Abstract
Background
After a 1999 National Cancer Institute (NCI) clinical alert was issued, chemoradiotherapy has become widely used in treating women with cervical cancer. Two subsequent systematic reviews found that interpretation of the benefits was complicated, and some important clinical questions were unanswered.
Patients and Methods
We initiated a meta-analysis seeking updated individual patient data from all randomized trials to assess the effect of chemoradiotherapy on all outcomes. We prespecified analyses to investigate whether the effect of chemoradiotherapy differed by trial or patient characteristics.
Results
On the basis of 13 trials that compared chemoradiotherapy versus the same radiotherapy, there was a 6% improvement in 5-year survival with chemoradiotherapy (hazard ratio [HR] = 0.81, P < .001). A larger survival benefit was seen for the two trials in which chemotherapy was administered after chemoradiotherapy. There was a significant survival benefit for both the group of trials that used platinum-based (HR = 0.83, P = .017) and non–platinum-based (HR = 0.77, P = .009) chemoradiotherapy, but no evidence of a difference in the size of the benefit by radiotherapy or chemotherapy dose or scheduling was seen. Chemoradiotherapy also reduced local and distant recurrence and progression and improved disease-free survival. There was a suggestion of a difference in the size of the survival benefit with tumor stage, but not across other patient subgroups. Acute hematologic and GI toxicity was increased with chemoradiotherapy, but data were too sparse for an analysis of late toxicity.
Conclusion
These results endorse the recommendations of the NCI alert, but also demonstrate their applicability to all women and a benefit of non–platinum-based chemoradiotherapy. Furthermore, although these results suggest an additional benefit from adjuvant chemotherapy, this requires testing in randomized trials.
INTRODUCTION
Cervical cancer is the second most common cancer among women worldwide and the main cancer affecting women in sub-Saharan Africa, Central America, and south-central Asia.1 A significant decline in incidence and mortality have been seen in North America, parts of Europe, Australia, and New Zealand, where screening programs have been implemented for some time.1-5
In 1999, after publication of five trials,6-10 the National Cancer Institute (NCI) issued an alert recommending that “concomitant (cisplatin-based) chemoradiotherapy should be considered instead of radiotherapy alone in women with cervical cancer.” This led to a change in the treatment for many women with cervical cancer.11,12 Two systematic reviews13-15 reported improved survival, progression-free survival, and recurrence rates with chemoradiotherapy. However, interpretation of the benefits were complicated by the use of different treatments on the control arms of the included studies,13 heterogeneity in trial results, and inconsistency in the definition of outcomes between trials.15 The authors concluded that an individual patient data (IPD) meta-analysis would be required to obtain time-to-event analyses of local and distant recurrence, more reliable estimates of effect in patient subgroups, and a better attribution of relative toxicities.
We therefore initiated a systematic review and meta-analysis that aimed to collect, validate, and reanalyze IPD from all relevant randomized trials.16 This permits time-to-event analyses and investigation of differences in the effect of chemoradiotherapy by trial or patient characteristics and, by seeking updated follow-up, provides the opportunity to look at these outcomes in the long-term. This IPD meta-analysis was initiated and coordinated by the Medical Research Council (United Kingdom) Clinical Trials Unit and carried out by the Chemoradiotherapy in Cervical Cancer Meta-Analysis Collaboration.
PATIENTS AND METHODS
The methods for this systematic review and meta-analysis followed a detailed, prespecified protocol (September 2004), a copy of which is available on request.
Trial Inclusion Criteria
Our inclusion criteria limited the main comparison to trials comparing concomitant chemoradiotherapy versus the same radiotherapy. However, given the importance to the NCI alert of two trials using hydroxyurea on the control arm9,10 and one trial that gave extended-field radiotherapy on the control arm,7,17 we analyzed these trials alongside the main comparison. For the main comparison, trials had to be properly randomized and should have aimed to randomly assign women with cervical cancer who had not received previous treatments likely to interfere with protocol treatments or comparisons. Trials should have been completed by the time of the final analyses (May 2007) and compared cytotoxic chemoradiotherapy (with or without surgery) with the same radiotherapy (with or without surgery). Chemotherapy should have been given on the experimental arm only. Trials were excluded if they used additional noncytotoxic treatments or only noncytotoxic radiosensitizers/radioprotectors on the experimental arm. Trials that used hydroxyurea as the sole chemotherapy agent have been considered in a prior systematic review18,19 and are not included here.
Trial Identification
To avoid publication bias, published and unpublished trials were included in the meta-analysis. We searched MEDLINE and CancerLit using an optimal search strategy,20 and also LILACS, the Physicians’ Data Query, and other trials registers. These were supplemented from reference lists of identified trial reports and review articles and from meeting proceedings (International Gynecologic Cancer Society and the Society for Gynecologic Oncology, 1994 through 2007). Furthermore, all participating investigators were asked to supplement our provisional list of trials. Searches were regularly updated until November 2007.
Data Collection
We sought to collect up-to-date information for all patients randomly assigned, including those excluded from investigators’ own analyses, on date of randomization, treatment allocation, tumor response, locoregional and distant progression/recurrence status, survival, cause of death, and acute and late toxicity. Baseline data on age, histology, International Federation of Gynecology and Obstetrics stage, tumor grade, performance status, and lymph node involvement were also sought. All data were checked for validity, consistency, and integrity of randomization and follow-up.16 Inconsistencies were resolved, and final database entries were validated by the responsible trial investigator, data manager, or statistician.
Definition of Outcomes
The primary outcome, overall survival, was defined as the time from randomization until death by any cause. Living patients were censored on the date of last follow-up. Locoregional progression/recurrence and metastases were supplied as classified in the individual trials. Locoregional disease-free survival was defined as the time from randomization until locoregional recurrence/progression or death by any cause. Patients alive with no locoregional disease were censored on the date of last follow-up. Metastases-free survival was defined as the time from randomization until first metastasis or death by any cause. Patients alive without metastases were censored on the date of last follow-up. In trials where only the first recurrence was recorded, patients with metastatic disease were censored in the analysis of locoregional recurrence, and those with locoregional disease were censored in the analysis of metastases. Overall disease-free survival was defined as the time from randomization until locoregional recurrence, metastasis, or death by any cause. Time to locoregional recurrence was defined as the time from randomization until the first local recurrence or progression; patients without local recurrence or progression were censored on the date of last follow-up or death. Time to metastases was defined as the time from randomization until first metastasis; patients without metastases were censored on the date of last follow-up or death. For trials that only recorded the first event, the methods of censoring described above were used.
Investigators were asked to supply acute and late toxicity data according to criteria used in their own trials. All trials used a five-grade system where 0 signifies no toxicity and 5 signifies death, making it reasonable to combine the results.
Analysis
All analyses (unless otherwise stated) were prespecified in the meta-analysis protocol and were performed on an intention-to-treat basis. For survival and recurrence outcomes, individual times to event were used to obtain hazard ratio (HR) estimates of treatment effect for individual trials, which were pooled across trials, using a stratified-by-trial, fixed-effect model.21 For binary outcomes of response and toxicity, the number of events and numbers of patients were used to calculate Peto odds ratio estimates of treatment effect21 for individual trials, which were pooled across trials using the stratified-by-trial, fixed-effect model.21 Trial results were also combined using the random effects approach.22
Three four-arm trials in the meta-analysis23-25 used a factorial design to assess the impact of two treatments at once, one of which was chemoradiotherapy. Each was split into two unconfounded comparisons of chemoradiotherapy versus radiotherapy and analyzed as separate trials (denoted A and B; Table 1). Two three-arm trials9,26 in the meta-analysis compared two different forms of chemoradiotherapy with a single control arm. The treatment arms were combined and compared with the control group for analysis; however, for the meta-analysis plot, each chemoradiotherapy arm is compared with the radiotherapy control arm as though they were two trials (A and B).
Table 1.
Characteristics of Included Studies
| Trial | Accrual Period | Stage | Affected Para-Aortic Nodes Excluded? | Comparison | Concomitant CT (dose in mg/m2) | CT Schedule
|
RT (Gy) | BRT(Gy to point A) | RT Duration (days) | No. of Patients | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cycles | Frequency (weeks) | ||||||||||
| Main analysis | |||||||||||
| *Thomas (a)24 | 1987-1995 | Ib (>5 cm) to IVa | No | RT v CTRT | IV FU 32 mg/m2 | 2 | 3 | 50 | 40 | < 56 | 116 |
| *Thomas (b)24 | 1987-1995 | Ib (>5 cm) to IVa | No | Hyperfractionated RT v hyperfractionated CTRT | IV FU 32 mg/m2 | 2 | 3 | 50 | 40 | < 56 | 118 |
| *†Lorvidhaya (a)25 | 1987-1994 | IIb, IIIb to IVa | No | RT v CTRT | MMC 10 mg/m2 | 2 | 4 | 50 | 28 | 49-56 | 475 |
| FU (oral) 4,200 mg | |||||||||||
| *†Lorvidhaya (b)25 | 1987-1994 | IIb, IIIb to IVa | No | RT + Adj CT v CTRT + Adj CT | MMC 10 mg/m2, FU (oral) 4,200 mg | 2 | 4 | 50 | 28 | 49-56 | 451 |
| 3 | 4 | ||||||||||
| Adj FU 5,600 mg (oral) | |||||||||||
| Onishi44 | 1988-1998 | IIb to IV | No | RT v CTRT | CDDP1: 100 mg/m2 | 2 | 2-3 | 50 | 24 | 45-55 | 49 |
| CDDP2: 50 mg/m2 | 3 | 1 | |||||||||
| CBDCA: 100 mg/m2 | 6 | 1 | |||||||||
| Roberts49 | 1991-2001 | Ib2, II to IVa | No | RT v CTRT | MMC 30 mg/m2 | 2 | 6 | IB2-IIB: 40‡ | 45-50 | Not specified | 248 |
| III-IVA: 46‡ | 40-45 | ||||||||||
| Peters8,46 SWOG8797 | 1991-1996 | Ia2 to IIa | No | S + RT v S + CTRT + CT | CDDP 70 mg/m2 | 4 | 3 | 49.3 | None | 42 | 268 |
| FU 4,000 mg/m2 | |||||||||||
| Pearcey43 | 1991-1996 | Ib-IIb (> 4 cm) III to IVa | No | RT v CTRT | CDDP 40 mg/m2 | 5 | 1 | 45 | 24-35 | 46-56 | 259 |
| Keys6 GOG0123 | 1992-1997 | Ib (bulky) | Yes | S + RT v S + CTRT | CDDP 40 mg/m2 | 6 | 1 | 45 | 30 | < 70 | 374 |
| *Chen (a)23 | 1993-1994 | IIb to III | No | RT v CTRT | CDDP 60 mg/m2 | 2 | 3 | 40 | 50 | 49 | 60 |
| FU 1,500 mg/m2 | |||||||||||
| VCR 2 mg/m2 | |||||||||||
| *Chen (b)23 | 1993-1994 | IIb to III | No | RT + hyperthermia v CTRT + hyperthermia | CDDP 60 mg/m2 | 2 | 3 | 40 | 50 | 49 | 60 |
| FU 1,500 mg/m2 | |||||||||||
| VCR 2 mg/m2 | |||||||||||
| Pras | 1995-1999 | Ib to IIa >4 cm | Yes | RT v CTRT | CDBCA 300 mg/m2 | 3 | 4 | 45 | 35 | > 56 | 54 |
| IIb to IVa | FU 2,400 mg/m2 | ||||||||||
| Leborgne | 1995-2004 | Ib2 to IVb | No | RT v CTRT | CDDP 80 mg/m2 | 2 | 4 | 40 | 42 | 40 | 340 |
| FU 2,400 mg/m2 | |||||||||||
| Garipagaoglu48 | 1996-1997 | IIb, IIIb | No | RT v CTRT | CDDP 120 mg/m2 | 2 | 3 | 46-50 | 20 | 61-62 | 44 |
| Kantardzic45 | 1996-1999 | IIb to III | No | RT v CTRT + CT | CDDP 40 mg/m2 | 6 | 3 | 46 | 25-30 | 56-60 | 80 |
| BLM 15 mg/m2 | 6 | 3 | |||||||||
| *Lanciano (a)26 GOG0165 | 1997-1998 | IIb, IIIb, IVa | Yes | RT v CTRT | CDDP 40 mg/m2 | 6 | 1 | 45 | 30 (HDR) or 40 (LDR) | < 56 | 50 |
| *Lanciano (b)26 GOG0165 | 1997-1998 | IIb, IIIb, IVa | Yes | RT v CTRT | FU 1,125 mg/m2 | 6 | 1 | 45 | 30 (HDR) or 40 (LDR) | < 56 | 51 |
| Lal50 | 2000-2006 | II to IV | No | RT v CTRT | CDDP 35 mg/m2 | 5 | 1 | 50 | 18 | 63 | 180 |
| Cikaric47 | 2002-2003 | IIb to IVa | No | RT v CTRT | CDDP 40 mg/m2 | 5 | 1 | 46 | 35 | 45 | 200 |
| Sensitivity analysis | |||||||||||
| Whitney10 GOG0085 | 1986-1990 | IIb to IVa | Yes | RT + HU v CTRT | CDDP 50 mg/m2 | 2 | 4 | 40.8 or 51 or 61.2 | 40 | < 70 | 388 |
| FU 4,000 mg/m2 | 40 | ||||||||||
| 0 | |||||||||||
| §Morris7,17 RTOG9001 | 1990-1997 | Ib to IIa (>4 cm or positive pelvic nodes) | Yes | RT v CTRT | CDDP 75 mg/m2 | 3 | 3 | 45 | 40 | < 56 | 403 |
| FU 4,000 mg/m2 | |||||||||||
| IIb to IVa | |||||||||||
| *Rose (a)9 GOG0120 | 1992-1997 | IIb to IVa | Yes | RT + HU v CTRT | CDDP 40 mg/m2 | 6 | 1 | 40.8 or 51 or 61.2 | 40 | 70 | 384 |
| 40 | |||||||||||
| 0 | |||||||||||
| *Rose (b)9 GOG0120 | 1992-1997 | IIb to IVa | Yes | RT + HU vCTRT + HU | CDDP 50 mg/m2 | 2 | 4 | 40.8 or 51 or 61.2 | 40 | 70 | 383 |
| FU 4,000 mg/m2 | 40 | ||||||||||
| HU (oral) 2 g/m2 | 1 | 6 | 0 | ||||||||
Abbreviations: CT, chemotherapy; RT, radiotherapy; BRT, brachytherapy; CTRT, chemoradiotherapy; IV, intravenous; FU, fluorouracil; MMC, mitomycin; Adj, adjuvant; CDDP, cisplatin; CDBCA, carboplatin; S, surgery; VCR, vincristine; BLM, bleomycin; HDR, high-dose rate; LDR, low-dose rate; HU, hydroxyurea; GOG, Gynecologic Oncology Group; RTOG, Radiation Therapy Oncology Group.
Three-arm and four-arm trials were analyzed as two separate trials.
After 673 patients were randomly assigned, FU was given 300 mg/day (oral) Monday through Friday for duration of external-beam radiotherapy.
With or without 8- to 10-Gy parametrial boost.
Extended-field external-beam radiotherapy (to para-aortic nodes) given on the control arm.
To explore the impact of trial characteristics on the effect of chemoradiotherapy, we prespecified analyses that grouped trials according to chemotherapy scheduling (chemotherapy entirely during radiotherapy, chemotherapy during and after radiotherapy); chemotherapy type (platinum-based chemotherapy, non–platinum-based chemotherapy); planned radiotherapy dose (optimal radiotherapy of ≥ 45 Gy external beam plus brachytherapy [any dose], sub-optimal radiotherapy of ≥ 45 Gy external beam without brachytherapy or < 45 Gy external beam with brachytherapy); planned radiotherapy duration (≤ 8 weeks, > 8 weeks). For the subset of trials that used cisplatin-based chemotherapy only, we also planned analyses of chemotherapy frequency (≤ 1 weekly cycles of chemotherapy, > 1 weekly cycles of chemotherapy) and chemotherapy dose-intensity (≤ 25 mg/m2/wk of cisplatin, > 25 mg/m2/wk of cisplatin). These analyses focused on the primary outcome of overall survival, with other outcomes carried out to support or refute any patterns found. For serious (grades 3 to 5) acute toxicity, trials were grouped according to their use of platinum-based chemoradiotherapy, non–platinum-based chemoradiotherapy, chemoradiotherapy plus additional chemotherapy, additional radiotherapy on the control arm, and additional hydroxyurea on the control arm, with HRs calculated for each group.
The effects of chemoradiotherapy within patient subgroups were investigated using similar stratified analyses. HRs were obtained for each predefined subgroup within each trial. These HRs were then combined to give overall HRs. χ2 tests for interaction or trend were used to investigate whether there were any substantial differences in the effect of concomitant chemoradiotherapy between different groups of trials or subgroups of patients.
Results are also presented as absolute differences, calculated from the overall HR and the control arm event rate.27 χ2 heterogeneity tests28 and the I2 statistic29 were used to assess statistical heterogeneity across trials. Kaplan-Meier curves30 are nonstratified. All P values are two-sided.
The main analyses described were limited to trials that compared concomitant chemotherapy and radical radiotherapy (with or without surgery) with the same radical radiotherapy (with or without surgery). However, to establish how sensitive the effect of chemoradiotherapy is to different trial designs and for completeness, the analyses were repeated including trials that used hydroxyurea or extended-field radiotherapy in the control arms.
Where IPD were not available, wherever possible, we calculated HRs and associated statistics from reported time-to-event analyses31,32 and considered the impact on the analyses of IPD.
RESULTS
Main Analysis
We identified 25 randomized trials that were eligible for the main analysis. We were unable to include data from 10 trials (1,113 patients), either because data could not be located33-38 (six trials, 814 patients) or because we were unable to make contact with the relevant investigators39-42 (four trials, 299 patients). Data were therefore available for 3,452 women from 15 trials23-26,43-50 (Leborgne, unpublished data; Pras, unpublished data). This includes 85% of women from trials that used cisplatin-based chemoradiotherapy and almost 80% of women from trials that used fluorouracil (FU)- and/or mitomycin-based (66% of all women who took part in trials of non–platinum-based) chemoradiotherapy. Data were obtained for 118 women (100%) who were excluded from the investigators’ original analyses and reinstated in the meta-analysis. Characteristics of the included trials are shown in Table 1.
The 15 available trials accrued 44 to 926 patients between May 1987 and June 2006. Eleven trials used platinum-based chemoradiotherapy, either as a single agent (eight trials) or in combination regimens (three trials). Three trials used nonplatinum regimens comprising either FU, mitomycin, or a combination of the two. One three-arm trial randomly assigned patients to receive chemoradiotherapy either with cisplatin or FU.26 Each of the trials aimed to prescribe external-beam radiation at a dose to the tumor of between 40 and 61.2 Gy, and all except one trial8 (which used primary hysterectomy) also used brachytherapy. The planned total duration of all radiotherapy (external-beam plus brachytherapy) was from 40 to 70 days across all trials. The median follow-up for living patients across all 15 trials was 5.2 years. Data on overall survival, disease-free survival, locoregional disease-free survival, and metastases-free survival were available for all trials, but tumor response was only available for two trials, preventing an analysis of this outcome.
Patient characteristics for the 15 trials are listed in Appendix Table A1 (online only). Data on age were provided for all trials, data on histology and stage were provided for 14 trials, data on performance status were provided for 12 trials, and data on grade were available for nine trials. Data on pelvic node involvement and iliac node involvement were available for six trials, with para-aortic node involvement available for nine trials. On the basis of the available data, women were mostly between 35 and 64 years of age, with good performance status. They had tumors that were largely of squamous cell histology (89%), stage IIb (36%), or stage III (36%), and moderately differentiated (35%). However, as there was generally no central pathology review, the precise definition of tumor grade may vary from trial to trial. Three trials excluded women with involved para-aortic nodes6,26 (Pras, unpublished data), and para-aortic nodal status was either uninvolved (48%) or unknown (51%) for the vast majority of the women from the remaining trials.
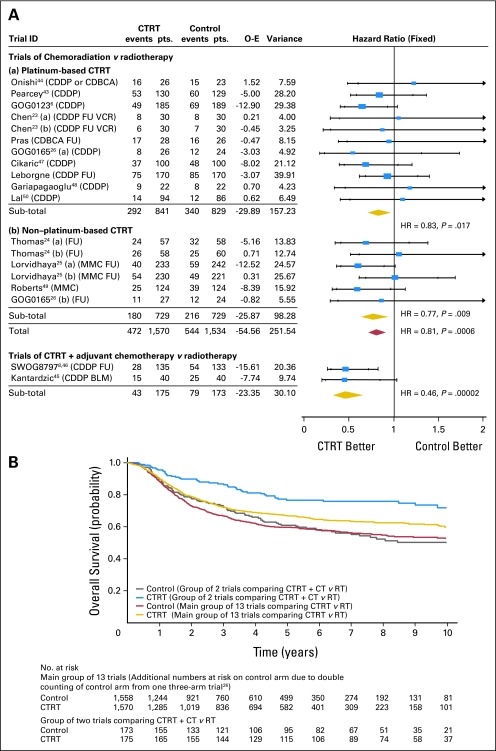
Overall survival data were supplied for 15 trials including 3,452 women, and 1,138 deaths have been recorded. Figure 1A shows the results for these trials, grouped according to whether chemoradiotherapy only was used or whether additional chemotherapy after chemoradiotherapy was administered. Although there was no evidence of statistical heterogeneity within each trial group (chemoradiotherapy only, P = .646, I2 = 0.00; chemoradiotherapy plus adjuvant chemotherapy, P = .945, I2 = 0.00), there was a large and significant difference between groups in the benefit of chemoradiotherapy (interaction P = .004). The HR for the two trials in which chemoradiotherapy plus adjuvant chemotherapy was administered is 0.46 (95% CI, 0.32 to 0.66; P = .00002), representing a 54% reduction in the risk of death and translating into an absolute benefit of 19% at 5 years (from 60% to 79%). However, the most reliable and unconfounded estimate of the effect of chemoradiotherapy alone is obtained from the 13 trials whose design did not include the use of additional chemotherapy. The HR of 0.81 (95% CI, 0.71 to 0.91) represents a highly significant (P = .0006), 19% relative reduction in the risk of death with chemoradiotherapy compared with radiotherapy and translates to an absolute survival benefit of 6% at 5 years (from 60% to 66%). The survival curves for these 13 trials and for the two trials in which adjuvant chemotherapy was used follow a similar pattern, although separation of the curves is greater with adjuvant chemotherapy, albeit that follow-up for one of the trials45 in this group of trials is somewhat less mature (median follow-up 2.35 years) than that for the main group of 13 trials (overall median follow-up, 4.77 years; Fig 1B). The results are similar when the random effects model22 was applied.
Fig 1.
(A) Hazard ratio (HR) plot for survival. Each trial is represented by a square, the center of which gives the hazard ratio for that trial. Size of square is proportional to the information in that trial. Ends of horizontal bars denote 99% CI and inner bars mark 95% CI. Trials are ordered chronologically by date of start of trials (oldest first). The shaded diamonds give the overall hazard ratio for the combined results of all trials; the center denotes the hazard ratio, and the extremities, the 95% CI. Trials of chemoradiation versus radiotherapy: HR = 0.81 (95% CI, 0.71 to 0.91), P = .0006; heterogeneity χ2 = 12.43, P = .646; I2 = 0.00. Trials of chemoradiotherapy + adjuvant chemotherapy versus radiotherapy: HR = 0.46 (95% CI, 0.32 to 0.66), P = .00002; heterogeneity χ2 = 0.00, P = .945; I2 = 0.00. Interaction test: χ2 = 8.39, df = 1, P = .004. (B) Kaplan-Meier curves for survival. GOG, Gynecologic Oncology Group; SWOG, Southwest Oncology Group; FU, fluorouracil; MMC, mitomycin; CDDP, cisplatin; CDBCA, carboplatin; VCR, vincristine; BLM, bleomycin; CTRT, chemoradiotherapy; O-E, observed minus expected events.
Subsequent prespecified analyses by trial group were therefore restricted to the 13 trials that had an unconfounded comparison of chemoradiotherapy versus radiation. We found no evidence of a difference in the size of the effect of chemoradiotherapy when trials were grouped according to the type of chemotherapy they had used (platinum-based or non–platinum-based), the planned radiotherapy dose, or the total planned duration of radiotherapy (Table 2). Similarly, for the eight trials that used cisplatin-based chemoradiotherapy, we found no evidence that the effect of chemoradiotherapy differed according to the cycle length or the dose-intensity of cisplatin used (Table 2). However, the power of these analyses, particularly those involving just the cisplatin-based chemoradiotherapy trials, is limited.
Table 2.
Results of Trial Group Analyses for Survival
| Variable | Main Analysis (13 trials)
|
||
|---|---|---|---|
| HR | 95% CI | Interaction P | |
| Planned chemotherapy type | |||
| Platinum based | 0.84 | 0.72 to 0.98 | |
| Nonplatinum based | 0.76 | 0.62 to 0.94 | .48 |
| Planned radiotherapy dose | |||
| ≥ 45 Gy + BRT | 0.78 | 0.68 to 0.89 | |
| < 45 Gy + BRT | 0.93 | 0.70 to 1.24 | .26 |
| Planned radiotherapy duration, weeks | |||
| ≤ 8 | 0.83 | 0.72 to 0.96 | |
| > 8 | 0.73 | 0.57 to 0.93 | .35 |
| Planned chemotherapy cycle length, weeks* | |||
| ≤ 1 | 0.74 | 0.60 to 0.92 | |
| > 1 | 0.95 | 0.72 to 1.25 | .16 |
| Planned cisplatin dose-intensity, mg/m2/wk* | |||
| ≤ 25 | 0.93 | 0.70 to 1.24 | |
| > 25 | 0.76 | 0.62 to 0.96 | .25 |
| Cisplatin regimen* | |||
| Single agent | 0.76 | 0.62 to 0.93 | |
| Combination | 0.93 | 0.70 to 1.24 | .25 |
| Chemotherapy regimen | |||
| Single agent | 0.75 | 0.63 to 0.88 | |
| Combination | 0.86 | 0.71 to 1.04 | .29 |
NOTE. Two trials in which additional adjuvant chemotherapy was administered on the treatment arm are excluded.
Results are based only on trials in which cisplatin-based chemoradiation was administered.
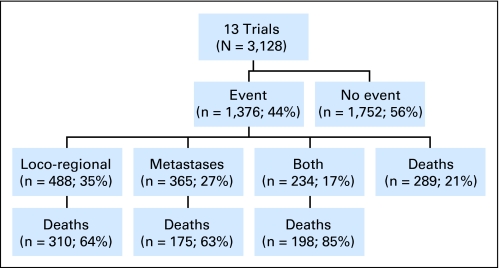
Data on overall disease-free survival, locoregional disease-free survival, and metastases-free survival were available from all of the 13 trials in the unconfounded comparison of chemoradiotherapy versus radiation. For disease-free survival, there were 1,376 events in total, of which 1,087 were recurrences or metastases and 289 were deaths (Appendix Fig A1, online only). The HR of 0.78 (95% CI, 0.70 to 0.87; P = .000005) translates to an absolute disease-free survival benefit of 8% at 5 years (from 50% to 58%). There were similar and significant absolute benefits of chemoradiotherapy on 5-year locoregional disease-free survival (9%; P = .000003), time to locoregional recurrence/progression (6%; P = .00009; Table 3), and metastases-free survival (7%; P = .0004). However, there was a smaller and less convincing improvement in time to metastases at 5 years (4%; P = .037; Table 3). Insufficient data were available to assess the impact of chemoradiotherapy on response.
Table 3.
Results of All Outcomes for the Main Analyses
| Survival Measure | Main Analysis (13 trials)
|
|||
|---|---|---|---|---|
| HR | 95% CI | P | Absolute 5-Year Survival Benefit (%) | |
| Overall disease-free survival | 0.78 | 0.70 to 0.87 | .000005 | 8 |
| Locoregional disease-free survival | 0.76 | 0.68 to 0.86 | .000003 | 9 |
| Metastases-free survival | 0.81 | 0.72 to 0.91 | .0004 | 7 |
| Locoregional disease-free interval | 0.74 | 0.64 to 0.86 | .00009 | 6 |
| Metastases-free interval | 0.83 | 0.71 to 0.99 | .037 | 4 |
NOTE. Two trials in which additional adjuvant chemotherapy was administered on the treatment arm are excluded.
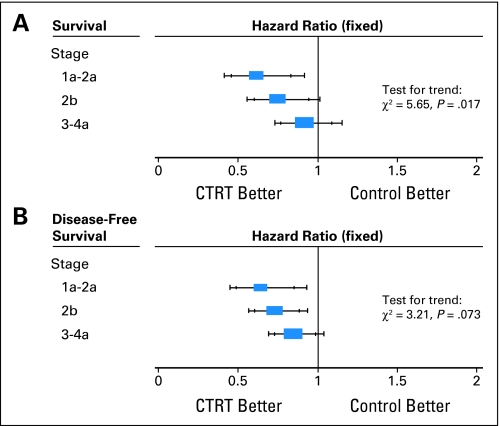
Patient subgroup analyses were similarly restricted to the 13 trials in the unconfounded group that were able to supply data. A planned analysis based on iliac node involvement was not completed because there were insufficient data (Appendix Table A1). Also, as most patients for whom data was supplied had good performance status and either unknown or negative para-aortic nodal status, there was little to gain from analyses of these subgroups. We found no evidence to suggest that the effect of chemoradiotherapy differed in groups of women defined by age, histology, tumor grade, or pelvic lymph node involvement, although the analyses by grade and pelvic node involvement were limited to eight and five trials, respectively (Appendix Table A2, online only). There was a suggestion of trend in the relative effect of chemoradiotherapy by tumor stage (P = .017), with the benefit of chemoradiotherapy decreasing with increasing stage. The HRs obtained for each stage translate to 5-year survival benefits of 10% for women with stages Ib to IIa cervical cancer, 7% for women with stage IIb cervical cancer, and 3% for women with stage III to IVa cancer. This trend, however, was not supported in the analysis of disease-free survival (test for trend, P = .073; Fig 2).
Fig 2.
(A) Survival and (B) disease-free survival by tumor stage (main group of 13 trials only). CTRT, chemoradiotherapy.
For trials for which IPD were not available, it was only possible to estimate HRs for survival31,32 for three35,36,41 of the 10 trials, two of which contributed to the main group of 13 trials and one trial to the group of trials that used additional chemotherapy after chemoradiotherapy. However, incorporating them into the meta-analysis did not materially change the results for either group (data not shown).
Sensitivity Analyses
Three trials were included in the sensitivity analysis of trials of different designs.9,10,17 Data were supplied for 1,366 women (100%), including 84 women who had been excluded from the investigator's original analyses. The three trials recruited 388 to 575 women between August 1986 and October 1997. All used platinum-based chemoradiotherapy; however, in one trial, extended-field radiotherapy was administered in the control arm,17 and in two trials, additional hydroxyurea was administered in the control arm.9,10 The trials planned to give 40.8 to 61.2 Gy of external-beam radiation plus brachytherapy. The planned total duration for all radiotherapy (external-beam and brachytherapy) was fewer than 56 days17 and fewer than 70 days.9,10 The median follow-up for living patients across these trials was 8.4 years. Characteristics of these trials are shown in Table 1. Patient characteristics in the three trials were broadly similar to those in the main analyses; however, women with para-aortic nodal involvement were actively excluded from each trial.
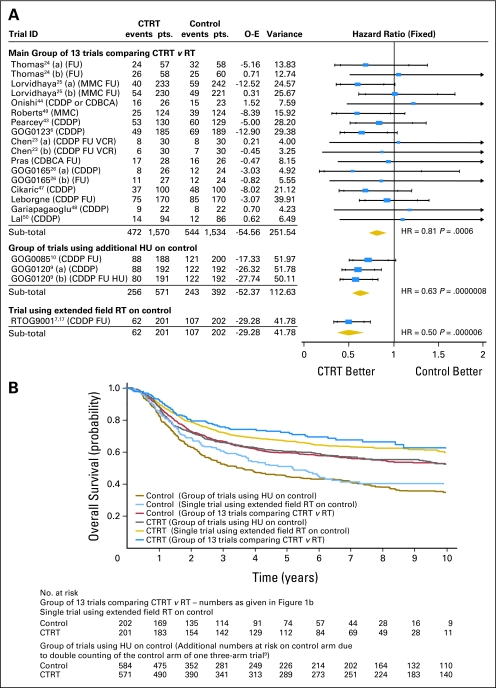
Inclusion of the three trials alongside the 13 trials of the main analysis substantially increased heterogeneity (P = .12; I2 = 28.28). Moreover, Figure 3A illustrates that the treatment effect observed in trials using hydroxyurea on control (HR, 0.63; 95% CI, 0.52 to 0.76; P = .0000008) differed from that in the main analysis (test for interaction, P = .029), with an absolute survival benefit of 15% (from 45% to 60%) at 5 years. The effect of the trial using extended-field radiotherapy on the control arm (HR, 0.50; 95% CI, 0.37 to 0.67; P = .000006) also differed from that in the main analysis (test for interaction, P = .004), with an absolute survival benefit of 21% (from 50% to 71%) at 5 years. Although these benefits seem greater, the control group survival for both groups is lower than that for the main group of 13 trials (Fig 3B). Because these trials differ from the trials in the main analysis in terms of both trial design and the size of the treatment effect, the best estimate of the effect of chemoradiotherapy over radiotherapy remains that from the unconfounded analysis of 6% at 5 years.
Fig 3.
(A) Hazard ratio (HR) plot for survival (sensitivity analysis). Main group of trials: HR = 0.81 (95% CI, 0.71 to 0.91), P = .0006; heterogeneity χ2 = 12.43, I2 = 0.00. Trials using HU on control arms: HR = 0.63 (95% CI, 0.54 to 0.74), P = .00000002; heterogeneity χ2 = 1.39, I2 = 0.00. Trials using additional radiotherapy (RT) on control: HR = 0.50 (95% CI, 0.37 to 0.67), P = .000006. (B) Kaplan-Meier curves for survival (sensitivity analysis). GOG, Gynecologic Oncology Group; RTOG, Radiation Therapy Oncology Group; FU, fluorouracil; MMC, mitomycin; CDDP, cisplatin; CDBCA, carboplatin; VCR, vincristine; BLM, bleomycin; HU, hydroxyurea; CTRT, chemoradiotherapy; O-E, observed minus expected events.
Analyses of Toxicity
Data on overall acute hematologic toxicity and GI toxicity were supplied for 16 trials. Data were available on WBC and genitourinary toxicity for 14 trials, hemoglobin toxicity for 13 trials, platelet toxicity for 12 trials, and skin toxicity for 10 trials.
Serious hematologic toxicity increased by approximately two- to 10-fold in individual trials. However, for the group of trials that used hydroxyurea on the control arm, a high level of serious hematologic toxicity was evident on both arms but slightly greater on the control arm (odds ratio = 0.74; 95% CI, 0.53 to 1.03; P = .075). A similar pattern of results was observed for WBC toxicity, as most of the 517 events (92%) recorded for overall hematologic toxicity were WBC toxicities. There was a significant increase in serious GI toxicity for the groups of trials using platinum-based chemoradiotherapy (P = .000002), chemoradiotherapy plus additional chemotherapy (P = .001), and additional radiotherapy on the control arm (P = .000002). This increase was not observed for the group of trials using non–platinum-based chemoradiotherapy (P = .465), where the event rate was low (approximately 2%) on both arms, or for the trials in which hydroxyurea was administered on the control arm (P = .591), where the event rate was high (approximately 10%) on both arms. For acute hemoglobin toxicity, acute platelet toxicity, genitourinary toxicity, and skin toxicity, few serious events were recorded, making formal analyses inappropriate.
Data on late toxicity were not recorded for the majority of trials in the meta-analysis. Data on late rectal toxicity were available for seven trials, late bladder toxicity for five trials, and late intestinal and late vaginal toxicity for only four trials. Furthermore, within these trials there were substantial missing data. Therefore, there were insufficient data available to assess whether serious late toxicity is affected by the type of treatment. The available data suggest that only a small number of women across all trials (1% to 3%) experienced serious late toxicities, including nine deaths, but these data may not represent the true levels of late toxicity across all trials.
DISCUSSION
Our findings are based on the results of 18 trials from 11 countries worldwide, including the five studies that formed the basis of the 1999 NCI alert, and include 4,818 women. On the basis of the 15 trials in the main analysis, there was clear evidence that adding chemotherapy to radiotherapy improves both overall and disease-free survival. For the group of trials in which chemoradiotherapy alone was used, there was a 6% absolute survival benefit and an 8% disease-free survival benefit at 5 years, with no evidence of heterogeneity. These analyses endorse the recommendations made in the NCI alert, but with far greater reliability and precision regarding the gains of chemoradiotherapy.
The benefit of chemoradiotherapy on survival and disease-free survival was supported by similar benefits on the other outcomes analyzed, although the evidence for time to metastases was less compelling. Chemoradiotherapy is thought to exert its major beneficial effects by improving local disease control. However, the benefit of chemoradiotherapy on metastases suggested previously15 and confirmed in this meta-analysis may indicate that it also has a modest systemic effect.
Larger benefits were seen for the trials in which additional chemotherapy was administered after chemoradiotherapy, with an absolute improvement of 19% at 5 years. However, this result is based on two relatively small trials of differing design and with less mature follow-up and is therefore not conclusive. Inclusion of published summary data from one unavailable trial36 does not materially alter the estimate of effect for this group. Furthermore, we cannot be certain that the larger benefit is not due to factors other than the additional chemotherapy administered after chemoradiotherapy. Nevertheless, the results are promising and may warrant a direct comparison with chemoradiotherapy alone.
Inclusion of trials that used additional treatments on the control arm in previous meta-analyses led to difficulties in interpretation13 and significant statistical heterogeneity.14 Analyzing these trials separately facilitates interpretation and minimizes heterogeneity. There were larger absolute survival benefits for the group of trials in which hydroxyurea was administered on the control arm and for the single trial in which extended-field radiotherapy was administered on the control arm. However, these trials all excluded women with surgically identified positive para-aortic nodes, compared with only three of 13 trials (529 patients) in the main analysis, thus including women who may have been more likely to benefit from chemoradiotherapy. Furthermore, this highly selected group of women is unlikely to be representative of the general population of women with cervical cancer. Patient selection may also explain why the benefits observed in this meta-analysis are smaller than had been previously reported.13-15 These benefits are, however, likely to be generalizable to more women with cervical cancer.
Importantly, this meta-analysis shows that the benefit associated with chemoradiotherapy may not depend on the use of platinum. Previous recommendations have been limited to platinum-based chemoradiotherapy,51 but this meta-analysis shows a significant benefit associated with nonplatinum regimens. However, as our results are not based on a direct comparison, we cannot be clear about the relative merits of platinum versus nonplatinum. The only randomized trial that has directly compared platinum (cisplatin) and non–platinum-based FU chemoradiotherapy closed early, because interim analyses suggested that FU-based chemoradiotherapy was unlikely to improve progression-free survival compared with cisplatin, even if full accrual had been completed. Furthermore, because it closed early, it was underpowered to detect a difference between the two chemoradiotherapy regimens.26 For women who are unable to tolerate cisplatin or when more easily tolerated chemotherapy is required, non–platinum-based chemoradiotherapy offers an additional option.
Other planned analyses by trial characteristics were hampered because most trials gave radiotherapy over 8 weeks or less in addition to weekly, high dose-intensity cisplatin-based chemotherapy, and so should be interpreted cautiously. Nevertheless, we found no evidence to suggest that the effect of chemoradiotherapy differs by any of the trial characteristics investigated. Currently, therefore, there is insufficient evidence to suggest that any one treatment type, dose, or schedule is better than any other.
The effect of chemoradiotherapy seems consistent across patient subgroups, defined by age, histology, grade, or pelvic node involvement. There was, however, the suggestion of a decreasing relative effect of chemoradiotherapy on survival with increasing tumor stage, with estimated absolute survival benefits of 10% (stage Ia to IIa), 7% (stage IIb), and 3% (stage III to IVa) at 5 years. Even if this trend occurred by chance, applying the overall HR (0.81) to each of the stage subgroups gives an improvement in 5-year survival for all stages, thus confirming that chemoradiotherapy benefits women with all stages of cervical cancer, although the size of the benefit may vary.
Although chemoradiotherapy increases some serious acute toxicity, particularly hematologic and GI toxicities, few of the trials in this meta-analysis measured late toxicity, and only one of the 28 trials eligible for inclusion in this meta-analysis reported quality-of-life outcomes.34 This highlights the need for prospective evaluations of treatment tolerability and quality of life in future trials that investigate the use of new or targeted therapies.
Although this meta-analysis provides the most comprehensive and up-to-date summary of the effects of chemoradiotherapy and is based on a large number of women from the large majority of the international trials, IPD from 10 trials were unavailable and might impact on these results. Nine of these trials, including 891 randomly assigned patients, would contribute to the main analysis. Although HR estimates based on the publications of three unavailable trials suggest that their inclusion would not change the results, and all of the unavailable data would only contribute 20% more data to the main analysis, it is possible that inclusion of IPD from these trials could modify our estimate of effect to some degree. Since the final analyses were completed, we have become aware of one completed trial that compared weekly cisplatin-based chemoradiotherapy with radiotherapy alone in 160 patients52 and one large ongoing trial of chemoradiotherapy versus radiotherapy (NC00193791), both from India. Once completed, we will seek inclusion of these trials in an updated analysis.
This meta-analysis provides an unconfounded estimate of the effect of chemoradiotherapy compared with radiotherapy. Adding chemotherapy to radiotherapy offers a modest, but significant, additional benefit on all outcomes and for all stages of disease. There is also the potential to use both platinum and nonplatinum regimens and to investigate whether additional chemotherapy offers additional benefits. With wider implementation of national screening and vaccination programs, it is likely that the incidence of cervical cancer will continue to decrease. However, financial and organizational difficulties, particularly in the developing world, mean that in countries unable to implement such programs, substantial numbers of women will continue to be affected by cervical cancer. Even access to radiotherapy continues to be a barrier to effective treatment in large parts of the world. Nevertheless, effective and affordable treatments, such as those used in this meta-analysis, provide a standard against which promising new drug regimens or novel treatment approaches should be compared.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Claire Vale, Jayne F. Tierney, Lesley A Stewart
Administrative support: Claire Vale
Provision of study materials or patients: David S. Alberts, Hongwei Chen, Slobodan Cikaric, Patricia J. Eifel, Melahat Garipagaoglu, Henry Keys, Nermina Kantardzic, Punita Lal, Rachelle Lanciano, Felix Leborgne, Vicharn Lorvidhaya, Hiroshi Onishi, Robert G. Pearcey, Elizabeth Pras, Kenneth Roberts, Peter G. Rose, Gillian Thomas, Charles W. Whitney
Collection and assembly of data: Claire Vale
Data analysis and interpretation: Claire Vale, Jayne F. Tierney, Lesley A. Stewart, Mark Brady, Ketayun Dinshaw, Anders Jakobsen, Mahesh K.B. Parmar, Gillian Thomas, Ted Trimble, David S. Alberts, Hongwei Chen, Slobodan Cikaric, Patricia J. Eifel, Melahat Garipagaoglu, Henry Keys, Nermina Kantardzic, Punita Lal, Rachelle Lanciano, Felix Leborgne, Vicharn Lorvidhaya, Hiroshi Onishi, Robert G. Pearcey, Elizabeth Pras, Kenneth Roberts, Peter G. Rose, Charles W. Whitney
Manuscript writing: Claire Vale, Jayne F. Tierney, Lesley A. Stewart, Mark Brady, Ketayun Dinshaw, Anders Jakobsen, Mahesh K.B. Parmar, Gillian Thomas, Ted Trimble
Final approval of manuscript: Claire Vale, Jayne F. Tierney, Lesley A. Stewart, Mark Brady, Ketayun Dinshaw, Anders Jakobsen, Mahesh K.B. Parmar, Gillian Thomas, Ted Trimble, David S. Alberts, Hongwei Chen, Slobodan Cikaric, Patricia J. Eifel, Melahat Garipagaoglu, Henry Keys, Nermina Kantardzic, Punita Lal, Rachelle Lanciano, Felix Leborgne, Vicharn Lorvidhaya, Hiroshi Onishi, Robert G. Pearcey, Elizabeth Pras, Kenneth Roberts, Peter G. Rose, Charles W. Whitney
Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration
Project management group.
Claire Vale, Jayne F. Tierney (MRC Clinical Trials Unit, United Kingdom), and Lesley A. Stewart (Centre for Research and Dissemination, United Kingdom).
International advisory group.
Mark Brady (Gynecologic Oncology Group Statistical & Data Center, United States), Ketayun Dinshaw (Tata Memorial Hospital, India), Anders Jakobsen (Vejle Hospital, Denmark), Mahesh K.B. Parmar (MRC Clinical Trials Unit, United Kingdom), Gillian Thomas (Toronto Sunnybrook Cancer Center, Canada), and Ted Trimble (National Cancer Institute-Cancer Therapy Evaluation Program, United States).
Collaborators (institutes) supplying individual patient data.
David S. Alberts (Arizona Cancer Center, Tucson, AZ), Hongwei Chen (First Teaching Hospital, China), Slobodan Cikaric (Institute for Oncology and Radiology of Serbia), Patricia J. Eifel (The University of Texas M. D. Anderson Cancer Center, Houston, TX), Melahat Garipagaoglu (Acýbadem Oncology and Neurological Science Hospital, Turkey), Henry Keys (Albany Medical College, Albany, NY); Nermina Kantardzic (Institut za onkologiju, Bosnia), Punita Lal (Sanjay Gandhi Postgraduate Institute of Medical Sciences, India), Rachelle Lanciano (Delaware County Memorial Hospital, Drexel Hill, PA), Felix Leborgne (Instituto de Radiología y Centro de Lucha Contra el Cáncer, Uruguay), Vicharn Lorvidhaya (Chiang Mai University, Thailand), Hiroshi Onishi (University of Yamanashi, Japan), Robert G. Pearcey (Cross Cancer Institute and University of Alberta, Alberta, Edmonton, Canada), Elizabeth Pras (University Medical Center Groningen and University of Groningen, Netherlands), Kenneth Roberts (Yale University School of Medicine, New Haven, CT), Peter G. Rose (Cleveland Clinic, Cleveland, OH), Gillian Thomas (Toronto Sunnybrook Cancer Center, Canada), and Charles W. Whitney (Christiana Care Health System, Wilmington, DE).
Acknowledgments
We thank all the women who took part in these trials and contributed to this research. The meta-analysis would not have been possible without the collaborating institutions that kindly supplied their trial data and those responsible for maintaining, updating, and preparing this data. We also thank the Patient Research Partners (Alison Nightingale, Andrea Whelan, Nicolette Spera, and Sue Davidson) for their comments and contributions throughout the project.
Appendix
Fig A1.
Event flow diagram (main analysis group of 13 trials only).
Table A1.
Patient Characteristics for 15 Trials in the Main Analyses
| Subgroup |
Chemoradiation(n = 1,745)
|
Control (n = 1,707)
|
Total (N = 3,452) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years* | |||||
| < 35 | 233 | 13 | 249 | 15 | 482 |
| 35-50 | 873 | 50 | 775 | 45 | 1,648 |
| > 50-65 | 538 | 31 | 555 | 33 | 1,093 |
| > 65 | 98 | 6 | 125 | 7 | 223 |
| Unknown | 3 | < 1 | 3 | < 1 | 6 |
| Median age | 47.00 | 47.00 | 47.00 | ||
| Range | 20-87 | 20-91 | 20-91 | ||
| Histology† | |||||
| Squamous cell | 1,541 | 88 | 1,531 | 89 | 3,072 |
| Adenocarcinoma | 87 | 5 | 90 | 5 | 177 |
| Adenosquamous | 51 | 3 | 32 | 2 | 83 |
| Other | 32 | 2 | 17 | 1 | 49 |
| Unknown | 34 | 2 | 37 | 2 | 71 |
| Stage‡ | |||||
| Ia-IIa | 413 | 24 | 417 | 24 | 830 |
| IIb | 625 | 36 | 601 | 35 | 1,226 |
| III-IVa | 667 | 38 | 658 | 39 | 1,325 |
| Unknown | 40 | 2 | 31 | 2 | 71 |
| Grade§ | |||||
| Well differentiated | 157 | 9 | 155 | 9 | 312 |
| Moderately well differentiated | 600 | 34 | 594 | 35 | 1,194 |
| Poorly/undifferentiated | 223 | 13 | 195 | 11 | 418 |
| Unknown | 765 | 44 | 763 | 45 | 1,528 |
| Performance status‖ | |||||
| 0 | 692 | 40 | 655 | 38 | 1,347 |
| 1 | 498 | 29 | 508 | 30 | 1,006 |
| 2 | 29 | 2 | 23 | 1 | 52 |
| 3-4 | 1 | 0 | 0 | 0 | 1 |
| Unknown | 525 | 30 | 521 | 31 | 1,046 |
| Pelvic node involvement¶ | |||||
| Involved | 191 | 11 | 173 | 10 | 364 |
| Not involved | 535 | 31 | 549 | 32 | 1,084 |
| Unknown | 1,019 | 58 | 985 | 58 | 2,004 |
| Para-aortic node involvement# | |||||
| Involved | 32 | 2 | 21 | 1 | 53 |
| Not involved | 823 | 47 | 801 | 47 | 1,624 |
| Unknown | 890 | 51 | 885 | 52 | 1,775 |
| Iliac node involvement** | |||||
| Involved | 50 | 3 | 55 | 3 | 105 |
| Not involved | 591 | 34 | 591 | 35 | 1,182 |
| Unknown | 1,104 | 63 | 1,061 | 62 | 2,165 |
Unknowns are largely as a result of trials not collecting data items requested. Data supplied for all trials, with very few missing from one trial.47
Data not supplied for one trial (Pras, unpublished data) and very few missing from three trials.41,44,47
Data not supplied for one trial (Pras, unpublished data) and very few missing from four further trials40,41 (Leborgne, unpublished data; Lal, unpublished data).
Data not supplied for six trials40,44,46,48 (Leborgne, unpublished data; Pras, unpublished data) and large proportion of data missing from five further trials41,42,47,49 (Lal, unpublished data).
Data not supplied for three trials41,47 (Leborgne, unpublished data) and with a large proportion of missing data from two further trials49 (Lal, unpublished data).
Data only supplied in full for one trial40 and with some missing data for five further trials41,44,47,49 (Lal, unpublished data).
Table A2.
Results of Patient Subgroups for the Main Analyses
| Patient Subgroup | Main Analysis (13 trials)
|
|
|---|---|---|
| χ2 | P | |
| Age (< 35, 35 to 49, 50 to 64, ≥ 65 years) | 0.61* | .436 |
| Stage (Ia to IIa, 2b, III to IVa) | 5.65* | .017 |
| Histology (squamous, adenosquamous, or adenocarcinoma) | 0.00 | .992 |
| Grade (well/moderately well differentiated, poorly differentiated) | 0.00 | .961 |
| Pelvic node involvement (not involved, involved) | 0.49 | .483 |
NOTE. Two trials in which additional adjuvant chemotherapy was administered on the treatment arm are excluded.
Test for trend (other results are for test for interaction).
published online ahead of print at www.jco.org on November 10, 2008.
Supported by the United Kingdom Medical Research Council and the United Kingdom National Coordinating Centre for Research Capacity Development (C.V.).
Presented in part at the 11th International Gynecological Cancer Society Conference, October 14-18, 2006, Santa Monica, CA (oral presentation); the 15th International Meeting of the European Society of Gynaecological Oncology, October 28-November 1, 2007, Berlin, Germany (poster presentation); and the British Gynecological Cancer Society Annual Conference, November 15-16, 2007, Belfast, United Kingdom (oral presentation).
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al: Global cancer statistics, 2002. CA Cancer J Clin 55:74-108, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society/National Cancer Institute of Canada: Canadian Cancer Statistics 2007, Toronto, Canada, 2007
- 3.Arbyn M, Raifu AO, Autier P, et al: Burden of cervical cancer in Europe: Estimates for 2004. Ann Oncol 18:1708-1715, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Australian Institute of Health and Welfare: Cervical Screening in Australia 2004-2005. Cancer Series No. 38. Cat no. CAN 33. Canberra, Australia, Australian Institute of Health and Welfare, 2007
- 5.Ries LAG, Harkins D, Krapcho M, et al (eds): SEER Cancer Statistics Review, 1975-2003. Bethesda, MD, National Cancer Institute, 2006. (based on November 2005 SEER data submission). http://seer.cancer.gov/csr/1975_2003/
- 6.Keys HM, Bundy BN, Stehman FB, et al: Cisplatin, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154-1161, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Morris M, Eifel PJ, Lu J, et al: Pelvic radiation with concurrent chemotherapy compared with plevic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 340:1137-1143, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Peters WA, Liu PY, Barrett RGW, et al: Cisplatin, 5-Fluorouracil plus radiation therapy are superior to radiation therapy as adjunctive therapy in high risk, early stage carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: Report of a Phase III inter group study. Presented at Soc Gynecol Oncol 30th Annual Meeting, San Fransisco, CA, February 5-9, 1999
- 9.Rose PG, Bundy BN, Watkins EB, et al: Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340:1144-1153, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Whitney CW, Sause W, Bundy BN, et al: Randomised comparison of fluorouracil plus cisplatin versus hydroxyurea in stage IIB/IVA in carcinoma of the cervix. J Clin Oncol 17:1339-1348, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Pearcey R, Miao Q, Kong W, et al: Impact of adoption of chemoradiotherapy on the outcome of cervical cancer in Ontario: Results of a population-based cohort study. J Clin Oncol 25:2383-2388, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Trimble E, Gius D, Harlan LC: Impact of NCI announcement upon use of chemoradiation for women with cervical cancer. J Clin Oncol 25:283s, 2007. (suppl; abstr 5537) [Google Scholar]
- 13.Lukka H, Hirte H, Fyles A, et al: Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer: A meta-analysis. Clin Oncol 14:203-212, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Green JA, Kirwan JM, Tierney JF, et al: Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 358:781-786, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Green J, Kirwan J, Tierney J, et al: Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 3:CD002225.pub2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart LA, Clarke MJ, on behalf of the Cochrane Working Party Group on Meta-analysis using Individual Patient Data: Practical methodology of meta-analyses (overviews) using updated individual patient data. Stat Med 14:2057-2079, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Eifel PJ, Winter K, Morris M, et al: Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of Radiation Oncology Group Trial (RTOG) 90-01. J Clin Oncol 22:872-880, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Symonds RP, Collingwood M, Kirwan J, et al: Concomitant hydroxurea plus radiotherapy versus radiotherapy for carcinoma of the uterine cervix: A systematic review. Cochrane Database Syst Rev 1:CD003918, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symonds RP, Collingwood M, Kirwan J, et al: Concomitant hydroxyurea plus radiotherapy versus radiotherapy for carcinoma of the uterine cervix: A systematic review. Cancer Treat Rev 30:405-414, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Dickersin K, Scherer R, Lefebvre C: Identifying relevant studies for systematic reviews, in Chalmers I, Altman DG (eds): Systematic Reviews. London, United Kingdom, BMJ Publishing Group, 1995, pp 17-36
- 21.Yusuf S, Peto R, Lewis JA, et al: Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis 27:335-371, 1985 [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7:177-188, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Chen H-W, Jei J, Wei L, et al: A randomized trial of hyperthermo-radiochemotherapy for uterine cervix cancer. Chinese J Oncol 24:249-251, 1997 [Google Scholar]
- 24.Thomas G, Dembo A, Ackerman I, et al: A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol 69:137-145, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Lorvidhaya V, Chitapanarux I, Sangruchi S, et al: Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: A randomized trial. Int J Radiat Oncol Biol Phys 55:1226-1232, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lanciano RM, Calkins A, Bundy BN, et al: Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervical cancer: A Gynecologic Oncology Group study. J Clin Oncol 23:8289-8295, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Parmar MKB, Machin D: Survival Analysis: A Practical Approach. Chichester, United Kingdom, John Wiley and Sons Ltd, 1995
- 28.Advanced Bladder Cancer Overview Collaboration: Does neo-adjuvant cisplatin-based chemotherapy improve the survival of patients with locally advanced bladder cancer: A meta-analysis of individual patient data from randomised clinical trials. Br J Urol 75:206-213, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, et al: Measuring inconsistency in meta-analyses. BMJ 327:557-560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P: Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 31.Parmar MKB, Torri V, Stewart L: Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815-2834, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Tierney JF, Stewart LA, Ghersi D, et al: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong LC, Choo YS, Choy D, et al: Long-term follow-up of potentiation of radiotherapy by cis-platinum in advanced cervical cancer. Gynecol Oncol 35:159-163, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Lira Puerto V, De la Huerta R, Cortes H, et al: Cisplatin (CDDP) plus radiotherapy (RT) vs radiotherapy alone in locally advanced cervical cancer. Proc Am Soc Clin Oncol 9:633a, 1990. (abstr) [Google Scholar]
- 35.Tseng C-J, Chang-Ting C, Chyong-Huey L, et al: A randomized trial of concurrent chemoradiotherapy versus radiotherapy in advanced carcinoma of the cervix. Gynecol Oncol 66:52-58, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Wong LC, Ngan ANY, Cheung ANY, et al: Chemoradiation and adjuvant chemotherapy in cervical cancer. J Clin Oncol 17:2055-2060, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Singh P, Dharmalingham SK, Tan MK, et al: Chemotherapy-radiotherapy combination in the treatment of carcinoma of the cervix. Southeast Asian J Trop Med Public Health 16:665-668, 1985 [PubMed] [Google Scholar]
- 38.Fernandez DJ, Vidyasagar MS, Rao KK, et al: Synchronous 5-fluorouracil, mitomycin-c and radiation therapy in the treatment of locally advanced carcinoma od the cervix. Proc 16th Annual Meeting of the Assoc Radiat Oncol of India, Kerala, India, February 2-4, 1995, pp 97-103
- 39.Singh TT, Singh IY, Sharma DT, et al: Role of chemoradiation in advanced cervical cancer. Indian J Cancer 40:101-107, 2003 [PubMed] [Google Scholar]
- 40.Bulnes R, Rivera R: Carcinoma Epidermoide del cervix uterino: Tratamiento con radiotherapia y quimioterapia asociadas. Prensa Med Argent 73:100-103, 1986 [Google Scholar]
- 41.Hernandez JRA, Rafael de la Huerta S, Canfield FM, et al: Cervicouterine cancer: Clinical Stage III. Combination treatment of radiotherapy and chemotherapy [in Spanish]. Ginecol Obstet Mex 59:238-242, 1991 [PubMed] [Google Scholar]
- 42.Dawel M, Brock A, Prager W, et al: Simultaneous chemoradiotherapy with carboplatin in inoperative cervical carcinoma: Results of a study [in German]. Strahlenther Onkol 173:545, 1997 [Google Scholar]
- 43.Pearcey R, Brundage M, Drouin P, et al: Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol 20:966-972, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Onishi H, Yagamushi M, Kuriyama K, et al: Effect of concurrent intra-arterial infusion of platinum drugs for patients with stage III or IV uterine cervical cancer treated with radiotherapy. Cancer J Sci Am 6:40-45, 2000 [PubMed] [Google Scholar]
- 45.Kantardzić N, Beslija S, Begić D: Comparison of parameters of myelotoxicity in patients treated with concomitant chemotherapy and radiotherapy versus radiotherapy alone. Med Arh 58:19-22, 2004 [PubMed] [Google Scholar]
- 46.Peters WA III, Liu PY, Barrett II RJ, et al: Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Cikaric S, Petrovic-Stupar S, Marjanov I, et al: Radiotherapy vs. radiotherapy + chemotherapy of advanced cervical cancer: Regression of tumour, early and late sequalaes, relapses of disease and 3 years survival (the third phase). Eur J Cancer 3:266, 2005. (suppl) [Google Scholar]
- 48.Garipağaoğlu M, Kayikcioglu F, Kose MF, et al: Adding concurrent low dose continuous infusion of cisplatin to radiotherapy in locally advanced cervical carcinoma: A prospective randomized pilot study. Br J Radiol 77:581-587, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Roberts KB, Urdaneta N, Raul V, et al: Interim results of a randomized trial of mitomycin C as an adjunct to radical radiotherapy in the treatment of locally advanced squamous-cell carcinoma of the cervix. Int J Cancer 90:206-223, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Lal P, Saibish Kumar E, Tiwari A, et al: Chemo-irradiation versus radiotherapy alone in locally advanced carcinoma of the uterine cervix: An ongoing phase III trial. Presented at the 23rd Annual Meeting of the European Society for Therapeutic Radiology and Oncology, Amsterdam, the Netherlands, October 24-28, 2004. (abstr 673)
- 51.National Cancer Institute: NCI Issues Clinical Announcement on Cervical Cancer: Chemotherapy plus Radiation Improves Survival, 1999. http://www.nih.gov/news/pr/feb99/nci22.htm
- 52.Mitra D, Ghosh B, Kar A, et al: Role of chemoradiotherapy in advanced carcinoma cervix. J Indian Med Assoc 104:432,434, 2006 [PubMed] [Google Scholar]