | 2.26 | .0186 |
| At 3 months | 51.8 | 2.88 | 59.1 | 3.05 | .0848 |
Abbreviations: PCI, prophylactic cranial irradiation; SD, standard deviation.
Multivariate test of no difference at any follow-up time point.
Fig 3.
Scales for (A) appetite loss, (B) constipation, (C) nausea/vomiting, (D) social functioning, (E) future uncertainty, (F) headaches, (G) motor dysfunction, and (H) weakness of legs. PCI, prophylactic cranial irradiation.
Table 1.
Compliance With Health-Related Quality-of-Life Assessments
| Time Point | Forms
|
% | Difference | |
|---|---|---|---|---|
| Expected | Received | |||
| Baseline | 286 | 268 | 93.7 | 0.144 |
| PCI | 143 | 137 | 95.8 | |
| Control | 143 | 131 | 91.6 | |
| At 6 weeks | 270 | 162 | 60.0 | 0.274 |
| PCI | 134 | 76 | 56.7 | |
| Control | 136 | 86 | 63.2 | |
| At 3 months | 235 | 128 | 54.5 | 0.340 |
| PCI | 120 | 69 | 57.5 | |
| Control | 115 | 59 | 51.3 | |
| At 6 months | 130 | 79 | 60.8 | 0.679 |
| PCI | 71 | 42 | 59.2 | |
| Control | 59 | 37 | 62.7 | |
| At 9 months | 82 | 38 | 46.3 | 0.948 |
| PCI | 45 | 21 | 46.7 | |
| Control | 37 | 17 | 45.9 | |
| At 12 months | 45 | 22 | 48.9 | 0.155 |
| PCI | 28 | 16 | 57.1 | |
| Control | 17 | 6 | 35.3 | |
Abbreviations: PCI, prophylactic cranial irradiation.
Table 2.
Mean Baseline Scores for the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire C30 by Treatment Group and Normative Reference Values
| HRQOL Score | Reference Data Normative (n = 1,956) | Reference Data ED-SCLC [19] (n = 271) | Control Arm (n = 131) | PCI Arm (n = 137) |
|---|---|---|---|---|
| Global health status/QOL | 75.3 | 56 | 66 | 67 |
| Functioning | ||||
| Physical | 89.9 | 57 | 66 | 68 |
| Role | 83.3 | 59 | 64 | 67 |
| Emotional | 82.8 | 66 | 80 | 79 |
| Cognitive | 86.5 | 77 | 88 | 90 |
| Social | 85.8 | 68 | 76 | 73 |
| Fatigue | 28.8 | 49 | 35 | 35 |
| Nausea/vomiting | 4 | 12 | 9 | 9 |
| Pain | 20.4 | 36 | 13 | 12 |
| Dyspnea | 14.3 | 49 | 28 | 31 |
| Insomnia | 20.4 | 43 | 24 | 23 |
| Appetite loss | 7.4 | 39 | 17 | 13 |
| Constipation | 10.7 | 28 | 14 | 15 |
| Diarhea | 9.4 | 8 | 7 | 6 |
Abbreviations: HRQOL, health-related quality of life; ED-SCLC, extensive-disease small-cell lung cancer; PCI, prophylactic cranial irradiation; QOL, quality of life.
Table 3.
Global Quality-of-Life Results With Data Cut-Off at 9 Months
| Assessment Time | PCI
|
Control
|
P for Treatment Difference | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Overall* | .1134 | ||||
| Baseline | 66.5 | 1.68 | 66.1 | 1.72 | .8633 |
| At 6 weeks | 60.2 | 2.34 | 67.9 | 2.25 | .0183 |
| At 3 months | 51.7 | 2.85 | 59.7 | 3.03 | .0554 |
| At 6 months | 52.8 | 3.41 | 52.8 | 3.67 | .9919 |
| At 9 months | 52.4 | 4.81 | 54.4 | 5.21 | .7764 |
Abbreviations: PCI, prophylactic cranial irradiation; SD, standard deviation.
Multivariate test of no difference at any follow-up time point.
Table 4.
Proportion of Patients Experiencing Severe Worsening From Baseline Up to 3 Months
| Parameter | Treatment
|
Total (N = 188)
|
||||
|---|---|---|---|---|---|---|
| PCI (n = 98)
|
Control (n = 90)
|
|||||
| No. | % | No. | % | No. | % | |
| Global health status | ||||||
| ≥ 20 points decrease | ||||||
| No | 64 | 65.3 | 70 | 77.8 | 134 | 71.3 |
| Yes | 34 | 34.7 | 20 | 22.2 | 54 | 28.7 |
| Hair loss | ||||||
| ≥ 20 points increase | ||||||
| No | 76 | 77.6 | 79 | 87.8 | 155 | 82.4 |
| Yes | 22 | 22.4 | 11 | 12.2 | 33 | 17.6 |
| Fatigue | ||||||
| ≥ 20 points increase | ||||||
| No | 50 | 51.0 | 66 | 73.3 | 116 | 61.7 |
| Yes | 48 | 49.0 | 24 | 26.7 | 72 | 38.3 |
| Role functioning | ||||||
| ≥ 20 points decrease | ||||||
| No | 63 | 64.3 | 68 | 75.6 | 131 | 69.7 |
| Yes | 35 | 35.7 | 22 | 24.4 | 57 | 30.3 |
| Cognitive functioning | ||||||
| ≥ 20 points decrease | ||||||
| No | 76 | 77.6 | 81 | 90.0 | 157 | 83.5 |
| Yes | 22 | 22.4 | 9 | 10.0 | 31 | 16.5 |
| Emotional functioning | ||||||
| ≥ 20 points decrease | ||||||
| No | 77 | 78.6 | 79 | 87.8 | 156 | 83.0 |
| Yes | 21 | 21.4 | 11 | 12.2 | 32 | 17.0 |
Abbreviation: PCI, prophylactic cranial irradiation.
published online ahead of print at www.jco.org on December 1, 2008.
Supported by Grants No. 5U10-CA11488-29 through 5U10 CA11488-37 from the National Cancer Institute (Bethesda, MD), as well as by the EORTC Charitable Trust. Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Data management was supported by the Dutch Cancer Society (CKTO).
Presented in part in abstract format at the 43rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2007 and the IASLC World Conference, Seoul, Korea, September 2-6, 2007.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00016211.
REFERENCES
- 1.Govindan R, Page N, Morgensztern D, et al: Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J Clin Oncol 24:4539-4544, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Groen HJM, Smit EF, Haaxma-Reiche H, et al: Carboplatin as second line treatment for recurrent or progressive brain metastases from small cell lung cancer. Eur J Cancer 29A:1696-1699, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Postmus PE, Haaxma-Reiche H, Gregor A, et al: Brain only metastases of small cell lung cancer; efficacy of whole brain radiotherapy: An EORTC phase II study. Radiother Oncol 46:29-32, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Postmus PE, Haaxma-Reiche H, Smit EF, et al: Treatment of brain metastases of small-cell lung cancer: Comparing teniposide and teniposide with whole brain radiotherapy: A phase III study of the EORTC Lung Cancer Cooperative Group. J Clin Oncol 18:3400-3408, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Slotman BJ, Faivre-Finn C, Kramer GW, et al: Prophylactic cranial irradiation in small cell lung cancer. N Engl J Med 357:664-672, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Aaronson NK, Ahmedzai S, Bergman B, et al: The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Osoba D, Aaronson NK, Muller M, et al: The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 5:139-150, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Efficace F, Bottomley A: Health related quality of life assessment methodology and reported outcomes in randomised controlled trials of primary brain cancers. Eur J Cancer 38:1824-1831, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Taphoorn MJ, Claassens L, Aaronson NK, et al: The EORTC QLQ-BN20 questionnaire for assessing the Health-Related Quality of Life (HRQoL) in brain cancer patients: A phase IV validation study on behalf of the EORTC QLG, BCG, ROG, NCIC-CTG. J Clin Oncol 26:99s, 2008. (suppl; abstr 2041) [Google Scholar]
- 10.Cull A, Sprangers M, Bjordal K, et al: Guidelines for Translating EORTC Questionnaires. Brussels, Belgium, Quality of Life Study Group Publications, EORTC Publications, 2002
- 11.Fayers P, Aaronson NK, Bjordal K, et al: EORTC QLQ-C30 Scoring Manual (ed 3). Brussels, Belgium, EORTC Publications, 2001
- 12.Bottomley A, Flechtner H, Efficace F, et al: Health related quality of life outcomes in cancer clinical trials: On behalf of the EORTC Data Center and Quality of Life Group. Eur J Cancer 41:1697-1709, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Osoba D, Rodrigues G, Myles J, et al: Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139-144, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Fayers PM, Weeden S, Curran D, on behalf of the EORTC Quality of Life Study Group: EORTC QLQ-C30 Reference Values. Brussels, Belgium, EORTC, 1998
- 15.Arriagada R, Monnet I, Riviere A et al: Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. Eur J Cancer 31A:83, 1995. (suppl 5) [Google Scholar]
- 16.Arriagada R, LeChevalier T, Borie F, et al: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 87:183-190, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Gregor A, Cull A, Stephens RJ, et al: Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: Results of a multicentre randomised trial (UKCCCR and EORTC). Eur J Cancer 33:1752-1758, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Auperin A, Arriagada R, Pignon JP, et al: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 341:476-484, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Meert AP, Paesmans M, Berghmans T, et al: Prophylactic cranial irradiation in small cell lung cancer: A systematic review of the literature with meta-analysis. BMC Cancer 1:1-9, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joly F, Vardy J, Pintilie M, Tannock IF: Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Ann Oncol 12:1935-1942, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.