Abstract
Purpose
Gonadotropin-releasing hormone (GnRH) agonists are associated with greater risk of coronary heart disease and myocardial infarction in men with prostate cancer, but little is known about potential impact on cardiovascular mortality. We assessed the relationship between GnRH agonists and cardiovascular mortality in a large randomized phase III trial of men treated with or without adjuvant goserelin after radiation therapy (RT) for locally advanced prostate cancer.
Patients and Methods
Between 1987 and 1992, 945 men with locally advanced prostate cancer were randomly assigned to RT and adjuvant goserelin or RT alone. Fine and Gray's regression was used to evaluate treatment effect on cardiovascular mortality. Covariates included age, prevalent cardiovascular disease (CVD), hypertension, diabetes mellitus (DM), body mass index, race, Gleason score, stage, acid phosphatase level, prostatectomy history, and nodal involvement.
Results
After a median follow-up of 8.1 years, there were 117 cardiovascular-related deaths but no treatment-related increase in cardiovascular mortality. At 9 years, cardiovascular mortality for men receiving adjuvant goserelin was 8.4% v 11.4% for men treated without adjuvant goserelin (Gray's P = .17). In multiple regression analyses, treatment arm was not significantly associated with increased risk of cardiovascular mortality (adjusted hazard ratio [HR] = 0.73; 95% CI, 0.47 to 1.15; P = .16; when censoring at time of salvage goserelin therapy, HR = 0.99; 95% CI, 0.58 to 1.69; P = .97). Traditional cardiac risk factors, including prevalent CVD and DM, were significantly associated with greater cardiovascular mortality.
Conclusion
GnRH agonists do not seem to increase cardiovascular mortality in men with locally advanced prostate cancer. Further studies are warranted to evaluate adverse effects of GnRH agonists in men with lower cancer-specific mortality.
INTRODUCTION
Several randomized trials demonstrated that adjuvant androgen deprivation therapy (ADT) with gonadotropin-releasing hormone (GnRH) agonists decreases cancer-specific and, in some cases, all-cause mortality for men with locally advanced or high-grade localized prostate cancer.1-6 On the basis of, in part, this evidence of improved survival, GnRH agonist therapy increased markedly in prostate cancer patients,7-9 including men with lower stage disease and older men with significant competing causes of mortality. Routine use of GnRH agonists increases the importance of understanding the unintended adverse effects of treatment.
A recent, large, claims-based analysis using Surveillance, Epidemiology, and End Results-Medicare data for 73,196 men with local or locoregional prostate cancer demonstrated that GnRH agonists are associated with greater risk of incident diabetes mellitus (DM), coronary heart disease, and admission for myocardial infarction (MI).10 Greater risk of DM and cardiovascular disease (CVD) was observed with short-term treatment and persisted with longer exposure to GnRH agonists. Several mechanisms may account for the association between GnRH agonists and greater risk for DM and CVD. GnRH agonists significantly increase fat mass,11-14 LDL cholesterol, and triglycerides11,15 and decrease insulin sensitivity.16 These adverse effects of GnRH agonists are suggestive of a metabolic syndrome,17,18 an independent risk factor for coronary heart disease and cardiovascular mortality.19,20
Although GnRH agonists have been associated with greater risk for coronary heart disease, there is limited information about GnRH agonists and cardiovascular mortality. To evaluate the relationship between GnRH agonists and cardiovascular mortality, we analyzed data from Radiation Therapy Oncology Group (RTOG) protocol 85-31, a large randomized trial of men treated with radiation therapy (RT) with or without adjuvant goserelin for locally advanced prostate cancer.
PATIENTS AND METHODS
RTOG 85-31 is a phase III trial designed to compare adjuvant ADT with goserelin, a GnRH agonist, plus external-beam RT versus RT alone in men with locally advanced prostate cancer.2,21
Patient Eligibility
All patients had histologically confirmed prostatic adenocarcinoma with either grossly palpable tumor beyond the prostate (clinical stage T3) or documented evidence of regional lymphatic involvement. Patients who underwent radical prostatectomy were eligible if there was penetration through the prostatic capsule to the resection margin and/or seminal vesicles. Karnofsky performance status had to be more than 60%. All institutional state and federal guidelines were followed. All patients provided written informed consent before enrollment.
Pretreatment Evaluation
Pretreatment evaluation included history and physical examination. Laboratory studies included serum acid phosphatase, CBC, serum testosterone, and, after July 1990, prostate-specific antigen measurement. Prostate-specific antigen determination was not mandatory at study inception because it was not widely available. Radiographic evaluation included chest x-ray and bone scan. Lymph node assessment was mandatory by lymphangiography, computed tomography, or lymphadenectomy.
Study Design
Patients were entered onto the study by telephone call to RTOG headquarters within the first week of RT. After confirmation of eligibility, patients were stratified by histologic differentiation determined by institutional pathologists (well differentiated or Gleason score of 2 to 5; moderately differentiated or Gleason score of 6 to 7; and poorly differentiated or Gleason score of 8 to 10), nodal involvement (none v below common iliacs v common iliac v para-aortic), acid phosphatase status (not elevated v elevated), and prior radical prostatectomy (no v yes). The random assignment scheme described by Zelen22 was used to achieve balance in treatment assignment among institutions using the stratification variables. Patients were randomly assigned either to RT and adjuvant goserelin (arm 1) or to RT alone followed by observation and goserelin only at relapse (arm 2).
Treatment
RT.
Details of RT technique, doses, and fields have been described previously.2,21 Of note, only a small percentage of patients received para-aortic irradiation (superior field border encompassing T11), and there was no significant difference between the treatment arms (8% in arm 1 v 9% in arm 2).
Drug therapy.
Patients assigned to arm 1 were treated with adjuvant goserelin acetate (Zoladex; AstraZeneca, Wilmington, DE; 3.6 mg subcutaneously in the anterior abdominal wall monthly), which was started during the last week of RT. Patients in arm 2 were treated with goserelin only for documented local and/or distant disease recurrence. In both arms, goserelin was continued indefinitely or until sign of disease progression.
In arm 1, the median duration of goserelin therapy was 4.2 years (range, 0.0 to 14.1 years). In arm 2, 298 (64%) of 468 patients received salvage GnRH agonist therapy at a median time interval of 3.0 years (range, 0.04 to 13.0 years) from the end of RT.
Data Collection and Analysis
Central review of RT delivered, calibration of machines, and review of materials on which diagnosis was based were performed for each patient as per RTOG/National Cancer Institute requirements.2
CVD Risk Factors
Information on CVD risk factors, including age, prevalent CVD, hypertension, DM, and body mass index (BMI) at baseline, was collected. BMI was categorized according to National Institutes of Health classifications, with a BMI less than 25 kg/m2 considered normal, a BMI of 25 to 29.9 kg/m2 considered overweight, and a BMI ≥ 30 kg/m2 considered obese.23
Follow-Up
Patients in both arms were evaluated every 3 months during the first year, every 4 months during the second and third years, every 6 months to year 5, and then annually for the remainder of their life.
Survival End Point
Cause of death was investigator defined and reported in follow-up case report forms by each institution. Protocol did not mandate a death certificate or autopsy report. Cardiovascular mortality was defined as death from coronary artery disease (CAD), CVD, congestive heart failure, cardiac arrest, cardiomyopathy, cardiovascular arrhythmia, MI, or sudden death. To exclude the possibility that our results would be sensitive to the definition of cardiovascular mortality, we performed additional analyses using alternative definitions by restricting the outcome to death from CAD, CVD, or cardiac arrest, and MI; and from MI only. We also considered a broader definition of death as a result of cardiovascular and cerebral events, including cerebrovascular accident, cerebral hemorrhage, cerebral infarction, stroke, and thrombotic occlusion. The end point of cardiovascular mortality was measured from date of random assignment to date of death or most recent follow-up through July 2003.
Statistical Methods
The χ2 test was used to compare pretreatment characteristics of patients at study entry. The cumulative incidence method24 was used to estimate time to cardiovascular mortality because it specifically adjusts for other competing causes of mortality. Gray's test25 was used for comparing cumulative incidence rates over time between treatment arms. Fine and Gray's regression analyses26 using χ2 test were performed to evaluate the solitary effect of each variable on cardiovascular mortality. To analyze whether treatment arm was independently associated with cardiovascular mortality while adjusting for other factors, multiple regression analyses were performed using Fine and Gray's regression model26 with the following categoric covariates: age (< 70 [reference level {RL}] v ≥ 70 years), race (black [RL] v white/other), CVD at registration (no [RL] v yes), hypertension (no [RL] v yes), DM (no [RL] v yes), baseline BMI (< 25 [RL] v ≥ 25 to 30 v ≥ 30 kg/m2), centrally reviewed Gleason score (2 to 6 [RL] v 7 v 8 to 10), clinical stage (A/B [RL] v C), acid phosphatase (not elevated [RL] v elevated), nodal involvement (no [RL] v yes), prostatectomy (no [RL] v yes), and treatment (arm 2 [RL] v arm 1). For the categoric variables, the cut points selected were made before data were examined and were based on established strata.2,23 Goodness of fit by scaled Schoenfeld-type residual plots indicated that the model adequately fits the data. Unadjusted and adjusted hazard ratios (HRs) were calculated for all covariates using Fine and Gray's regression model with associated 95% CIs and P values. All statistical comparisons were two-sided, and a P < .05 was considered significant. To eliminate any potential impact of salvage GnRH agonist therapy on the outcome, additional analyses were performed that censored patients at time of initiation of such salvage therapy. Further analyses were performed using a data set with imputed missing values for 304 patients using the multiple imputation method (10 imputations) with Markov chain Monte Carlo estimation.27,28 Missing at random assumptions were made. The Markov chain Monte Carlo sampler retained 5,000 samples after the first 1,000 samples burned-in. Jeffrey's prior was assigned for prior distributions. There was no autocorrelation between samples, and the posterior distributions were converged. SAS software (SAS Institute, Cary, NC) and software R (http://www.r-project.org/) were used for all analyses.
RESULTS
Pretreatment Characteristics
Between February 1987 and April 1992, a total of 945 eligible patients were enrolled. Four hundred seventy-seven patients were assigned to adjuvant goserelin (arm 1), and 468 patients were assigned to no adjuvant goserelin (arm 2). Median age was 70 years. Pretreatment characteristics, including CVD risk factors, were similar between treatment arms (Table 1).
Table 1.
Pretreatment Characteristics
| Characteristic | Arm 1 (n = 477)
|
Arm 2 (n = 468)
|
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | |||||
| < 70 | 230 | 48 | 223 | 48 | .86 |
| ≥ 70 | 247 | 52 | 245 | 52 | |
| Prevalent CVD | |||||
| No | 342 | 72 | 345 | 74 | .45* |
| Yes | 133 | 28 | 120 | 26 | |
| Unknown | 2 | < 1 | 3 | 1 | |
| Prevalent HTN | |||||
| No | 323 | 68 | 309 | 66 | .58* |
| Yes | 152 | 32 | 157 | 34 | |
| Unknown | 2 | < 1 | 2 | < 1 | |
| Prevalent DM | |||||
| No | 392 | 82 | 373 | 80 | .24* |
| Yes | 36 | 8 | 45 | 10 | |
| Unknown | 49 | 10 | 50 | 11 | |
| BMI | |||||
| Missing | 74 | 16 | 83 | 18 | |
| Available | 403 | 84 | 385 | 82 | |
| BMI category, kg/m2 | |||||
| < 25 | 132 | 33 | 109 | 28 | .40 |
| ≥ 25 to < 30 | 200 | 50 | 202 | 52 | |
| ≥ 30 | 71 | 18 | 74 | 19 | |
| Race | |||||
| White | 429 | 90 | 422 | 90 | .71† |
| Black | 43 | 9 | 39 | 8 | |
| Other | 5 | 1 | 7 | 2 | |
| Prostatectomy | |||||
| No | 406 | 85 | 400 | 85 | .88 |
| Yes | 71 | 15 | 68 | 15 | |
| Nodal involvement | |||||
| No | 337 | 71 | 345 | 74 | .29 |
| Yes | 140 | 29 | 123 | 26 | |
| Acid phosphatase | |||||
| Not elevated | 318 | 67 | 316 | 68 | .78 |
| Elevated | 159 | 33 | 152 | 32 | |
| Gleason score (central) | |||||
| Missing | 41 | 9 | 42 | 9 | |
| Available | 436 | 91 | 426 | 91 | |
| 2-6 | 125 | 29 | 129 | 30 | .82 |
| 7 | 172 | 39 | 160 | 38 | |
| 8-10 | 139 | 32 | 137 | 32 | |
| Clinical stage | |||||
| A/B | 141 | 30 | 127 | 27 | .41 |
| C | 336 | 70 | 341 | 73 | |
Abbreviations: CVD, cardiovascular disease; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index.
Comparison of no v yes.
Comparison of white/other v black.
Cardiovascular Mortality
Median follow-up time was 8.1 years (range, 0.2 to 15.1 years) for all eligible patients and 11.1 years (range, 0.4 to 15.0 years) for surviving patients. There was a total of 574 deaths; 117 (20.4%) were categorized as cardiovascular deaths.
Univariate Analyses
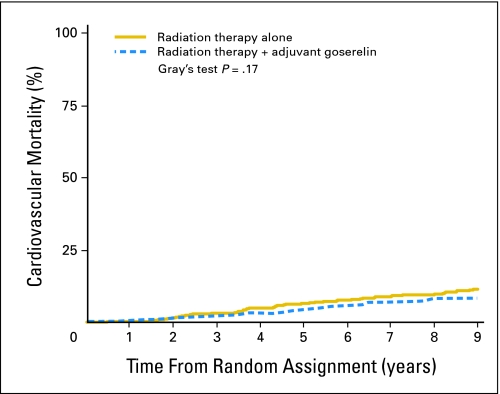
In univariate analyses, there was no treatment-related increase in cardiovascular mortality (Table 2). At 9 years, cardiovascular mortality rate for men treated with adjuvant goserelin on arm 1 was 8.4% v 11.4% for men treated without adjuvant goserelin on arm 2 (Gray's P = .17). The corresponding unadjusted HR was 0.77 (95% CI, 0.53 to 1.11; P = .16). Figure 1 graphically displays time to cardiovascular mortality by treatment arm. Similar results were observed in additional analyses that censored patients at time of initiation of salvage goserelin therapy (data not shown). Established CVD risk factors, including age, prevalent CVD, and DM, were significantly associated with greater cardiovascular mortality. In addition, prostatectomy and advanced clinical stage were significantly associated with increased cardiovascular mortality.
Table 2.
Univariate Analyses of Cardiovascular Mortality
| Factor | No. of Patients | No. of Treatment Failures | Cumulative Incidence
|
Fine and Gray's Models
|
||||
|---|---|---|---|---|---|---|---|---|
| 9-Year Failure Rate (%) | 95% CI | P* | Unadjusted HR | 95% CI | P† | |||
| Treatment arm | ||||||||
| Arm 2 | 468 | 65 | 11.4 | 8.4 to 14.3 | ||||
| Arm 1 | 477 | 52 | 8.4 | 5.8 to 11.0 | .17 | 0.77 | 0.53 to 1.11 | .16 |
| Age, years | ||||||||
| < 70 | 453 | 38 | 6.0 | 3.7 to 8.2 | ||||
| ≥ 70 | 492 | 79 | 13.6 | 10.5 to 16.8 | .0001 | 2.08 | 1.41 to 3.06 | .0002 |
| Prevalent CVD | ||||||||
| No | 687 | 54 | 6.5 | 4.5 to 8.4 | ||||
| Yes | 253 | 62 | 18.8 | 13.9 to 23.6 | < .0001 | 3.24 | 2.25 to 4.66 | < .0001 |
| Prevalent HTN | ||||||||
| No | 632 | 69 | 8.4 | 6.1 to 10.6 | ||||
| Yes | 309 | 47 | 12.8 | 9.1 to 16.6 | .09 | 1.36 | 0.94 to 1.97 | .10 |
| Prevalent DM | ||||||||
| No | 765 | 85 | 8.8 | 6.7 to 10.9 | ||||
| Yes | 81 | 21 | 22.4 | 13.2 to 31.6 | .0003 | 2.40 | 1.49 to 3.86 | .0003 |
| BMI, kg/m2 | ||||||||
| < 25 | 241 | 30 | 10.0 | 6.0 to 13.9 | ||||
| ≥ 25 to < 30 | 402 | 53 | 11.0 | 7.8 to 14.1 | 1.05 | 0.67 to 1.65 | .82 | |
| ≥ 30 | 145 | 20 | 9.5 | 4.6 to 14.5 | .94 | 1.10 | 0.63 to 1.93 | .73 |
| Race | ||||||||
| Black | 82 | 12 | 10.0 | 3.4 to 16.7 | ||||
| Other | 863 | 105 | 9.8 | 7.8 to 11.9 | .61 | 0.86 | 0.48 to 1.55 | .62 |
| Prostatectomy | ||||||||
| No | 806 | 109 | 10.8 | 8.6 to 13.0 | ||||
| Yes | 139 | 8 | 4.5 | 1.0 to 8.1 | .017 | 0.42 | 0.21 to 0.87 | .019 |
| Nodal involvement | ||||||||
| No | 682 | 92 | 10.7 | 8.3 to 13.1 | ||||
| Yes | 263 | 25 | 7.7 | 4.5 to 11.0 | .048 | 0.66 | 0.42 to 1.02 | .06 |
| Acid phosphatase | ||||||||
| Not elevated | 634 | 78 | 9.4 | 7.1 to 11.8 | ||||
| Elevated | 311 | 39 | 10.8 | 7.2 to 14.3 | .83 | 1.03 | 0.70 to 1.51 | .89 |
| Gleason score | ||||||||
| 2-6 | 254 | 37 | 10.5 | 6.7 to 14.3 | ||||
| 7 | 332 | 41 | 8.2 | 5.2 to 11.3 | 0.87 | 0.56 to 1.36 | .54 | |
| 8-10 | 276 | 29 | 10.4 | 6.8 to 14.1 | .52 | 0.74 | 0.45 to 1.20 | .22 |
| Clinical stage | ||||||||
| A/B | 268 | 19 | 5.7 | 2.9 to 8.6 | ||||
| C | 677 | 98 | 11.6 | 9.1 to 14.1 | .001 | 2.20 | 1.34 to 3.60 | .002 |
Abbreviations: HR, hazard ratio; CVD, cardiovascular disease; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index.
P value determined using Gray's test statistic.
P value determined using χ2 test.
Fig 1.
Time to cardiovascular mortality by treatment arm for all eligible patients.
Multiple Regression Analyses
In multiple regression analyses, prevalent CVD and DM were significantly associated with greater cardiovascular mortality (Table 3). Adjuvant goserelin treatment was not associated with cardiovascular mortality (adjusted HR = 0.73; 95% CI, 0.47 to 1.15; P = .16). Notably, there were no interaction effects between treatment arm and other covariates (data not shown). Similar results were observed in additional analyses that censored patients at time of initiation of salvage goserelin therapy (Table 3). In censored analyses, traditional cardiovascular risk factors, including prevalent CVD and DM, were associated with greater cardiovascular mortality, whereas adjuvant goserelin treatment was not (adjusted HR = 0.99; 95% CI, 0.58 to 1.69; P = .97).
Table 3.
Multiple Regression Analyses of Cardiovascular Mortality Without and With Censoring at Time of Salvage GnRH Agonist Therapy (N = 641)
| Covariate | Without Censoring
|
With Censoring
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment arm: arm 2 v arm 1 | 0.73 | 0.47 to 1.15 | .16 | 0.99 | 0.58 to 1.69 | .97 |
| Age: < 70 v ≥ 70 years | 1.57 | 0.95 to 2.59 | .08 | 1.39 | 0.79 to 2.45 | .26 |
| Prevalent CVD: no v yes | 2.60 | 1.65 to 4.11 | < .0001 | 2.92 | 1.71 to 4.98 | < .0001 |
| Prevalent HTN: no v yes | 1.32 | 0.85 to 2.04 | .22 | 1.57 | 0.96 to 2.57 | .08 |
| Prevalent DM: no v yes | 2.54 | 1.49 to 4.34 | .0006 | 2.92 | 1.57 to 5.43 | .0007 |
| BMI, kg/m2 | ||||||
| < 25 | — | — | — | — | ||
| ≥ 25 to < 30 | 0.94 | 0.58 to 1.54 | .81 | 0.89 | 0.49 to 1.61 | .70 |
| ≥ 30 | 0.87 | 0.45 to 1.67 | .67 | 0.92 | 0.42 to 2.01 | .83 |
| Race: black v other | 1.25 | 0.60 to 2.59 | .55 | 2.05 | 0.74 to 5.63 | .17 |
| Prostatectomy: no v yes | 0.45 | 0.14 to 1.47 | .18 | 0.33 | 0.09 to 1.28 | .11 |
| Nodal involvement: no v yes | 1.31 | 0.65 to 2.63 | .45 | 0.84 | 0.35 to 2.03 | .70 |
| Acid phosphatase: not elevated v elevated | 1.15 | 0.72 to 1.84 | .57 | 1.26 | 0.73 to 2.19 | .41 |
| Gleason score | ||||||
| 2-6 | — | — | — | — | ||
| 7 | 0.81 | 0.49 to 1.34 | .41 | 0.72 | 0.40 to 1.30 | .28 |
| 8-10 | 0.62 | 0.35 to 1.10 | .10 | 0.55 | 0.28 to 1.11 | .10 |
| Clinical stage: A/B v C | 1.13 | 0.46 to 2.83 | .79 | 0.57 | 0.20 to 1.61 | .29 |
Abbreviations: GnRH, gonadotropin-releasing hormone; HR, hazard ratio; CVD, cardiovascular disease; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index.
Our primary analysis was based on 641 patients with complete multivariable data. To address the possibility that our results may have been affected by the exclusion of patients with missing data, additional analyses were performed using imputed missing values (Table 4). Consistent with the primary analyses, adjuvant goserelin was not significantly associated with time to cardiovascular mortality, whereas prevalent CVD and DM were significant in both uncensored and censored analyses.
Table 4.
Multiple Regression Analyses of Cardiovascular Mortality Without and With Censoring at Time of Salvage GnRH Agonist Therapy (N = 945 using imputed missing values)
| Covariate | Without Censoring
|
With Censoring
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment arm: arm 2 v arm 1 | 0.76 | 0.52 to 1.09 | .14 | 0.97 | 0.63 to 1.48 | .88 |
| Age: < 70 v ≥ 70 years | 1.59 | 1.04 to 2.42 | .032 | 1.47 | 0.91 to 2.37 | .12 |
| Prevalent CVD: no v yes | 2.70 | 1.84 to 3.95 | < .0001 | 2.97 | 1.91 to 4.60 | < .0001 |
| Prevalent HTN: no v yes | 1.09 | 0.75 to 1.58 | .67 | 1.15 | 0.75 to 1.74 | .53 |
| Prevalent DM: no v yes | 1.79 | 1.10 to 2.89 | .018 | 1.83 | 1.07 to 3.12 | .027 |
| BMI, kg/m2 | ||||||
| < 25 | — | — | — | — | ||
| ≥ 25 to < 30 | 1.10 | 0.71 to 1.70 | .68 | 1.07 | 0.64 to 1.79 | .81 |
| ≥ 30 | 1.09 | 0.62 to 1.92 | .80 | 1.21 | 0.63 to 2.32 | .57 |
| Race: black v other | 0.86 | 0.47 to 1.55 | .60 | 1.25 | 0.58 to 2.69 | .57 |
| Prostatectomy: no v yes | 0.77 | 0.30 to 1.98 | .59 | 0.61 | 0.20 to 1.86 | .38 |
| Nodal involvement: no v yes | 1.17 | 0.68 to 2.02 | .57 | 0.88 | 0.46 to 1.70 | .71 |
| Acid phosphatase: not elevated v elevated | 1.01 | 0.68 to 1.51 | .96 | 1.10 | 0.70 to 1.74 | .67 |
| Gleason score | ||||||
| 2-6 | — | — | — | — | ||
| 7 | 0.94 | 0.61 to 1.46 | .80 | 0.80 | 0.48 to 1.31 | .37 |
| 8-10 | 0.82 | 0.50 to 1.35 | .43 | 0.74 | 0.41 to 1.34 | .32 |
| Clinical stage: A/B v C | 1.67 | 0.80 to 3.45 | .17 | 1.11 | 0.49 to 2.54 | .80 |
Abbreviations: GnRH, gonadotropin-releasing hormone; HR, hazard ratio; CVD, cardiovascular disease; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index.
We also considered the possibility that our results were sensitive to the definition of cardiovascular mortality. To address this issue, we performed further analyses using alternative definitions based on a more limited composite of causes of cardiovascular death (CAD, CVD, cardiac arrest, and MI) and then further restricted the definition to death as a result of MI only. We also considered a broader definition of death as a result of cardiovascular and cerebral events, including cerebrovascular accident, cerebral hemorrhage, cerebral infarction, stroke, and thrombotic occlusion (an additional 28 events). Similar results were observed in both univariate and multiple regression analyses irrespective of the definition used (data not shown). Specifically, treatment arm was not significantly associated with cardiovascular mortality, whereas traditional cardiac risk factors, including prevalent CVD and DM, were consistently associated with cardiovascular mortality.
Subgroup Analyses
On the basis of the observation that advanced age, prevalent CVD, and DM were associated with cardiovascular mortality, we evaluated the effect of treatment in men ≥ 70 years old and in men with prevalent CVD or DM. As displayed in Table 5, there was no significant treatment-related effect on cardiovascular mortality in these subgroups of high-risk patients. Results were similar in additional analyses that censored patients at time of salvage goserelin therapy (data not shown).
Table 5.
Univariate Analyses of Cardiovascular Mortality by Treatment Arm for Subgroups of Patients With Age ≥ 70 Years, Prevalent CVD, or DM
| Subgroup | No. of Patients | No. of Failures | Cumulative Incidence
|
Fine and Gray's Models
|
||||
|---|---|---|---|---|---|---|---|---|
| 9-Year Failure Rate (%) | 95% CI | P* | Unadjusted HR | 95% CI | P† | |||
| Age ≥ 70 years | ||||||||
| Arm 2 | 245 | 40 | 14.3 | 9.7 to 18.9 | ||||
| Arm 1 | 247 | 39 | 13.0 | 8.6 to 17.3 | .74 | 0.94 | 0.60 to 1.45 | .77 |
| Prevalent CVD | ||||||||
| Arm 2 | 120 | 36 | 24.3 | 16.5 to 32.0 | ||||
| Arm 1 | 133 | 26 | 13.7 | 7.8 to 19.7 | .086 | 0.63 | 0.38 to 1.03 | .065 |
| Prevalent DM | ||||||||
| Arm 2 | 45 | 11 | 22.2 | 9.9 to 34.6 | ||||
| Arm 1 | 36 | 10 | 22.7 | 8.5 to 36.8 | .67 | 1.18 | 0.51 to 2.74 | .70 |
Abbreviations: CVD, cardiovascular disease; DM, diabetes mellitus; HR, hazard ratio.
P value determined using Gray's test statistic.
P value determined using χ2 test.
DISCUSSION
A recent claims-based analysis linked GnRH agonists with a greater risk for incident coronary heart disease and MI.10 Using data from a large, multicenter, prospective, randomized controlled trial with long follow-up, we found that adjuvant goserelin was not associated with increased cardiovascular mortality in men with locally advanced prostate cancer. Specifically, the 9-year cardiovascular mortality rate for men treated with adjuvant goserelin was 8.4% v 11.4% for men treated without adjuvant goserelin. The lack of an apparent treatment-related detrimental effect was similarly seen after censoring patients at time of salvage GnRH agonist therapy and when using alternative definitions of cardiovascular mortality. Our results also confirmed that established cardiovascular risk factors, such as prevalent CVD and DM, are associated with increased risk of cardiovascular mortality. Within subgroups of men with highest risk, there remained no apparent treatment-related increase in cardiovascular mortality.
To the best of our knowledge, these are the first analyses using data from a large prospective study to directly address the potential relationship between GnRH agonists and cardiovascular mortality. Our results are consistent with other published reports. In a prospective randomized controlled trial (RTOG 92-02), long-term adjuvant treatment with GnRH agonists was associated with greater noncancer mortality than short-term therapy, although there seemed to be no difference when classified as cardiovascular death.5,5a Notably, there was an imbalance between the groups, with the long-term arm having a higher rate of prevalent CVD that the short-term arm (55% v 44%, respectively). A retrospective study suggested that neoadjuvant GnRH agonist therapy in men treated for early-stage prostate cancer with prostate brachytherapy was associated with worse overall survival compared with hormone-naïve men, although cancer-specific mortality was similar.29 In that series, CVD was the single largest cause of death in both groups without any obvious discrepancy between the groups (representing 24% and 22% of overall mortality in patients who did and did not receive GnRH agonist therapy, respectively). In European Organisation for Research and Treatment of Cancer trial 30891,30 in which 985 men with localized prostate cancer not suitable for local curative treatment were treated with immediate or deferred ADT (orchiectomy or GnRH agonist), overall survival favored the immediate arm seemingly as a result of fewer noncancer deaths. Specifically, death from CVD was 17.9% in the immediate arm compared with 19.7% in the deferred arm. In a recent pooled data analysis31 of three trials using varying courses of short-term GnRH agonist therapy, it was suggested that a subset of men age 65 years or older who received 6 months of ADT experienced shorter times to fatal MIs compared with men in this age group who did not receive ADT; notably, this study did not show any difference in total number of fatal MIs (18 v 16 MIs, respectively). Furthermore, compared with our study, the analysis was limited by a lower number of events (approximately one third), shorter follow-up, short treatment duration, and lack of information on known CVD risk factors.
The absence of an apparent increase in cardiovascular mortality in our study and other trials does not exclude the possibility that GnRH agonists increase noncancer mortality. Men with prostate cancer have higher rates of noncancer death than the general population,32 and GnRH agonists may contribute to this through multiple mechanisms. In the recent claims-based Surveillance, Epidemiology, and End Results-Medicare analysis that first reported an association between GnRH agonists and incident nonfatal coronary heart disease and MI,10 the effect was modest (16% and 11% increased risk, respectively) and may not translate into an apparent increase in cardiovascular mortality. Notably, the analyses by Keating et al10 also showed a greater risk for other adverse events, including incident DM (44% increased risk), which may independently contribute to noncancer, noncardiac mortality. GnRH agonists also decrease bone mineral density33,34 and increase fracture risk in men with prostate cancer,35,36 another possible cause of death.37 In addition, GnRH agonists may lead to declines in hemoglobin,38 and anemia has been shown to be a prognostic factor for survival in men with hormone-refractory prostate cancer.39 Other adverse effects such as fatigue and psychological distress40 impact quality of life and overall frailty. Thus, GnRH agonists have the potential to impact noncancer mortality through several mechanisms.
Additional studies are necessary to assess potential effects of neoadjuvant/adjuvant GnRH agonist therapy on cardiovascular mortality in men with earlier stage prostate cancer and lower rates of cancer-specific mortality. In our population of men with locally advanced disease, there was a significant rate of prostate cancer–specific mortality (35%). Cardiovascular mortality represented approximately 20% of all deaths in our study, whereas it represents closer to 30% of all deaths in the general male population.41 Especially among men with earlier stage disease and favorable prognosis in whom the role for GnRH agonists has not been clearly defined, treatment decisions need to carefully weigh potential risks and benefits.
Our study has substantial strengths. First, it is one of few prostate cancer trials with a control arm of no hormone therapy. Second, our study was large, with 945 patients, more than 11 years of follow-up for living patients, and 117 cardiovascular deaths. Although it is possible that we could have missed a small adverse treatment effect, the clinical significance of any such small effect may be questionable. Third, although we used investigator-defined cause of death as our study outcome, our ascertainment of cardiovascular mortality seems reliable given the strong association with traditional CVD risk factors, including prevalent CVD and DM, and consistency of results using alternative definitions of cardiovascular mortality. Data were not collected on each individual investigator, and thus, controlling for each investigator was not feasible. Fourth, we had information on a number of known CVD risk factors, and importantly, rates of prevalent CVD were similar between the treatment arms. We did lack detailed information on other risk factors including hyperlipidemia and certain lifestyle factors, such as smoking, diet, and physical activity, as well as CVD severity and use of cardiac medications. It is unlikely that there would have been any imbalance between the arms with respect to these other factors, however, given the size and randomized nature of our study. Fifth, the study's follow-up requirements for both arms seem to substantially exceed routine follow-up for adult men without cancer; accordingly, the possibility that any incremental difference in intensity of oncology follow-up for administration of a GnRH agonist led to a decrease in cardiovascular mortality in arm 1 seems remote and is unlikely to have affected the outcome. Finally, we observed consistency in our results when applying alternative definitions of cardiovascular mortality, when censoring at the time of salvage GnRH agonist therapy, and when imputing missing data.
In summary, although GnRH agonists are associated with greater risk of incident coronary heart disease and MI, we found no evidence that adjuvant long-term treatment with GnRH agonists increased cardiovascular mortality in men with locally advanced prostate cancer. Additional studies are needed to assess the potential relationship between GnRH agonists and cardiovascular mortality in men with lower cancer-specific mortality.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jason A. Efstathiou, Matthew R. Smith
Provision of study materials or patients: William U. Shipley, Gerald E. Hanks, Miljenko V. Pilepich, Howard M. Sandler
Collection and assembly of data: Kyounghwa Bae
Data analysis and interpretation: Jason A. Efstathiou, Kyounghwa Bae, William U. Shipley, Howard M. Sandler, Matthew R. Smith
Manuscript writing: Jason A. Efstathiou, Matthew R. Smith
Final approval of manuscript: Jason A. Efstathiou, Kyounghwa Bae, Matthew R. Smith
published online ahead of print at www.jco.org on December 1, 2008.
Supported in part by an NIH K24 Midcareer Investigator Award (5K24CA121990-02; M.R.S.) and grants from the Prostate Cancer Foundation (M.R.S.).
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Bolla M, Collette L, Blank L, et al: Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 360:103-106, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Pilepich MV, Winter K, Lawton CA, et al: Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 61:1285-1290, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Messing EM, Manola J, Sarosdy M, et al: Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 341:1781-1788, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Denham JW, Steigler A, Lamb DS, et al: Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol 6:841-850, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hanks GE, Pajak TF, Porter A, et al: Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 21:3972-3978, 2003 [DOI] [PubMed] [Google Scholar]
- 5a.Efstathiou JA, Bae K, Shipley WU, et al: Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: Analysis of RTOG 92-02. Eur Urol 54:816-824, 2008 [DOI] [PubMed] [Google Scholar]
- 6.D'Amico AV, Manola J, Loffredo M, et al: 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 292:821-827, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Park S, Meng MV, Elkin EP, et al: Androgen deprivation use with external beam radiation for prostate cancer: Results from CaPSURE. J Urol 174:1802-1807, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Shahinian VB, Kuo YF, Freeman JL, et al: Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 103:1615-1624, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Delorenzo MA, Walker-Corkery ES, et al: The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: A population-based cohort study. BJU Int 98:973-978, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating NL, O'Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24:4448-4456, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Finkelstein JS, McGovern FJ, et al: Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 87:599-603, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Smith MR: Changes in body composition during hormonal therapy for prostate cancer. Clin Prostate Cancer 2:18-21, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Smith MR: Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology 63:742-745, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Smith JC, Bennett S, Evans LM, et al: The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86:4261-4267, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Eri LM, Urdal P, Bechensteen AG: Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol 154:100-104, 1995 [PubMed] [Google Scholar]
- 16.Smith MR, Lee H, Nathan DM: Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 91:1305-1308, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Basaria S, Muller DC, Carducci MA, et al: Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 106:581-588, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Braga-Basaria M, Dobs AS, Muller DC, et al: Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 24:3979-3983, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Després JP, Lamarche B, Mauriege P, et al: Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 334:952-957, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Lakka HM, Laaksonen DE, Lakka TA, et al: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709-2716, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Pilepich MV, Caplan R, Byhardt RW, et al: Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol 15:1013-1021, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obes Res 6:51S-209S, 1998. (suppl 2) [PubMed] [Google Scholar]
- 24.Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data. New York, NY, John Wiley & Sons, 1980
- 25.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 26.Fine J, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 27.Rubin DB: Multiple Imputation for Nonresponse in Surveys. New York, NY, John Wiley & Sons, 1987
- 28.Li KH: Imputation using Markov chains. J Stat Comput Sim 30:57-79, 1988 [Google Scholar]
- 29.Beyer DC, McKeough T, Thomas T: Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 61:1299-1305, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Studer UE, Whelan P, Albrecht W, et al: Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol 24:1868-1876, 2006 [DOI] [PubMed] [Google Scholar]
- 31.D'Amico AV, Denham JW, Crook J, et al: Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 25:2420-2425, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Brown BW, Brauner C, Minnotte MC: Noncancer deaths in white adult cancer patients. J Natl Cancer Inst 85:979-987, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Stoch SA, Parker RA, Chen L, et al: Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab 86:2787-2791, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Smith MR, Goode M, Zietman AL, et al: Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: Effects on bone mineral density and body composition. J Clin Oncol 22:2546-2553, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Smith MR, Lee WC, Brandman J, et al: Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 23:7897-7903, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Shahinian VB, Kuo YF, Freeman JL, et al: Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352:154-164, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Seeman E: The structural basis of bone fragility in men. Bone 25:143-147, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Strum SB, McDermed JE, Scholz MC, et al: Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol 79:933-941, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Vollmer RT, Kantoff PW, Dawson NA, et al: Importance of serum hemoglobin in hormone refractory prostate cancer. Clin Cancer Res 8:1049-1053, 2002 [PubMed] [Google Scholar]
- 40.Herr HW, O'Sullivan M: Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol 163:1743-1746, 2000 [PubMed] [Google Scholar]
- 41.Anderson RN, Smith BL: Deaths: Leading causes for 2002. Natl Vital Stat Rep 53:1-92, 2005 [PubMed] [Google Scholar]