Abstract
Multidrug resistance pumps (MDRs) protect microbial cells from both synthetic and natural antimicrobials. Amphipathic cations are preferred substrates of MDRs. Berberine alkaloids, which are cationic antimicrobials produced by a variety of plants, are readily extruded by MDRs. Several Berberis medicinal plants producing berberine were found also to synthesize an inhibitor of the NorA MDR pump of a human pathogen Staphylococcus aureus. The inhibitor was identified as 5′-methoxyhydnocarpin (5′-MHC), previously reported as a minor component of chaulmoogra oil, a traditional therapy for leprosy. 5′-MHC is an amphipathic weak acid and is distinctly different from the cationic substrates of NorA. 5′-MHC had no antimicrobial activity alone but strongly potentiated the action of berberine and other NorA substrates against S. aureus. MDR-dependent efflux of ethidium bromide and berberine from S. aureus cells was completely inhibited by 5′-MHC. The level of accumulation of berberine in the cells was increased strongly in the presence of 5′-MHC, indicating that this plant compound effectively disabled the bacterial resistance mechanism against the berberine antimicrobial.
Keywords: multidrug resistance, efflux inhibitor
Bacteria have evolved numerous defenses against antimicrobial agents, and drug-resistant pathogens are on the rise (1). A general and effective defense is conferred by ubiquitous multidrug resistance pumps (MDRs), membrane translocases that extrude structurally unrelated toxins from the cell (2–5). Preferred substrates of most MDRs are synthetic hydrophobic cations such as quaternary ammonium antiseptics (6, 7). We have identified a group of cationic berberine alkaloids as natural substrates of MDR pumps (6). We suggested that berberine alkaloids represent a possibly larger group of cationic toxins that fueled the evolution of MDRs (7). Considering that microbial MDRs can render berberine alkaloids essentially ineffective, we reasoned that plants would benefit from making an MDR inhibitor. Here we show that Berberis fremontii, a berberine producer (8) used in Native American traditional medicine (9, 10), synthesizes a potent MDR inhibitor. Structural determination identified the substance as 5′-methoxyhydnocarpin (5′-MHC). Efflux of berberine from pathogenic Staphylococcus aureus expressing the NorA MDR pump that confers resistance to quinolones and antiseptics (6, 11, 12) was inhibited completely by 5′-MHC. This is a clear example of synergy between components of a medicinal plant described at a molecular level.
Materials and Methods
Cell Culturing and Susceptibility Testing.
S. aureus 4222 parent strain and the norA mutant KLE 820 (6) were cultured in Mueller–Hinton (MH) broth. Cells (105/ml) were inoculated into MH broth and dispensed at 0.2 ml/well in microtiter plates. All tests were done in triplicate by following National Center for Clinical Laboratory Standards recommendations. Briefly, minimal inhibitory concentrations (MIC) were determined by serial 2-fold dilution of test compounds. MIC was defined as a concentration of an antimicrobial that completely prevented cell growth during an 18-hr incubation at 37°C. Growth was assayed with a microtiter plate reader (Bio-Rad) by absorption at 600 nm.
Measurement of Active Transport.
Cells were cultured with aeration at 37°C to an OD600 of 1.8, pelleted, and washed twice with 20 mM Hepes/NaOH (pH 7.0) buffer. Cells then were resuspended in 1 ml of Hepes buffer at an OD600 of 0.3 containing 10 μM CCCP and 10 μg/ml ethidium bromide followed by incubation at 37°C for 30 min (6). The cells were centrifuged, washed, and resuspended at an OD600 of 0.15 in Hepes buffer, and fluorescence was measured with a Perkin–Elmer LS-5B luminescence spectrometer at 530-nm excitation and 600-nm emission wavelengths. Measurement of berberine efflux was performed by following a similar procedure with excitation at 355 nm and emission at 517 nm. The concentration of berberine for cell loading was 30 μg/ml.
Isolation of MDR Inhibitors and Structure Determination.
Dried, ground leaves (188 g) of B. fremontii were submerged in 1,200 ml of hexanes at room temperature for 24 hr and filtered from this inactive extract. The leaves then were treated similarly with 1,000 ml of chloroform for 24 hr, and the chloroform was removed in vacuo at 30–40°C to leave 1.4 g of dark black-green residue. Extract (1.4 g) was subjected to flash chromatography over silica gel with 9:l chloroform/methanol as eluting solvent. Twenty fractions were taken, the solvent was evaporated, and the fractions were weighed and tested for activity. Material from the active fractions was subjected to further separation on silica gel columns with chloroform/ethyl acetate/acetone/acetic acid, 7:1:2:0.1, and/or on reverse-phase silica gel columns by using acetonitrile/water, 70:30, with addition of a drop of diluted acetic acid. Structure determination was by NMR, UV light, and MS in comparison with literature values (13, 14).
Results and Discussion
Isolation of an MDR Inhibitor.
The alkaloid berberine (Fig. 1) is a common component of a variety of plant species, particularly in the family Berberidaceae (15). Berberine exhibits relatively weak antibiotic properties (16), apparently because of its efflux by MDRs (6). A bioassay-driven purification was used to detect possible MDR inhibitors accompanying berberine in Berberis repens, B. aquifolia, and B. fremontii. S. aureus, a major human pathogen largely responsible for nosocomial infections, was used as a target. The rationale to detect MDR inhibitory activity was to test the combined action of a plant extract (the nonalkaloid fraction) with berberine added at a subinhibitory concentration. Extracts that inhibited cell growth in the presence of berberine and had no activity when added alone were likely to contain an MDR inhibitor. Chloroform extracts of leaves from the three species had no antimicrobial activity at >500 μg/ml, but inhibited S. aureus growth completely in the presence of 30 μg/ml berberine, a concentration one-eighth the MIC for this substance. Isolation of an MDR inhibitor from B. fremontii (Fig. 2) provides an example. A chloroform extract from leaves had an activity of around 100 μg/ml in the presence of berberine. The extract was purified further by silica gel chromatography, and 20 fractions were collected. Activity was present in two peaks—fraction 5 and fractions 8–9 (not shown). Activity of the material from fraction 5 was 3 μg/ml in the presence of 30 μg/ml berberine, and it had no activity alone at 100 μg/ml. Further purification and characterization of the inhibitor from fraction 5 are presented in this paper. Reverse-phase chromatography produced a pure compound, and its structure was determined. The compound is 5′-MHC. We estimate that the content of 5′-MHC in B. fremontii was 0.05–0.1% of dry leaf weight. 5′-MHC was identified similarly from B. repens and B. aquifolia.
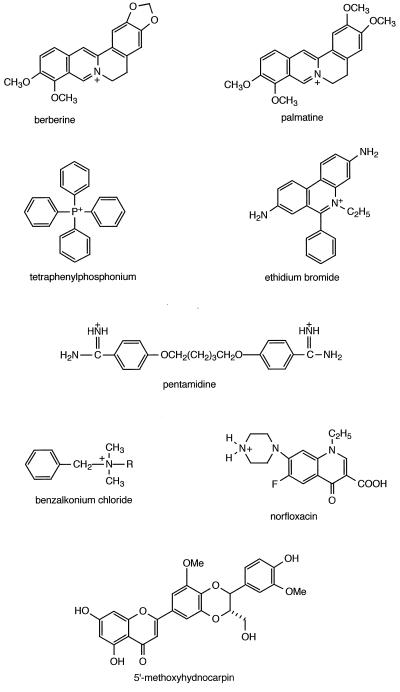
Figure 1.
Structural formulas of NorA substrates and inhibitors. Substrates that are weak bases are shown in their cationic form. 5′-MHC is the MDR inhibitor identified in this study.
Figure 2.
Medicinal plants producing berberine and the MDR inhibitor 5′-MHC. (Top) B. fremontii. (Middle) B. repens. (Bottom) B. aquifolia.
5′-MHC was reported previously only once (13) as a minor component of Hydnocarpus wightiana of the family Flacourtaceae. Nothing has been reported in the literature on the bioactivity of 5′-MHC. Known substrates of NorA MDR are hydrophobic cations (Fig. 1). The 7-OH group of 5′-MHC has a pKa of 7.3 (17), meaning that the substance exists as a mixture of an anion and a neutral molecule at physiological pH and certainly is not a typical MDR substrate. The neutral/anionic 5′-MHC might be a noncompetitive inhibitor interacting with the MDR in a manner distinctly different from its cationic substrates. Any prospective microbial MDR inhibitor to be used in medicine should be devoid of activity against P-glycoprotein MDR that is responsible for multidrug resistance of tumors and plays a role in toxin extrusion from normal cells (18). Interestingly, flavonoids with alkylated 7-O groups were active against P-glycoprotein, whereas 7-OH forms were completely inactive, apparently because of acidic properties of this group (19). The 7-OH containing 5′-MHC is likely to be a specific microbial MDR inhibitor.
Characterization of Inhibitory Activity of 5′-MHC.
5′-MHC potentiated the action of NorA substrates (Table 1). Norfloxacin had an MIC of 1 μg/ml in the wild type and 0.25 μg/ml in the NorA mutant strain. Addition of 5′-MHC to the wild type brought down the norfloxacin MIC to 0.25 μg/ml, indicating complete inhibition of efflux of this antibiotic by the plant MDR inhibitor. Addition of 5′-MHC to the NorA mutant strain had no effect, suggesting that NorA is the only pump extruding norfloxacin. This is consistent with norA being the only gene identified that affects norfloxacin transport (2). However, one cannot exclude the possibility of there being another MDR that extrudes norfloxacin and is insensitive to 5′-MHC. A similar pattern was observed with tetraphenylphosphonium (note that 2-fold differences in MIC might not be significant). With ethidium bromide, pentamidine, and benzalkonium chloride, the MIC of the NorA mutant strain was notably lower as compared with the wild type with 5′-MHC. This suggests that 5′-MHC does not completely inhibit efflux of these substances by the MDR. In P-glycoprotein, there is evidence for at least two binding sites (or subsites) with different inhibitor and substrate-binding properties (20). We might be encountering a similar phenomenon with NorA, 5′-MHC, pentamidine, and benzalkonium chloride. 5′-MHC, when tested with berberine and palmatine, appeared to completely inhibit NorA—the MIC was similar to the NorA mutant strain tested with these alkaloids. Unexpectedly, MIC for the alkaloids dropped further when 5′-MHC was added to the norA strain. This would suggest that S. aureus harbors an additional MDR pump that is rather specific for berberine alkaloids and is sensitive to 5′-MHC. Ethidium bromide is a planar molecule resembling berberine alkaloids, and 5′-MHC potentiated its action against the norA strain, suggesting that an additional MDR can be involved in this case as well.
Table 1.
Potentiation of NorA substrates by 5′-MHC in inhibiting growth of S. aureus
| Minimal inhibitory concentration, μg/ml
|
||||
|---|---|---|---|---|
| Wild type | NorA− | |||
| Drug | + 5′-MHC, 10 μg/ml | + 5′-MHC, 10 μg/ml | ||
| Norfloxacin | 1 | 0.25 | 0.25 | 0.25 |
| EtdBr | 4 | 0.5 | 0.5 | 0.125 |
| Tetraphenylphosphonium | 16 | 4 | 4 | 2 |
| Pentamidine | 64 | 4 | 0.5 | 0.25 |
| Benzalkonium chloride | 1 | 0.125 | 0.03125 | 0.0625 |
| Berberine | 256 | 16 | 32 | 2 |
| Palmatine | >256 | 64 | 64 | 2 |
A more detailed examination of a dose–response inhibition of cell growth is shown in Fig. 3a. When combined with subinhibitory amounts of berberine, 5′-MHC caused complete inhibition of growth at a concentration of 1 μg/ml. Berberine alone showed poor antimicrobial activity, and 5′-MHC alone had no antimicrobial activity at a concentration above 500 μg/ml. Synthetic inhibitors of bacterial MDR pumps have been identified by screening compound libraries (21). The only currently known natural inhibitor of bacterial MDRs is reserpine (22), an antihypertension alkaloid that has a 20-fold lower activity as compared with 5′-MHC. Reserpine initially was found to inhibit human P-glycoprotein, and it inhibits the bacterial ABC transporter LmrA that functionally complements human P-glycoprotein (23).
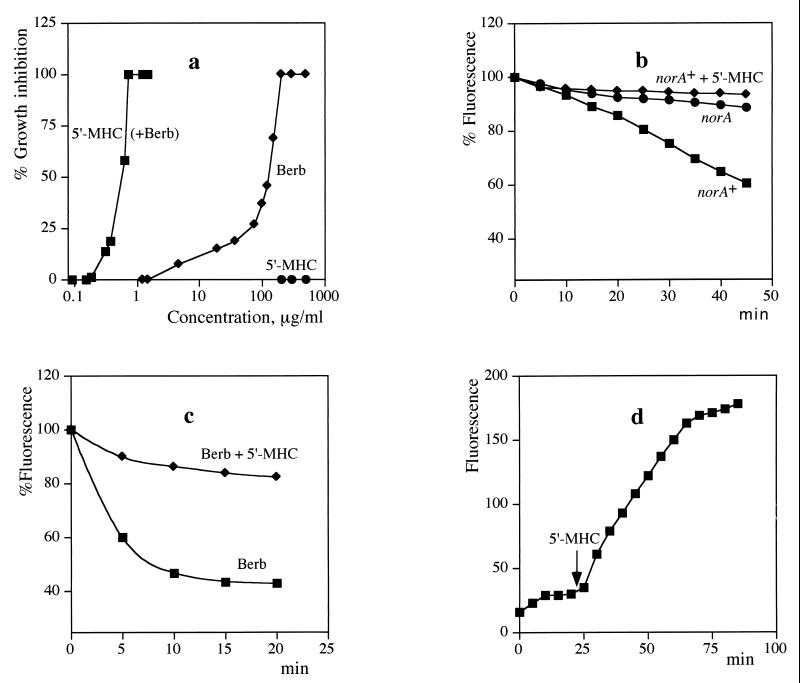
Figure 3.
Synergistic action of berberine and 5′-MHC. (a) Growth inhibition of S. aureus. Berberine was present at a concentration of 30 μg/ml when combined with 5′-MHC. Measurements were performed in triplicate, and the average values are shown. (b) Inhibition of NorA transport activity by 5′-MHC. S. aureus cells were loaded with EtdBr and washed, and efflux was measured in the presence of 100 mM formate, a respiratory substrate. 5′-MHC was added at a final concentration of 10 μg/ml. (c) Cells were loaded with berberine and efflux was measured in the presence of formate. (d) Uptake of berberine added at time 0 by cells in the presence of formate. A small increase of fluorescence produced by 5′-MHC alone was subtracted from the plot.
Next, the ability of 5′-MHC to inhibit NorA was measured directly, after extrusion of ethidium bromide (EtdBr). S. aureus cells deenergized with a protonophore (CCCP) can be loaded with EtdBr. EtdBr bound to DNA has a high level of fluorescence, and extrusion of EtdBr causes a decrease in fluorescence. Efflux of EtdBr was significant in wild-type cells expressing the NorA MDR (Fig. 3b). The rate of efflux will be affected by two opposing forces—accumulation of the permeant cation driven by the membrane potential (24) and extrusion by an MDR pump. The rate of efflux was low in a mutant with a disrupted norA gene (Fig. 3b). Addition of 5′-MHC completely inhibited NorA-dependent efflux of EtdBr in the wild type.
Berberine is a planar cationic molecule (Fig. 1) that resembles EtdBr and binds to DNA (25). The DNA binding apparently contributes to the antimicrobial activity of berberine. Similar to EtdBr, DNA-bound berberine has increased fluorescence. We took advantage of this property of berberine to directly examine the action of 5′-MHC on berberine efflux. Cells were loaded with berberine as described for EtdBr. Efflux of berberine was more rapid as compared with EtdBr. 5′-MHC effectively blocked berberine efflux (Fig. 3c). In a natural setting, a multidrug pump will decrease the rate of entry of berberine into the cell. The experiment shown in Fig. 3d emulates this situation. Berberine was added to energized cells of S. aureus, and a rapid accumulation was observed. After the cellular level of berberine reached a steady-state, 5′-MHC was added and a further, much larger uptake was observed.
Permeant cations like EtdBr and tetraphenylphosphonium were introduced originally to measure the membrane potential in mitochondria by following their uptake (24). Similar measurements in bacteria are inaccurate, as we now realize because of extrusion by MDRs, as the comparison of initial berberine uptake and accumulation in the presence of 5′-MHC clearly demonstrate. Applying inhibitors like 5′-MHC might revive this potentially useful method of measuring the membrane potential in bacteria.
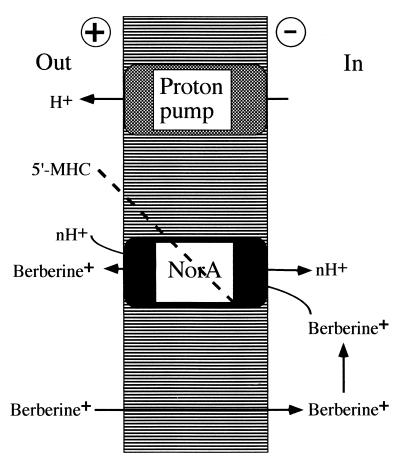
The two phases of the Fig. 3d plot perhaps reflect the sequence of evolutionary events, starting with bacteria having considerable resistance to berberine, followed by development of MDR inhibitors by the plant that overcame this resistance. Our experiments show how two different components of a medicinal plant can act in synergy, with one compound disabling a resistance mechanism and potentiating the antimicrobial activity of the antibiotic substance (Fig. 4). “Synergy” is a popular concept in the field of herbal medicine, suggesting that plant extracts contain compounds potentiating each other's action. Possible synergy would explain many failed attempts to isolate single, active compounds from medicinal plants. Solid, mechanistically supported evidence for this concept, however, has been lacking (26). It is hoped that this study will stimulate investigations at the molecular level of possible medicinal plant synergisms. Interestingly, 5′-MHC has been reported previously as a minor component of chaulmoogra oil from seeds of Hydnocarpus trees (13). Chaulmoogra oil has been used as the main treatment for leprosy in Indian and Chinese traditional medicine and, subsequently, in the West before the era of sulfones and antibiotics (27). Hydnocarpic acid had been identified as the principle active ingredient of the oil and showed antimycobacterial activity, apparently acting as an antagonist of biotin (28). The substance was marketed by Burroughs Wellcome as Alepol. It seems possible that Hydnocarpus seeds combine a synergistic couple of an antimicrobial, hydnocarpic acid and an MDR inhibitor, 5′-MHC, or its analog hydnocarpin (which is also present in the plant). Hydnocarpic acid is a lipophilic compound and would be a typical substrate for such broad-spectrum MDRs as EmrAB (29, 30) or RND and ABC pumps. By extracting the “active ingredient” from the oil, Western medicine might have missed the second essential component of the synergistic couple.
Figure 4.
A model of synergistic action of berberine and an MDR inhibitor that are both produced by B. fremontii. Berberine accumulates in the cell driven by the membrane potential. The NorA pump extrudes berberine. The MDR inhibitor 5′-MHC blocks the NorA pump, potentiating the antibiotic action of berberine.
The NorA pump of S. aureus is a member of the Major Facilitator family of drug/proton antiporters that are widely spread among Gram-positive, Gram-negative bacteria and yeast and are found in Archaea (31, 32). Substrates of most MF MDRs are hydrophobic cations and hydrophilic quinolones (probably transported in the form of protonated bases), in the case of NorA. S. aureus is likely to encounter natural cationic antimicrobials such as berberines when the microbe is persisting in the environment. Berberis species are not known to be infected by bacterial pathogens, apparently because of the presence of effective antimicrobials like berberine and 5′-MHC. Our current survey of Berberis species using NorA as a target has established the presence of the MDR inhibitor 5′-MHC and at least one additional compound (unpublished data). It seems that plants producing antimicrobials may have developed a variety of MDR inhibitors against different MDR pumps of plant pathogens. Because of their broad specificity, MDRs provide a ready-made resistance mechanism for the newest synthetic antibiotics such as quinolones. In a sense, various pathogens already have developed a resistance mechanism to current and future antimicrobials. Emulating nature's strategy and potentiating antibiotics with MDR inhibitors can be an effective strategy against drug-resistant microorganisms.
Acknowledgments
This work was supported by grants from the National Institutes of Health to K.L., the Howard Hughes Medical Institute to L.Z., and the National Science Foundation and Colorado State University Agricultural Experiment Station to F.R.S.
Abbreviations
- MDR
multidrug resistance pump
- MIC
minimal inhibitory concentration
- 5′-MHC
5′-methoxyhydnocarpin
- EtdBr
ethidium bromide
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030540597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030540597
References
- 1.Levy S B. Sci Am. 1998;278:46–53. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 2.Lewis K. In: Transport of Molecules Across Microbial Membranes. Broome-Smith J K, Baumberg S, Stirling C J, Ward F B, editors. Vol. 58. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 15–40. [Google Scholar]
- 3.Nikaido H. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen I T, Brown M H, Skurray R A. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lolkema J S, Poolman B, Konings W N. Curr Opin Microbiol. 1998;1:248–253. doi: 10.1016/s1369-5274(98)80018-0. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh P C, Siegel S A, Rogers B, Davis D, Lewis K. Proc Natl Acad Sci USA. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis K. Curr Biol. 1999;9:R403–R407. doi: 10.1016/s0960-9822(99)80254-1. [DOI] [PubMed] [Google Scholar]
- 8.Suess T R, Stermitz F R. J Nat Prod. 1981;44:680–687. doi: 10.1021/np50018a013. [DOI] [PubMed] [Google Scholar]
- 9.Vestal P A. Papers of the Peabody Museum (Harvard) Vol. 50 1952. [Google Scholar]
- 10.Moore M. Medicinal Plants of the Pacific West. Santa Fe, NM: Red Crane Books; 1993. [Google Scholar]
- 11.Neyfakh A A, Borsch C M, Kaatz G W. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng E Y, Trucksis M, Hooper D C. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranganathan K R, Seshadri T R. Indian J Chem. 1974;12:993. [Google Scholar]
- 14.Parthasarathy M R, Ranganathan K R, Sharma D K. Phytochemistry. 1979;18:506–508. [Google Scholar]
- 15.Bhakuni D S, Jain S. In: The Alkaloids: Chemistry and Pharmacology. Brossi A, editor. Vol. 28. New York: Academic; 1986. pp. 95–174. [Google Scholar]
- 16.Amin A H, Subbaiah T V, Abbasi K M. Can J Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 17.Markham K R, McGhie T K. Polyphenols Com. 1996;96:13–14. [Google Scholar]
- 18.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 19.Ferte J, Kuhnel J M, Chapuis G, Rolland Y, Lewin G, Schwaller M A. J Med Chem. 1999;42:478–489. doi: 10.1021/jm981064b. [DOI] [PubMed] [Google Scholar]
- 20.Dey S, Ramachandra M, Pastan I, Gottesman M M, Ambudkar S V. Proc Natl Acad Sci USA. 1997;94:10594–10599. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markham P N, Westhaus E, Klyachko K, Johnson M E, Neyfakh A A. Antimicrob Agents Chemother. 1999;43:2404–2408. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neyfakh A A, Bidnenko V E, Chen L B. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Veen H W, Callaghan R, Soceneantu L, Sardini A, Konings W N, Higgins C F. Nature (London) 1998;391:291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 24.Liberman E A, Topaly V P, Tsofina L M, Jasaitis A A, Skulachev V P. Nature (London) 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 25.Jennings B R, Ridler P J. Biophys Struct Mech. 1983;10:71–79. doi: 10.1007/BF00535543. [DOI] [PubMed] [Google Scholar]
- 26.Duke J A, Bogenschutz-Godwin M J. The Synergy Principle at Work in Plants, Pathogens, Insects, Herbivores, and Humans. Boca Raton, FL: CRC; 1998. [Google Scholar]
- 27.Norton S A. J Am Acad Dermatol. 1994;31:683–686. doi: 10.1016/s0190-9622(08)81744-6. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen P L, Levy L. Antimicrob Agents Chemother. 1973;3:373–379. doi: 10.1128/aac.3.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomovskaya O, Lewis K. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colmer J A, Fralick J A, Hamood A N. Mol Microbiol. 1998;27:63–72. doi: 10.1046/j.1365-2958.1998.00657.x. [DOI] [PubMed] [Google Scholar]
- 31.Paulsen I T, Sliwinski M K, Saier M H., Jr J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen I T, Sliwinski M K, Nelissen B, Goffeau A, Saier M H., Jr FEBS Lett. 1998;430:116–125. doi: 10.1016/s0014-5793(98)00629-2. [DOI] [PubMed] [Google Scholar]