Abstract
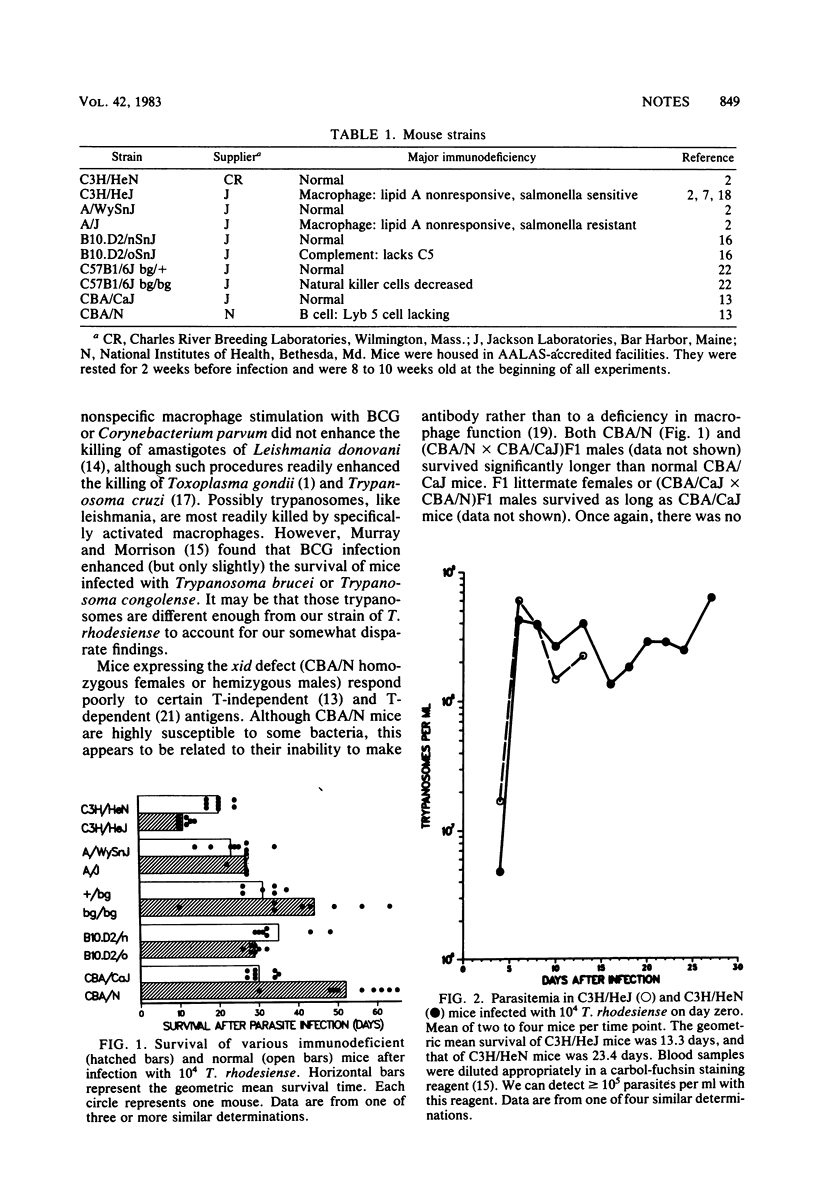
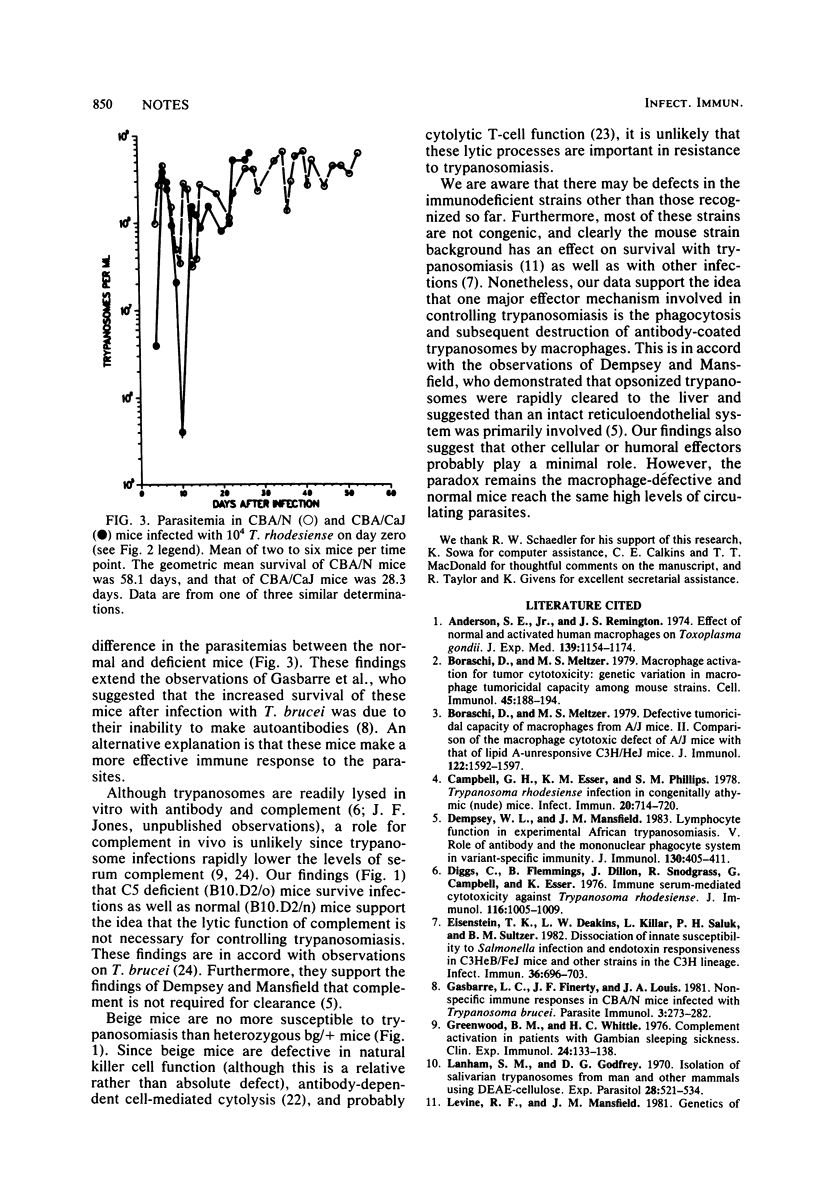
By using mice with naturally occurring defects, we have shown that an intact macrophage system is crucial to survival with the pathogenic protozoan Trypanosoma rhodesiense, since a defect in these cells decreased survival by half. Deficiencies in natural killer cell function or complement levels had no effect on survival. However, the capacity to survive trypanosomiasis was not related to the levels of parasitemia achieved during infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from A/J mice. II. Comparison of the macrophage cytotoxic defect of A/J mice with that of lipid A-unresponsive C3H/HeJ mice. J Immunol. 1979 Apr;122(4):1592–1597. [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Macrophage activation for tumor cytotoxicity: genetic variation in macrophage tumoricidal capacity among mouse strains. Cell Immunol. 1979 Jun;45(1):188–194. doi: 10.1016/0008-8749(79)90375-7. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Esser K. M., Phillips S. M. Trypanosoma rhodesiense infection in congenitally athymic (nude) mice. Infect Immun. 1978 Jun;20(3):714–720. doi: 10.1128/iai.20.3.714-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. L., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983 Jan;130(1):405–411. [PubMed] [Google Scholar]
- Diggs C., Flemmings B., Dillon J., Snodgrass R., Campbell G., Esser K. Immune serum-mediated cytotoxicity against Trypanosoma rhodesiense. J Immunol. 1976 Apr;116(4):1005–1009. [PubMed] [Google Scholar]
- Eisenstein T. K., Deakins L. W., Killar L., Saluk P. H., Sultzer B. M. Dissociation of innate susceptibility to Salmonella infection and endotoxin responsiveness in C3HeB/FeJ mice and other strains in the C3H lineage. Infect Immun. 1982 May;36(2):696–703. doi: 10.1128/iai.36.2.696-703.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarre L. C., Finerty J. F., Louis J. A. Non-specific immune responses in CBA/N mice infected with Trypanosoma brucei. Parasite Immunol. 1981 Autumn;3(3):273–282. doi: 10.1111/j.1365-3024.1981.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C. Complement activation in patients with Gambian sleeping sickness. Clin Exp Immunol. 1976 Apr;24(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Zitron I. M., Mond J. J., Ahmed A., Scher I., Paul W. E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Murray M., Morrison W. I. Non-specific induction of increased resistance in mice to Trypanosoma congolense and Trypanosoma brucei by immunostimulants. Parasitology. 1979 Dec;79(3):349–366. doi: 10.1017/s0031182000053750. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Press J. L. The CBA/N defect defines two classes of T cell-dependent antigens. J Immunol. 1981 Apr;126(4):1234–1240. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Saxena R. K., Saxena Q. B., Adler W. H. Defective T-cell response in beige mutant mice. Nature. 1982 Jan 21;295(5846):240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Levine R. F., Mansfield J. M. Cloning of African trypanosomes in mice immunosuppressed by cyclophosphamide treatment. Am J Trop Med Hyg. 1982 Nov;31(6):1098–1102. doi: 10.4269/ajtmh.1982.31.1098. [DOI] [PubMed] [Google Scholar]


