Abstract
Purpose
To determine the impact of adjuvant androgen deprivation therapy (ADT) for patients who have node-positive prostate cancer in the prostate-specific antigen (PSA) era.
Patients and Methods
We used linked Surveillance, Epidemiology and End Results-Medicare data to construct a cohort of men who underwent radical prostatectomy (RP) between 1991 and 1999 and who had positive regional lymph nodes. We classified men as receiving adjuvant ADT if they received ADT within 120 days of RP, and we compared them to the men who had not received adjuvant ADT. We used propensity scores to balance potential confounders of receiving adjuvant ADT (ie, tumor characteristics, extent of nodal disease, demographics, receipt of radiation therapy) and Cox proportional hazard methods to measure the impact of adjuvant ADT on overall survival (OS), stratified by propensity score quintile. We conducted a sensitivity analysis that used 90, 150, 180, and 365 days as the definition for adjuvant ADT.
Results
A total of 731 men were identified, 209 of whom received ADT within 120 days of RP. There was no statistically significant difference in OS between the adjuvant ADT and non-ADT group (HR, 0.97; 95% CI, 0.71 to 1.27). There was no statistically significant survival difference with 90, 150, 180, and 365 days as the adjuvant ADT definition.
Conclusion
Deferring immediate ADT in men with positive lymph nodes after RP may not significantly compromise survival. Because observational studies should be considered hypothesis-generating studies, these results should be validated in a prospective fashion in a similar patient population.
INTRODUCTION
Although androgen deprivation therapy (ADT) has a well-defined role in patients who have metastatic disease1 or high-risk, localized disease and who are undergoing radiotherapy,2 its role in patients who have node-positive disease after radical prostatectomy (RP) is controversial. Messing et al3,4 reported the results of a randomized, controlled clinical trial of men who had node-positive prostate cancer after RP that compared life-long adjuvant ADT that was started immediately after RP with ADT that was initiated at the time of metastatic disease. The study reported a significant advantage in progression-free survival (PFS) and overall survival (OS) that favored adjuvant ADT.3,4 However, development of clinical metastases, rather than biochemical recurrence, was the indication for treatment in the delayed arm in this study. This is in contrast to the contemporary practice post-operative prostate-specific antigen (PSA) surveillance to detect biochemical recurrence (BCR). Because BCR occurs at a median of 8 years before the onset of radiologic and other evidence of metastatic disease,5 this long interval allows physicians the opportunity to initiate therapy before the onset of metastatic disease on the basis of an assessment of patients’ risks for disease progression and prostate cancer–specific mortality (PCSM).
Therefore, the benefit of adjuvant ADT in contemporary node-positive patients is unclear. Given the potential for long-term adverse effects associated with ADT, such as osteoporosis,6 cardiovascular disease, diabetes,7 and mood disorders,8 it is important to understand the impact of adjuvant ADT on OS. If adjuvant ADT is not associated with an improvement in OS compared with treatment at BCR, or sometime thereafter, then delaying the initiation of ADT until BCR may spare patients significant treatment-related toxicities.
To better characterize the role of adjuvant ADT on OS in this setting, we examined a population-based sample of men who had node-positive prostate cancer after RP.
PATIENTS AND METHODS
Data Source
We used data from the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database. SEER is a population-based cancer registry that encompasses approximately 14% of the US population and that is administered by the National Cancer Institute. SEER includes information on tumor histology, size, and grade.9 Approximately 97% of individuals in SEER who were 65 years and older were successfully linked to their Medicare claims.
The original study population included 111,640 men between 65 and 80 years of age who had an incident prostate cancer diagnosis between 1991 and 1999. Men were excluded if they were diagnosed at autopsy or death or had Medicare entitlement on the basis of end-stage renal disease. Because Medicare does not contain complete claims information for individuals in managed care, men were excluded if they were enrolled on a health maintenance organization from 90 days before diagnosis to 180 days after diagnosis; this represented approximately 21% of the original cohort. The project was approved by the institutional review boards at the Fox Chase Cancer Center and the University of Pennsylvania.
Variable Definitions
SEER-reported tumor grades were well differentiated (Gleason score of 2 to 4), moderately differentiated (Gleason score of 5 to 7) or poorly differentiated (Gleason Score of 8 to 10), anaplastic or unknown. Twenty-one patients who were classified as having anaplastic or unknown tumors were included in the poorly differentiated group.
Tumor Stage
We used clinical extension information provided by SEER to determine tumor stage for patients who were diagnosed from 1991 to 1995. In this category, operative/pathology information is given priority over clinical information in determining stage. In 1995, SEER began to report pathologic information separately, and this pathologic extension category was used to determine tumor stage for patients who were diagnosed from 1995 to 1999. For patients whose pathologic extension was categorized as unknown, we used the corresponding clinical extension. We categorized patients as T2c or less, T3a, or T3b to T4. We included 12 patients who had unknown clinical stage in the T3b to T4 category, and we excluded nine patients who had metastatic disease.
Definition of Node-Positive Disease
Only patients who had positive regional lymph nodes were included in this analysis. SEER described regional lymph nodes as N1 (single node < 2 cm), N2 (single node 2 to 5 cm or multiple nodes, none > 5 cm), N3 (single or multiple lymph nodes, at least one of which is > 5 cm), or regional nodes NOS. Five patients in our data set were described as having at least one lymph node greater than 5 cm; they were included in the regional nodes NOS in this analysis. Patients with distant lymph nodes, lymph nodes NOS, or unknown were excluded.
Covariates
Comorbid disease.
Comorbidities were identified by searching Medicare inpatient and outpatient claims and Part B claims during the 90 days before diagnosis. Comorbidities were identified by using a modification of the methods described by Elixhauser.10 In this analysis, cancer was not considered a comorbidity; however, stroke and coronary heart disease were included on the basis of their relatively high prevalence. In our propensity score models, we calculated the odds of receiving ADT on the basis of the number of comorbidities.
Demographics.
Age, marital status, ethnicity, year of diagnosis, and SEER registry were provided by SEER. Patients were classified as living in a rural area if they lived in a county of fewer than 20,000 residents; the remaining patients were classified as living in an urban area. Because SEER does not provide individual patient level socioeconomic status (SES), we used median household income per census tract and percent census tract with a four-year college education as proxies for SES. Eighteen patients did not have SES data. However, these characteristics were balanced between the adjuvant ADT and no-ADT arms in the baseline analysis (Table 1). The analyses with and without SES had similar results, so we did not use SES in our final models.
Table 1.
Patient Characteristics
| Characteristic | No Adjuvant Treatment
|
Adjuvant Treatment
|
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No. of patients | 522 | 209 | |||
| Median age at diagnosis | 69.9 | 69.5 | .161 | ||
| Year of diagnosis | .061 | ||||
| 1991-1993 | 319 | 61.11 | 120 | 57.42 | |
| 1994-1996 | 134 | 25.67 | 47 | 22.49 | |
| 1997-1999 | 69 | 13.22 | 42 | 20.10 | |
| Ethnicity | .544 | ||||
| White | 477 | 91.38 | 186 | 89.00 | |
| African-American | 26 | 4.98 | 12 | 5.74 | |
| Other | 19 | 3.64 | 11 | 5.26 | |
| Marital status | .127 | ||||
| Single/other | 83 | 15.90 | 24 | 11.48 | |
| Married | 439 | 84.10 | 185 | 88.52 | |
| SEER site | < .005 | ||||
| San Francisco | 67 | 12.84 | 20 | 9.57 | |
| Connecticut | 30 | 5.75 | 8 | 3.83 | |
| Detroit | 53 | 10.15 | 19 | 9.09 | |
| Hawaii | 8 | 1.53 | 7 | 3.35 | |
| Iowa | 63 | 12.07 | 40 | 19.14 | |
| New Mexico | 40 | 7.66 | 9 | 4.31 | |
| Seattle | 80 | 15.33 | 27 | 12.92 | |
| Utah | 70 | 13.41 | 13 | 6.22 | |
| Atlanta | 15 | 2.87 | 15 | 7.18 | |
| San Jose-Monterey | 31 | 5.94 | 7 | 3.35 | |
| Los Angeles | 65 | 12.45 | 44 | 21.05 | |
| Comorbidities | .011 | ||||
| 0 | 257 | 51.3 | 94 | 40.87 | |
| 1 | 135 | 26.95 | 71 | 30.87 | |
| 2 | 60 | 58.82 | 42 | 18.26 | |
| ≥ 3 | 49 | 9.78 | 23 | 10 | |
| Tumor size | .437 | ||||
| ≤ T2c | 156 | 29.89 | 53 | 25.36 | |
| T3a | 119 | 22.80 | 48 | 22.97 | |
| T3b-T4 or unknown | 247 | 47.32 | 108 | 51.67 | |
| Tumor grade | .009 | ||||
| Well differentiated | 11 | 2.11 | < 5 | < 2.4 | |
| Moderately differentiated | 259 | 49.62 | 78 | 37.32 | |
| Poorly differentiated | 252 | 48.28 | 127 | 60.77 | |
| Nodal status | < .005 | ||||
| Single node < 2 cm | 153 | 29.31 | 31 | 14.83 | |
| Single node 2-5 cm or multiple nodes | 67 | 12.84 | 75 | 35.89 | |
| Regional nodes NOS | 302 | 57.85 | 103 | 49.28 | |
| Receipt of radiation | 77 | 14.75 | 18 | 8.61 | .026 |
| Median income, $† | 40,654 | 39,058 | .3563 | ||
| % with college education† | 26.65 | 26.5 | .922 | ||
| Residence | .458 | ||||
| Metropolitan | 404 | 77.39 | 167 | 79.90 | |
| Nonmetropolitan | 118 | 22.61 | 42 | 20.10 | |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; NOS, not otherwise specified.
P values are for χ2 tests for all variables, with the exception of age, median income, and percent with college education. The t-test was used for those covariates.
Households, by census tracts: eighteen participants did not have information about median income or college education available. These variables were not included in the final model.
Treatment
Treatment was determined by searching Medicare files for the appropriate International Classification of Diseases-9 and Healthcare Common Procedure Coding System codes for RP and radiation therapy during the 6 months after the date of diagnosis. Codes for ADT were assessed for the first 3 years past diagnosis. Because SEER provides the month of diagnosis only, we assumed that all patients were diagnosed on the 15th of the month, and we included an additional 15 days in the treatment time windows. Medicare files included the inpatient claims (Part A), the carrier or physician file (Part B) and the outpatient claims file.
Definition of Adjuvant ADT
The indication for ADT (adjuvant v salvage) was not available in Medicare claims. Therefore, in our primary analysis, we used receipt of ADT within 120 days after surgery as our definition of adjuvant ADT. This is less stringent than the time frame used in the study (12 weeks) by Messing et al,3,4 but it may better reflect practice patterns in a nonclinical trial population. All other patients, including those who received ADT after 120 days or those who never received ADT, were included in the no-ADT group. We tested various definitions of adjuvant ADT, which ranged between 90 to 365 days, in our sensitivity analysis.
Survival
OS was defined as the interval from the date of RP to the date of death according to Medicare. Patients who were alive at the end of the study period (December 30, 2002) were censored at that point and contributed the time interval from their date of diagnosis to the end of the study in the survival analysis. Prostate cancer–specific survival was determined by using cause-of-death information provided by SEER.
Statistical Analysis
Summary statistics were constructed by using frequencies and proportions for categoric variables and means and medians for continuous variables. We used propensity scores to balance observed covariates between the adjuvant ADT and no-ADT arms.
Propensity scores are the probability that a patient received adjuvant ADT on the basis of his observed covariates. We calculated propensity scores by using multivariable logistic regression. Receipt of adjuvant ADT was the outcome of interest; age, SEER site, year of diagnosis, stage, tumor grade, marital status, receipt of radiation, and comorbidities were independent variables. Propensity scores then were used to group patients into quintiles, and we used χ2 tests and t tests to determine that the covariates were balanced within quintiles.
We measured the impact of receiving ADT on with using a Cox Proportional Hazards regression and prostate cancer–specific survival with a Competing Risk Proportional Hazards Regression.11 In both models, we controlled for propensity score as the only independent variable using a restricted cubic spline with five knots. Restricted cubic splines are flexible functions that allow for nonlinear relationships in models.12 The propensity score was not statistically significant (P = .93). We tested proportionality of hazards for the treatment effect by including treatment-interacted-with-time as a time-dependent covariate in the primary models of interest.
To test our definition of adjuvant therapy, we performed sensitivity analyses by using alternate time periods to define receipt of adjuvant ADT: 90, 150, 180, and 365 days OS. The statistics were performed with STATA 8.0 (STATA Corp, College Station, TX) and R version 2.5.1. (R Foundation for Statistical Computing; http://www.r-project.org).
RESULTS
A total of 23,545 patients were identified as having undergone RP between 1991 and 1999. A total of 819 (3.5%) had regional lymph node metastasis at the time of surgery. We excluded patients who received ADT before RP (n = 50), patients who had metastatic disease (n = 9), and patients without available RP dates (n = 29). The final cohort for analysis was 731 patients.
In our primary analysis of 120 days from RP as the definition of adjuvant ADT, 188 (25.7%) of patients received ADT. Baseline characteristics are lsited in Table 1. Use of adjuvant ADT varied by year of diagnosis, SEER site, number of comorbidities, grade, nodal status, and receipt of radiation therapy within 6 months of diagnosis. There was no statistically significant difference in the use of ADT by ethnicity, marital status, tumor stage, SES, or place of residence.
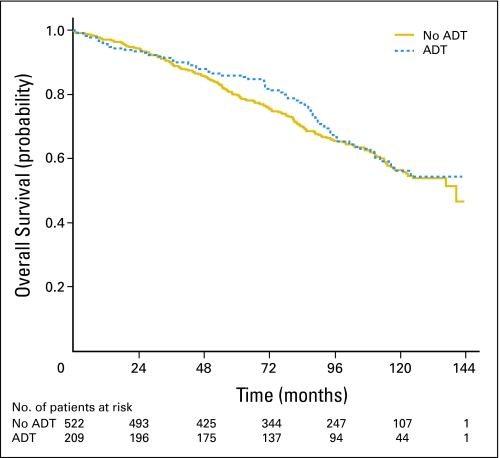
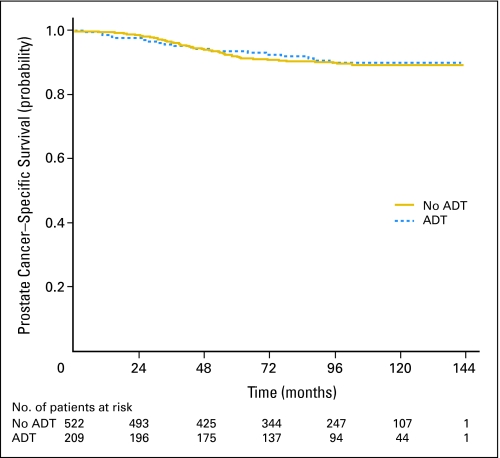
At the end of the study period, 269 patients (36.8%) had died. Seventy-one patients (9.7%) had died as a result of prostate cancer. Ten-year OS and PCSM were similar in both groups (Figs 1 and 2) After adjustment of the propensity score, there was no statistically significant difference in OS (HR, 0.95; 95% CI, 0.71 to 1.27; Table 2). In addition, there was no statistically significant difference in PCSM (subdistribution HR, 0.97; 95% CI, 0.56 to 1.68).
Fig 1.
Overall survival (Kaplan-Meier). ADT, androgen deprivation therapy.
Fig 2.
Prostate cancer–specific survival estimated from cumulative incidence. ADT, androgen deprivation therapy.
Table 2.
Overall Survival According to Alternate Definitions for Adjuvant ADT
| Survival (days) | Adjuvant ADT Use
|
HR for Death in Adjuvant ADT Group | 95% CI | |
|---|---|---|---|---|
| No. of Patients With No Adjuvant ADT | No. of Patients With Adjuvant ADT | |||
| 90 | 543 | 188 | 0.97 | 0.72 to 1.32 |
| 120 | 522 | 209 | 0.95 | 0.71 to 1.27 |
| 150 | 511 | 220 | 1.02 | 0.77 to 1.36 |
| 180 | 501 | 230 | 1.06 | 0.81 to 1.41 |
| 365 | 473 | 258 | 1.18 | 0.90 to 1.54 |
Abbreviations: ADT, androgen deprivation therapy; HR, hazard ratio.
We then performed sensitivity analysis by repeating the analyses with various definitions of adjuvant ADT: 90, 150, 180, and 365 days. The propensity score adjusted results are listed in Tables 2 and 3. As listed, there was no significant OS difference with 90, 150, 180, and 365 days as the adjuvant ADT definition. There was no difference in prostate cancer–specific survival using 90, 150, and 180 days. However, the use of 365 days as the adjuvant ADT definition resulted in a higher risk of prostate cancer–specific death in the adjuvant arm (HR, 1.96; 95% CI, 1.16 to 3.29).
Table 3.
Prostate-Cancer Specific Survival According to Alternate Definitions for ADT
| Survival (days) | Adjuvant ADT Use
|
Subdistribution HR for Death in Adjuvant ADT Group | 95% CI | |
|---|---|---|---|---|
| No. of Patients With No Adjuvant ADT | No. of Patients With Adjuvant ADT | |||
| 90 | 543 | 188 | 1.22 | 0.69 to 2.16 |
| 120 | 522 | 209 | 0.97 | 0.56 to 1.68 |
| 150 | 511 | 220 | 1.15 | 0.67 to 1.97 |
| 180 | 501 | 230 | 1.53 | 0.90 to 2.61 |
| 365 | 473 | 258 | 1.96 | 1.16 to 3.29 |
Abbreviations: ADT, androgen deprivation therapy; HR, hazard ratio.
DISCUSSION
The role of ADT in patients with node-positive prostate cancer after RP is controversial. Messing et al3,4 found significant improvements in both PCSM and OS in the adjuvant ADT arm.3,4 However, Schroeder et al13 recently reported the results of EORTC 30846, which examined the role of immediate versus delayed ADT in patients who had node-positive disease and who did not undergo RP. There was no difference in OS between the early and deferred ADT arms. Whether the difference was a result of the removal of the primary tumor in the study by Messing et alX or the higher rate of clinical T3 disease in the study by Schroeder et al13 is not known. Given these conflicting results, the 2006 American Society of Clinical Oncology Practice Guidelines do not give firm recommendations regarding the role of ADT in patients with node-positive disease, and they note that any benefit in prostate cancer–specific survival may be offset by an increase in non-PCSM.14
Observational studies can be helpful to provide clinical guidance when clinical trial data is limited or equivocal. These results suggest that the delay of ADT in patients who have node-positive prostate cancer after RP may not adversely impact OS. We believe that these results add to the currently available data and should help treatment decisions, especially in patients who are concerned about ADT-associated toxicity.
Our results may differ from those of Messing et al3,4 for several reasons. The most likely is the difference is indication for initiation of ADT in the groups that were initially observed. In the study by Messing et al,3,4 patients were only started on ADT if they developed clinical metastases, which are associated with a high risk of both cancer-specific and overall mortality.5,15 However, in the early 1990s, routine post-RP monitoring of PSA became routine, and patients in our series likely were followed for BCR. If treatment with ADT at the time of BCR is successful in the treatment of micrometastatic disease and in the prevention of the onset of metastatic disease and subsequent death from prostate cancer, it is possible that earlier detection and treatment of BCR may have led to the improved outcomes in the delayed arm in our series.
Even among patients with BCR, outcomes vary significantly. Risk factors for PCSM (ie, PSA recurrence within 3 years, Gleason score ≥ 8, PSA doubling time < 9 months)16 may guide clinicians in the selection of patients for treatment with ADT; others with indolent disease may elect to be observed. Therefore, even among patients with node-positive disease, it may be possible to defer adjuvant therapy and to spare some the toxicities associated with ADT.
Our results also may be different from those reported by Messing et al3,4 because of baseline differences between the two study populations. The group in our study is older than those enrolled on the trial by Messing et al (median age, 69 v 65.6 years). In addition, clinical trial participants tend to be healthier than the general population.17 Therefore, if ADT-associated toxicities, such as osteoporosis, metabolic syndrome, and cardiovascular disease, have greater effects on patients who are older or sicker, the benefits of ADT on PCSM may be offset by an increased non–cancer-specific mortality. Therefore, any possible benefit of ADT must be balanced against the known long-term toxicities of ADT.14
Our results also may have differed from those reported by Messing et al3,4 because they were diagnosed during the 1990s, when PSA screening increased in use. This length-time bias may have resulted in patients who had earlier-stage, lower-volume disease than those seen in the study by Messing et al. Therefore, our cohort may better represent contemporary patients diagnosed the PSA era, when patients are more likely to have early-stage disease and, consequently, a lower chance of micrometastatic disease. This would result in a smaller benefit from adjuvant ADT than those in the study by Messing et al.
Our study has several strengths. Our population-based estimates of the prevalence of node-positive disease after RP are similar to prior reports of between 0.87% and 10%.18,19 We are able to report OS, rather than to rely on intermediate markers of survival, such as BCR, which may not correlate well with OS. However, patients diagnosed during the later portion of our study had shorter follow-up; given the long natural history of prostate cancer, it will be important to follow these patients to determine if their outcomes differ from earlier patients.
Our study highlights the significant uncertainty that surrounds the role of adjuvant therapy for men with high-risk, localized prostate cancer. Although ADT has a well-defined role for patients who have locally advanced prostate cancer and who are undergoing definitive radiation therapy,2,20 it has not been effective in the neoadjuvant setting before RP.21,22 This apparent dichotomy may support an interaction between radiation therapy and ADT that is not present in patients who undergo RP and who receive neoadjuvant ADT.23 This may also explain the lack of benefit of adjuvant ADT seen in this study.
This study has several limitations. SEER-Medicare does not provide information about the indication for ADT. Patients who received salvage ADT for biochemical, local, or distant recurrence likely will have worse clinical outcomes than those who received ADT in the adjuvant setting. On the other hand, patients who did not receive ADT during the first 3 years after diagnosis likely had less aggressive disease and would be expected to have longer survival. We have attempted to address this limitation by analyzing the data as an intention-to-treat design, in which patients who received adjuvant ADT (within 120 days of RP) were compared with all other patients in the cohort. We then tested our definition of adjuvant therapy by performing sensitivity analyses with 90, 150, 180, and 365 days; we found no statistically significant difference in OS when we used any of the examined definitions. This supports our a priori cutoff of 120 days as the definition of adjuvant ADT. Similar results were seen for prostate cancer–specific survival, in which there was no significant difference with 90, 150, and 180 days. However, when we extended the definition to 365 days, the adjuvant arm did worse (HR for PCSM, 1.96; 95% CI, 1.16 to 3.29). This probably occurred because, according to this extended definition, some of these patients likely had early relapse and were treated with the salvage regimen. Therefore, they were likely at a higher risk of PCSM than those who did not receive ADT. This may reflect a benefit in PCSM, because treatment for early relapse is offset by ADT-related toxicity, which resulted in no difference in OS.
Although we have attempted to control for known confounders, it is impossible to adjust for unmeasured confounders, such as performance status or patient preferences, in an observational study such as this. However, all men are presumably healthy enough to undergo RP, which reduces the possibility that unmeasured imbalances in performance status alone explained our results. It is possible that patients who received adjuvant ADT may have been more motivated to seek out aggressive care; however, despite this potential bias, there was no evidence of improved survival in the group that received adjuvant ADT. In addition, our cohort did not include patients who were enrolled in health maintenance organizations. If outcomes for prostate cancer vary by insurance status, it is possible that our results may not be generalizable to patients covered by managed care.
In conclusion, this study suggests that OS in men with node-positive prostate cancer after RP may not be significantly harmed by delaying the initiation of ADT. As with an observational study, these results should be considered hypothesis generating and should be confirmed in prospective clinical trials in a similar patient population, which should include treatment at the time of BCR in the delayed treatment arm rather than at the onset of metastases.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Stephen J. Freedland, AstraZeneca (C), GTx Inc (C); J. Sanford Schwartz, Sanofi-aventis (C) Stock Ownership: None Honoraria: Stephen J. Freedland, AstraZeneca Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Yu-Ning Wong, Stephen J. Freedland, J. Sanford Schwartz, Katrina Armstrong
Financial support: Katrina Armstrong
Administrative support: Katrina Armstrong
Provision of study materials or patients: Katrina Armstrong
Collection and assembly of data: Yu-Ning Wong, Katrina Armstrong
Data analysis and interpretation: Yu-Ning Wong, Stephen J. Freedland, Brian L. Egleston, Gary R. Hudes, Katrina Armstrong
Manuscript writing: Yu-Ning Wong, Stephen J. Freedland, Gary R. Hudes, Katrina Armstrong
Final approval of manuscript: Yu-Ning Wong, Stephen J. Freedland, Brian L. Egleston, Gary R. Hudes, J. Sanford Schwartz, Katrina Armstrong
Acknowledgments
We thank the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services Inc and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Treatment Codes
| Treatment | Codes |
|---|---|
| Radical prostatectomy | 60.3 60.4 60.5 60.6 60.62 55,821 55,801 55,810 55,812 55,815 55,831 55,840 55,842 55,845 |
| External-beam radiation | 9221 9222 9223 9224 9225 9226 9229 77,305 77,310 77,315 77,321 77,332 77,333 77,334 77,336 77,370 77,261 77,262 77,263 77,280 77,285 77,290 77,295 77,299 77,300 77,401 77,402 77,403 77,404 77,405 77,406 77,407 77,408 77,409 77,410 77,411 77,412 77,413 77,414 77,415 77,416 77,417 77,418 77,419 77,420 77,421 77,422 77,423 77,424 77,425 77,426 77,427 77,428 77,429 77,430 77,431 77,520 77,521 77,522 77,523 77,524 77,525 |
| Brachytherapy | 922 9227 55,860 55,865 55,862 55,859 77,326 77,327 77,328 77,331 77,750 77,751 77,752 77,753 77,754 77,755 77,756 77,757 77,758 77,759 77,760 77,761 77,762 77,763 77,764 77,765 77,766 77,767 77,768 77,769 77,770 77,771 77,772 77,773 77,774 77,775 77,776 77,777 77,778 77,779 77,780 77,781 77,782 77,783 77,784 77,785 77,786 77,787 77,788 77,789 77,790 77,791 77,792 77,793 77,794 77,795 77,796 77,797 77,798 77,799 C1715 C1716 C1717 C1718 C1719 C1728 C2632 C2633 C2634 C2635 C2636 Q3001 |
| Hormonal therapy | |
| Injectable medications | 99.24 11,980 J1950 J9217 J9218 J9219 C9430 J9202 S9560 J141 J1000 J1380 J1390 J0970 J1056 C9216 S0165 J3315 |
| Orchiectomy | 62.4 62.41 62.42 54,520 54,522 54,530 54,535 |
published online ahead of print at www.jco.org on December 1, 2008.
Supported by Grants No. P50-CA105461 and P30-CA006927 from the Center for Population Health and Health Disparities at the University of Pennsylvania, Public Health Services, Comprehensive Cancer Center Program at Fox Chase.
Presented in part at the Prostate Cancer Symposium, February 22-24, 2007, Orlando, FL and at the 43rd Annual meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
This study used the linked Surveillance, Epidemiology, and End Results–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Adib RS, Anderson JB, Ashken MH, et al: Immediate versus deferred treatment for advanced prostatic cancer: Initial results of the Medical Research Council trial. Br J Urol 79:235-246, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Gonzalez D, Warde P, et al: Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337:295-300, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Messing EM, Manola J, Yao J, et al: Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 7:472-479, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Messing EM, Manola J, Sarosdy M, et al: Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 341:1781-1788, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Pound CR, Partin AW, Eisenberger MA, et al: Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281:1591-1597, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Morote J, Morin JP, Orsola A, et al: Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology 69:500-504, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Keating NL, O'Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24:4448-4456, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Sharifi N, Gulley JL, Dahut WL: Androgen deprivation therapy for prostate cancer. JAMA 294:238-244, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Potosky AL, Harlan LC, Stanford JL, et al: Prostate cancer practice patterns and quality of life: The prostate cancer outcomes study. J Natl Cancer Inst 91:1719-1724, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Elixhauser ASC, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 36:8-27, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 12.Harrell F: Regression Modeling Strategies. New York, NY, Springer, 2001
- 13.Schröder FH KK, Fossa SD, Hoekstra W, Karthaus PP, Debois M, Collette L: Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: Results of European Organisation for the Research and Treatment of Cancer 30846—A phase III study. J Urol 172:923-927, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Loblaw DA, Virgo KS, Nam R, et al: Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 25:1596-1605, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ryan CJ EE, Cowan J, Carroll PR: Initial treatment patterns and outcome of contemporary prostate cancer patients with bone metastases at initial presentation. Cancer 110:81-86, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Humphreys EB, Mangold LA, et al: Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 294:433-439, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Elting LS, Cooksley C, Bekele BN, et al: Generalizability of cancer clinical trial results: Prognostic differences between participants and nonparticipants. Cancer 106:2452-2458, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kawakami J, Meng MV, Sadetsky N, et al: Changing Patterns of Pelvic Lymphadenectomy for Prostate Cancer: Results From CaPSURE. J Urol 176:1382-1386, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Swanson G, Thompson IM, Basler, J: Current status of lymph node-positive prostate cancer. Cancer 107:439-450, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hanks GE, Pajak TF, Porter A, et al: Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group protocol 9202. J Clin Oncol 21:3972-3978, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, Schelin S, Pedersen K: Three-month neoadjuvant hormonal therapy before radical prostatectomy: A 7-year follow-up of a randomized controlled trial. BJU International 90:561-566, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Soloway MS, Pareek K, Sharifi R, et al: Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 167:112-116, 2002 [PubMed] [Google Scholar]
- 23.Ryan CJ, Small EJ: Early versus delayed androgen deprivation for prostate cancer: New fuel for an old debate. J Clin Oncol 23:8225-8231, 2005 [DOI] [PubMed] [Google Scholar]