Abstract
Purpose
Inhibitors of the mammalian target of rapamycin (mTOR) kinase have shown clinical activity in several lymphoma subtypes. Sirolimus, an mTOR inhibitor, also has activity in the treatment and prophylaxis of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem-cell transplantation (HSCT). We hypothesized that the use of sirolimus for GVHD prophylaxis in patients with lymphoma might lead to improved survival after transplantation through a decreased incidence of disease progression.
Patients and Methods
We retrospectively analyzed 190 patients who underwent transplantation for lymphoma. We compared the outcomes of patients who received sirolimus for GVHD prophylaxis with those of patients who received transplantation with a combination of a calcineurin inhibitor and methotrexate without sirolimus.
Results
Overall survival (OS) after transplantation was significantly superior in the sirolimus group, which was confirmed in multivariable analysis. The benefit was restricted to patients undergoing reduced-intensity conditioning (RIC) HSCT (3-year OS, 66% for sirolimus group v 38% for no-sirolimus group; P = .007; hazard ratio [HR] for mortality in multivariable analysis = 0.5, P = .042). Patients who received sirolimus had a similar incidence of nonrelapse mortality but a decreased incidence of disease progression compared with patients who did not receive sirolimus (3-year cumulative incidence of progression, 42% v 74%, respectively; P < .001; HR for progression in multivariable analysis = 0.4, P = .01). The effect of sirolimus persisted after adjusting for the occurrence of GVHD. No such survival advantage was apparent in a similar comparison of patients who underwent transplantation for diseases other than lymphoma.
Conclusion
This study suggests that sirolimus can independently decrease the risk of lymphoma progression after RIC HSCT, paving the way for prospective clinical trials.
INTRODUCTION
Sirolimus (rapamycin) is a naturally occurring triene macrolide with antiviral, antifungal, antineoplastic, and immunosuppressive properties.1 It binds to FKBP-12 to form an immunosuppressive complex that inhibits the mammalian target of rapamycin (mTOR), resulting in the downregulation of T-cell proliferation and activation.2 On the basis of its immunosuppressive activity, sirolimus has been used in solid organ transplantation to maintain graft tolerance.3 Moreover, sirolimus has shown activity in the treatment of both acute4 and chronic5-7 graft-versus-host disease (GVHD) after hematopoietic stem-cell transplantation (HSCT). Over the last 7 years, we have explored the role of sirolimus for the prophylaxis of GVHD and have performed transplantations in patients on successive clinical trials or treatment plans with a GVHD prophylaxis regimen based on the combination of sirolimus and tacrolimus, with or without low-dose methotrexate.8-10
Independent of the use of sirolimus in transplantation, there has been recent interest in using mTOR inhibitors for the treatment of lymphoma.11 Inhibition of mTOR has shown preclinical activity against chronic lymphocytic leukemia,12 Hodgkin's lymphoma (HL),13 anaplastic large-cell lymphoma,13 diffuse large B-cell lymphoma (DLBCL),14 primary effusion lymphoma,15 post-transplantation lymphoproliferative disease,16 and acute lymphoblastic leukemia,17,18 among others. Furthermore, two mTOR inhibitors, temsirolimus and everolimus, have demonstrated promising clinical activity against relapsed or refractory mantle-cell lymphoma.19,20 Preliminary studies have also suggested that everolimus may be useful in the treatment of DLBCL,21 HL,22 and Waldenström macroglobulinemia.23
On the basis of the accumulating evidence of antilymphoma activity for mTOR inhibitors, we hypothesized that sirolimus might improve HSCT outcomes for patients with lymphoma. To test this hypothesis, we retrospectively studied 190 lymphoma patients who underwent transplantation at our institution and compared the outcomes of patients who received sirolimus as part of their GVHD prophylaxis regimen with the outcomes of patients who did not.
PATIENTS AND METHODS
Patients
We studied 190 consecutive adult patients with lymphoma who underwent allogeneic stem-cell transplantation at the Dana-Farber/Brigham and Women's Hospital transplantation program between October 2000 (the date at which the first lymphoma patient underwent transplantation with sirolimus) and July 2006. We only considered for analysis patients who received one of the following GVHD prophylaxis regimens: a calcineurin inhibitor (cyclosporine or tacrolimus) plus methotrexate; tacrolimus plus sirolimus; or tacrolimus plus sirolimus plus methotrexate. Institutional review board approval was obtained from the Office for the Protection of Research Subjects at Dana-Farber/Harvard Cancer Center in accordance with the principles of the Declaration of Helsinki.
Transplantation
Patients received transplantation under several treatment and investigational protocols over the 6-year period covered by this study. All patients receiving reduced-intensity conditioning (RIC) were evaluated for myeloablative transplantation and considered to have contraindications to that approach. Relative contraindications to myeloablative transplantation included prior myeloablative transplantation, age more than 50 to 55 years, significant organ dysfunction or infection, prior chest radiotherapy, prior myeloablative regimen (including autologous transplantation), and disease status. However, the choice of conditioning regimen intensity always ultimately rested with the treating clinician.
Seventy patients were enrolled onto one of six protocols, whereas 120 patients received transplantation off-protocol. All protocols were designed to examine the role of sirolimus as GVHD prophylaxis in various settings. The three conventional-intensity protocols used cyclophosphamide (3,600 mg/m2) and total-body irradiation (14 Gy in seven fractions). Patients who received transplantation off-protocol with conventional-intensity conditioning received the same regimen, except for six patients who received a combination of busulfan and cyclophosphamide. The two RIC protocols used fludarabine (120 mg/m2) plus intravenous low-dose busulfan (3.2 mg/kg); patients who received transplantation off-protocol with RIC received the same regimen. Finally, one protocol for umbilical cord blood transplantation used antithymocyte globulin, fludarabine, and melphalan for conditioning. There was no other difference in treatment between on-protocol and off-protocol treatments.
For all patients who received sirolimus for prophylaxis, the drug was administered orally at the same dose (12 mg loading dose on day −3, followed by 4 mg daily) to a target trough level of 3 to 12 ng/mL. Tacrolimus was dosed intravenously or orally to achieve a target trough level of 5 to 10 ng/mL. For patients on-protocol, the recommendation was to taper off immunosuppressive medications by 6 months (or 6 to 9 months for cord blood recipients), although the actual taper was left to the discretion of the treating clinician.
Patients received bone marrow, peripheral-blood stem cells, or umbilical cord blood from matched or mismatched, related or unrelated donors. Acute GVHD was graded according to the modified consensus scale.24 Supportive care for all patients consisted of Pneumocystis jiroveci prophylaxis and varicella zoster virus/herpes simplex virus prophylaxis. Viral load monitoring was performed for cytomegalovirus, with pre-emptive treatment in cases of reactivation.
Statistics
Patient baseline characteristics were reported descriptively and compared using Fisher's exact test or the Wilcoxon rank sum test. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. OS was defined as the time from stem-cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the time last seen alive. PFS was defined as the time from stem-cell infusion to lymphoma relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without lymphoma relapse or progression were censored at the time last seen alive and progression free. The log-rank test was used for comparisons of Kaplan-Meier curves. Cumulative incidence curves for nonrelapse death and progression or relapse with or without death were constructed reflecting time to progression and time to nonrelapse death as competing risks. Time to progression and time to nonrelapse death were measured from the date of stem-cell infusion. Competing risks analysis was also used to determine the cumulative incidence of GVHD, considering death without GVHD as a competing risk. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method.25 Potential prognostic factors for OS, PFS, progression, and nonrelapse mortality (NRM) were examined in the proportional hazards model as well as in the competing risks regression model.26 The impact of GVHD on outcome was examined using the proportional hazards model with GVHD as a time-dependent variable. Interaction terms including interaction with time were examined in the proportional hazards regression model. Proportional hazards assumption for each variable of interest was tested. All calculations were performed using SAS 9.1 (SAS Institute, Cary, NC) and R Project (version 2.4.1; http://www.r-project.org/).
RESULTS
Patient Characteristics
The baseline characteristics of the 190 patients included in this study are listed in Table 1. One hundred forty-two patients received sirolimus as part of their GVHD prophylaxis regimen; of those, 102 (72%) received tacrolimus, sirolimus, and methotrexate, whereas 40 (28%) received tacrolimus and sirolimus without methotrexate. Forty-eight patients underwent transplantation using a combination of a calcineurin inhibitor (mostly tacrolimus) and methotrexate without sirolimus. On average, patients in the sirolimus group received transplantation later than patients in the no-sirolimus group (median year of HSCT, 2004 v 2003, respectively; P < .001). The groups were otherwise well matched with respect to baseline characteristics, even when considering conventional-intensity and reduced-intensity transplantations separately (Table 1). Of note, 36% of patients had received a prior autologous stem-cell transplantation. Most patients (88%) received peripheral-blood stem cells, and 40% of patients received their graft from a matched related donor. Sixty-six percent of patients were conditioned with an RIC regimen.
Table 1.
Baseline Characteristics of the Patients
| Variable | Conventional-Intensity Conditioning
|
Reduced-Intensity Conditioning
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Sirolimus (n = 25)
|
Sirolimus (n = 39)
|
P | No Sirolimus (n = 23)
|
Sirolimus (n = 103)
|
P | |||||
| No. | %* | No. | %* | No. | %* | No. | %* | |||
| GVHD prophylaxis | ||||||||||
| Tac/Siro/Mtx | 19 | 49 | 83 | 81 | ||||||
| Tac/Siro | 20 | 51 | 20 | 19 | ||||||
| CnI/Mtx | 25 | 100 | 23 | 100 | ||||||
| Age, years | NS | NS | ||||||||
| Median | 40 | 43 | 47 | 51 | ||||||
| Range | 21-56 | 22-58 | 20-67 | 18-67 | ||||||
| Disease | ||||||||||
| CLL/SLL | 2 | 8 | 8 | 21 | NS | 8 | 35 | 37 | 36 | NS |
| Low-grade B-cell NHL† | 5 | 20 | 9 | 23 | NS | 2 | 9 | 15 | 15 | NS |
| Aggressive B-cell NHL‡ | 13 | 52 | 11 | 28 | NS | 3 | 13 | 13 | 13 | NS |
| Mantle-cell lymphoma | 2 | 8 | 4 | 10 | NS | 1 | 4 | 9 | 9 | NS |
| T-cell lymphoma§ | 2 | 8 | 3 | 8 | NS | 0 | 0 | 3 | 3 | NS |
| Hodgkin's lymphoma | 1 | 4 | 2 | 5 | NS | 9 | 39 | 26 | 25 | NS |
| Disease status at HSCT | ||||||||||
| Sensitive | 22 | 88 | 28 | 72 | NS | 18 | 78 | 78 | 76 | NS |
| CR | 6 | 24 | 4 | 10 | NS | 2 | 9 | 25 | 24 | NS |
| PR | 16 | 64 | 24 | 62 | NS | 16 | 70 | 53 | 51 | NS |
| Refractory | 3 | 12 | 11 | 28 | NS | 4 | 17 | 25 | 24 | NS |
| SD | 1 | 4 | 2 | 5 | NS | 2 | 9 | 9 | 9 | NS |
| PD | 2 | 8 | 9 | 23 | NS | 2 | 9 | 16 | 16 | NS |
| Untreated relapse | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | NS | |
| No. of prior therapies | NS | NS | ||||||||
| Median | 3 | 3 | 4 | 4 | ||||||
| Range | 1-5 | 1-5 | 2-7 | 1-9 | ||||||
| Prior ASCT | 6 | 24 | 0 | 0 | .002 | 10 | 43 | 53 | 51 | NS |
| Months from ASCT to HSCT | NA | NS | ||||||||
| Median | 20 | NA | 21 | 20 | ||||||
| Range | 9-35 | NA | 12-67 | 2-170 | ||||||
| Donor HLA | ||||||||||
| Matched | ||||||||||
| MRD | 15 | 60 | 17 | 44 | NS | 9 | 39 | 35 | 34 | NS |
| MUD | 9 | 36 | 21 | 54 | NS | 14 | 61 | 53 | 51 | NS |
| Mismatched | 1 | 4 | 1 | 3 | NS | 0 | 0 | 15 | 15 | NS |
| MMRD | 1 | 4 | 0 | 0 | NS | 0 | 0 | 1 | 1 | NS |
| MMUD | 0 | 0 | 1 | 3 | NS | 0 | 0 | 14 | 14 | NS |
| Graft source | ||||||||||
| Bone marrow‖ | 6 | 24 | 4 | 10 | NS | 1 | 4 | 4 | 4 | NS |
| Peripheral blood | 19 | 76 | 35 | 90 | NS | 22 | 96 | 92 | 89 | NS |
| Umbilical cord blood | 0 | 0 | 7 | 7 | NS | |||||
| CMV seropositivity | ||||||||||
| Recipient | 4 | 16 | 16 | 41 | NS | 12 | 52 | 41 | 40 | NS |
| Donor¶ | 8 | 31 | 17 | 44 | NS | 21 | 34 | 38 | 37 | NS |
| Sex match: female to male | 5 | 20 | 9 | 23 | NS | 5 | 22 | 31 | 30 | NS |
| Year of HSCT | NS | < .001 | ||||||||
| Median | 2003 | 2003 | 2003 | 2004 | ||||||
| Range | 2000-2005 | 2000-2006 | 2002-2005 | 2002-2006 | ||||||
Abbreviations: GVHD, graft-versus-host disease; Tac, tacrolimus; Siro, sirolimus; Mtx, methotrexate; CnI, calcineurin inhibitor (cyclosporine or tacrolimus); NS, not significant; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; NHL, non-Hodgkin's lymphoma; HSCT, hematopoietic stem-cell transplantation; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; ASCT, autologous stem-cell transplantation; MRD, matched related donor; MUD, matched unrelated donor; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; CMV, cytomegalovirus.
Percentages may not add to 100 because of rounding.
Including follicular lymphoma, marginal zone lymphoma, and lymphoplasmacytic lymphoma.
Including diffuse large B-cell lymphoma, mediastinal large-cell lymphoma, transformed low-grade lymphoma, Burkitt's lymphoma, and lymphoblastic lymphoma.
Excluding cutaneous T-cell lymphoma.
One patient received combined marrow and peripheral blood.
Data were not available for two patients.
OS and PFS
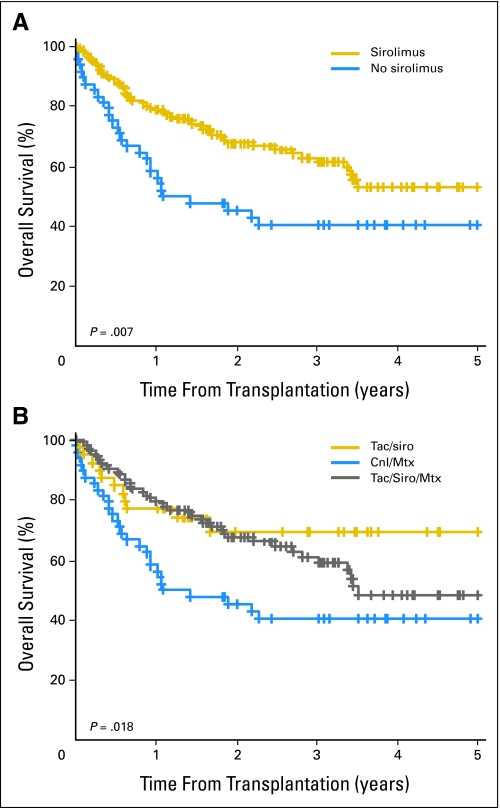
Median follow-up time for survivors was 31 months (range, 12 to 82 months) in the sirolimus group and 42 months (range, 22 to 66 months) in the no-sirolimus group. OS by sirolimus use is shown in Figure 1A. Three-year OS rate was 63% (95% CI, 54% to 72%) in the sirolimus group compared with 41% (95% CI, 26% to 55%) in the no- sirolimus group (P = .007). As shown in Figure 1B, patients receiving tacrolimus and sirolimus alone had a similar survival to patients receiving tacrolimus, sirolimus, and methotrexate, whereas patients receiving a calcineurin inhibitor and methotrexate had a lower survival; this suggests that the benefit of sirolimus is independent of the use of methotrexate.
Fig 1.
Overall survival (A) stratified by sirolimus use and (B) stratified by graft-versus-host disease prophylaxis group. Tac, tacrolimus; Siro, sirolimus; CnI, calcineurin inhibitor; Mtx, methotrexate.
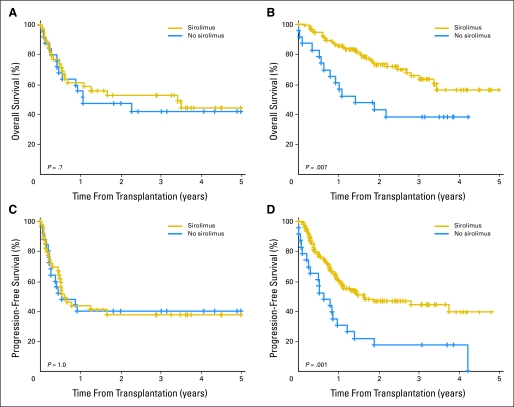
In the multivariable proportional hazards model for OS, including all patients in the cohort, the hazard ratio (HR) for mortality associated with sirolimus was 0.6 (P = .048). However, when patients were stratified by conditioning regimen intensity, the survival benefit of sirolimus was only apparent in patients receiving RIC HSCT (Fig 2). Therefore, we restricted further analyses to the 126 patients who underwent RIC HSCT (Table 1). OS for those patients is shown in Figure 2B, and PFS is shown in Figure 2D. Three-year OS rate was 66% (95% CI, 55% to 76%) in the sirolimus group compared with 38% (95% CI, 18% to 58%) in the no-sirolimus group (P = .007); the corresponding figures for 3-year PFS rate were 44% (95% CI, 34% to 55%) and 17% (95% CI, 2% to 33%), respectively (P = .001). Table 2 lists the results of multivariable OS and PFS analyses for patients who received RIC HSCT. The HR for mortality associated with sirolimus use was 0.5 (P = .042); for progression or death, the HR was 0.5 (P = .01). Of note, even though, on average, patients in the sirolimus group received transplantation later, the date of transplantation was not statistically significant when included in the proportional hazards models.
Fig 2.
Overall and progression-free survival by sirolimus use, stratified by conditioning regimen intensity. (A) Overall survival with conventional conditioning. (B) Overall survival with reduced-intensity conditioning. (C) Progression-free survival with conventional conditioning. (D) Progression-free survival with reduced-intensity conditioning.
Table 2.
Multivariable Analyses for Patients Receiving Reduced-Intensity Conditioning
| Variable | Overall Survival
|
Progression-Free Survival
|
||
|---|---|---|---|---|
| HR | P | HR | P | |
| GVHD prophylaxis | ||||
| CnI/Mtx | 1.0 | Reference | 1.0 | Reference |
| Tac/Siro ± Mtx | 0.5 | .042 | 0.5 | .01 |
| Age > 50 years | 1.5 | .3 | 0.8 | .6 |
| Disease | ||||
| Indolent NHL/CLL | 0.9 | .7 | 0.9 | .8 |
| All others* | 1.0 | Reference | 1.0 | Reference |
| Chemotherapy refractory | 0.8 | .5 | 0.9 | .8 |
| Prior therapy | ||||
| > 2 lines | 1.3 | .6 | 1.3 | .5 |
| Prior ASCT | 1.9 | .16 | 1.6 | .2 |
| Donor HLA match | ||||
| MRD | 1.0 | Reference | 1.0 | Reference |
| Non-MRD | 1.6 | .2 | 0.9 | .6 |
| Graft source† | ||||
| PB/BM | 1.0 | Reference | 1.0 | Reference |
| Umbilical cord blood | 0.9 | .9 | 2.0 | .2 |
| CMV serostatus | ||||
| Donor and recipient negative | 1.0 | Reference | 1.0 | Reference |
| Donor or recipient positive | 1.4 | .3 | 1.0 | .99 |
| Female to male | 1.2 | .5 | 1.0 | .9 |
| Year of HSCT | 0.8 | .2 | 0.9 | .2 |
Abbreviations: HR, hazard ratio; Reference, reference group; GVHD, graft-versus-host disease; CnI, calcineurin inhibitor (cyclosporine or tacrolimus); Mtx, methotrexate; Tac, tacrolimus; Siro, sirolimus; NHL, non-Hodgkin's lymphoma; CLL, chronic lymphocytic leukemia; ASCT, autologous stem-cell transplantation; MRD, matched related donor; PB, peripheral-blood stem cells; BM, bone marrow; CMV, cytomegalovirus.
Including mantle-cell lymphoma.
The PB and BM groups were considered together given the small number of patients receiving marrow after reduced-intensity conditioning.
GVHD
The inclusion of sirolimus in the GVHD prophylaxis regimen was associated with a nonsignificant lower risk of acute GVHD. For patients receiving RIC HSCT, the 100-day cumulative incidence of grade 2 to 4 acute GVHD was 14% in the sirolimus group compared with 22% in the no-sirolimus group (P = .6); the corresponding figures for grade 3 to 4 acute GVHD were 6% and 13%, respectively (P = .4). To ensure that acute GVHD did not act as a confounder, we included grade 2 to 4 acute GVHD as a time-dependent covariate in the proportional hazards model for OS. The HR for mortality associated with grade 2 to 4 acute GVHD was 2.7 (P = .005), but the survival benefit of sirolimus remained unchanged (HR = 0.5, P = .045).
The 2-year cumulative incidence of chronic GVHD was 63% in the sirolimus group compared with 48% in the no-sirolimus group (P = .2). When chronic GVHD was added as a time-dependent covariate in the model (with or without acute GVHD), the HR for mortality associated with sirolimus was similarly unchanged (HR = 0.4, P = .018). Thus, the survival benefit of sirolimus seemed to be independent of the occurrence of acute or chronic GVHD in our cohort.
Progression and NRM
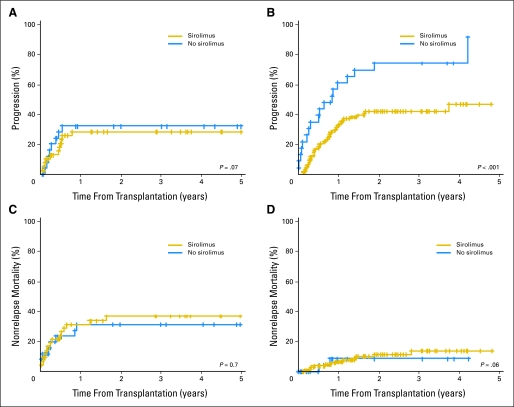
Figure 3 shows the cumulative incidences of disease progression (Figs 3A and 3B) and NRM (Figs 3C and 3D) for patients who received conventional-intensity conditioning (Figs 3A and 3C) and RIC (Figs 3B and 3D). For patients who received RIC HSCT, the 3-year cumulative incidence of disease progression was 42% (95% CI, 32% to 52%) in the sirolimus group compared with 74% (95% CI, 55% to 93%) in the no-sirolimus group (P < .001). The 3-year cumulative incidence of NRM was 14% (95% CI, 6% to 22%) in the sirolimus group compared with 9% (95% CI, 0% to 21%) in the no-sirolimus group (P = .6). We also performed competing risks regression analyses for progression and NRM using the same covariates as mentioned earlier. The results are listed in Table 3. Sirolimus (with or without methotrexate) was associated with a significantly lower risk of relapse compared with a calcineurin inhibitor plus methotrexate (HR = 0.4, P = .01). In contrast, sirolimus did not affect the incidence of NRM (HR = 1.2, P = .9).
Fig 3.
Cumulative incidence of progression and nonrelapse mortality by sirolimus use, stratified by conditioning regimen intensity. (A) Progression with conventional conditioning. (B) Progression with reduced-intensity conditioning. (C) Nonrelapse mortality with conventional conditioning. (D) Nonrelapse mortality with reduced-intensity conditioning.
Table 3.
Multivariable Analyses for Progression and Nonrelapse Mortality for Patients Receiving Reduced-Intensity Conditioning
| Variable | Progression
|
Nonrelapse Mortality
|
||
|---|---|---|---|---|
| HR | P | HR | P | |
| GVHD prophylaxis | ||||
| CnI/Mtx | 1.0 | Reference | 1.0 | Reference |
| Tac/Siro ± Mtx | 0.4 | .01 | 1.2 | .9 |
| Age > 50 years | 0.6 | .2 | 1.5 | .6 |
| Disease | ||||
| Indolent NHL/CLL | 1.2 | .7 | 0.4 | .16 |
| All others* | 1.0 | Reference | 1.0 | Reference |
| Chemorefractory | 0.6 | .13 | 3.2 | .074 |
| Prior therapy | ||||
| > 2 lines | 2.2 | .092 | 0.3 | .054 |
| Prior ASCT | 1.0 | .9 | 4.5 | .16 |
| Donor HLA match | ||||
| MRD | 1.0 | Reference | 1.0 | Reference |
| Non-MRD | 0.7 | .14 | 4.1 | .068 |
| Graft source† | ||||
| PB/BM | 1.0 | Reference | 1.0 | Reference |
| Umbilical cord blood | 1.8 | .2 | 1.4 | .7 |
| CMV serostatus | ||||
| Donor and recipient negative | 1.0 | Reference | 1.0 | Reference |
| Donor or recipient positive | 1.0 | .99 | 1.0 | .99 |
| Female to male | 0.7 | .3 | 2.5 | .14 |
| Year of HSCT | 0.9 | .5 | 0.8 | .2 |
Abbreviations: HR, hazard ratio; Reference, reference group; GVHD, graft-versus-host disease; CnI, calcineurin inhibitor (cyclosporine or tacrolimus); Mtx, methotrexate; Tac, tacrolimus; Siro, sirolimus; NHL, non-Hodgkin's lymphoma; CLL, chronic lymphocytic leukemia; ASCT, autologous stem-cell transplantation; MRD, matched related donor; PB, peripheral-blood stem cells; BM, bone marrow; CMV, cytomegalovirus.
Including mantle-cell lymphoma.
The PB and BM groups were considered together given the small number of patients receiving marrow after reduced-intensity conditioning.
We also built proportional hazards models for progression and NRM using acute grade 2 to 4 and chronic GVHD as time-dependent covariates. Chronic GVHD was associated with a decreased risk of progression (HR = 0.3, P < .0001) and a trend towards decreased NRM (HR = 0.4, P = .14). Grade 2 to 4 acute GVHD did not affect progression (HR = 1.1, P = .9) but was associated with increased NRM (HR = 5.2, P = .021). In those analyses, sirolimus use remained associated with a significantly lower risk of progression (HR = 0.4, P = .002) and had no significant effect on NRM (HR = 0.8, P = .8). Thus, the effect of sirolimus on progression seemed to be independent of the occurrence of acute or chronic GVHD.
We also analyzed progression and NRM in the sirolimus and calcineurin inhibitor/methotrexate groups using acute GVHD as a predictor instead of GVHD prophylaxis regimen. As expected, grade 2 to 4 acute GVHD was associated with significantly higher NRM (HR = 3.9, P = .021) but had no significant effect on disease progression (HR = 0.9, P = .8). This further argues that the benefit of sirolimus is independent of its effect on acute GVHD because reducing the incidence of acute GVHD should lower the risk of NRM but not that of progression.
Impact of Histology
We tested the impact of sirolimus within each lymphoma histology. Again, we only considered patients receiving RIC HSCT because this group seemed to be the one in which sirolimus impacted survival. The small numbers of patients involved in most groups precluded the use of multivariable models and demand caution in interpreting these exploratory results; nonetheless, this analysis suggested that the survival benefit of sirolimus might be most marked for low-grade B-cell non-HL (including chronic lymphocytic leukemia; HR for mortality associated with sirolimus use = 0.4, P = .044) and HL (HR = 0.3, P = .039). The small number of patients receiving RIC HSCT for mantle-cell lymphoma prevented us from reliably assessing the impact of sirolimus in this subgroup.
Comparison With Nonlymphoma Patients
To ascertain whether the effect of sirolimus on HSCT outcome was specific to lymphoma, we performed a similar outcome comparison for 562 patients who received transplantation in the same time frame for diseases other than lymphoma. Among them, 196 patients received sirolimus for GVHD prophylaxis, and 293 did not. There was no significant difference in OS (3-year OS, 43% in the sirolimus group v 41% in the no-sirolimus group; P = .4) or PFS (3-year PFS, 38% in the sirolimus group v 36% in the no-sirolimus group; P = .7). This held true even if we only considered the 154 patients receiving RIC HSCT (3-year OS, 35% for sirolimus v 34% for no sirolimus, P = .06; and 3-year PFS, 26% for sirolimus v 20% for no sirolimus, P = .3).
DISCUSSION
Our analysis demonstrates a robust survival benefit in patients with lymphoma receiving sirolimus as part of their GVHD prophylaxis after RIC HSCT. This benefit was highly significant both statistically and clinically, with an HR for mortality of 0.5 in multivariable analysis. It must be remembered that our study is retrospective and is subject to the cautions and limitations inherent to any such analysis. In particular, we cannot entirely exclude the possibility that the patients receiving sirolimus differed in unmeasured ways from those not receiving it, despite the apparent similarities of their baseline characteristics.
We attempted to dissect the basis for the apparent survival benefit of sirolimus. Although sirolimus led to a (nonsignificant) lower incidence of acute GVHD, this did not account for its effect on survival; the possible anticytomegalovirus effect of sirolimus27 also did not account for the effect on survival because the effect was similar in seronegative patient-recipient pairs and in seropositive pairs (data not shown). Moreover, we found that sirolimus-containing regimens, compared with the traditional regimen of calcineurin inhibitor plus methotrexate, resulted in a significant decrease in the rate of progression or relapse without a significant effect on NRM. This supports the starting hypothesis that sirolimus may confer a benefit based on its antilymphoma activity. Finally, sirolimus did not confer a survival advantage in a comparable cohort of patients who received transplantation for diseases other than lymphoma. This last observation is particularly important because it argues against baseline confounding (eg, by time of transplantation, by selection of patients for clinical trial entry, and so on).
The benefit of sirolimus seemed entirely restricted to patients receiving an RIC regimen. This may be best understood in the context of the comparison of conventional-intensity conditioning versus RIC HSCT for lymphoma. Several retrospective comparisons have suggested that conventional HSCT is associated with a lower relapse rate than RIC HSCT, but that this benefit may be offset by an increased NRM.28,29 This was also the case in our cohort (data not shown). Thus, the cytotoxicity of a conventional regimen may be necessary for the cure of some (although not all) patients, but it exposes all patients to its increased toxicity. The antilymphoma activity of sirolimus may partly substitute for the cytotoxicity of a conventional conditioning regimen in patients receiving RIC HSCT, and because its use does not increase NRM, the net effect is a survival benefit. In contrast, patients receiving a full-intensity regimen may not derive any additional antitumor benefit from sirolimus and thus may not gain from its use.
The clinical role of mTOR inhibitors in lymphoma is still under active investigation. Thus far, the most robust trial data apply to mantle-cell lymphoma.19,20 Although mTOR inhibitors also show promise for DLBCL,21 HL,22 and Waldenström macroglobulinemia,23 those studies are less mature. The small sample size of our study did not allow definitive conclusions regarding which lymphoma histologies may particularly benefit from the use of sirolimus as GVHD prophylaxis. As we learn more about the activity of mTOR inhibitors against lymphoma, we may be better able to rationally explore their use in the transplantation setting.
In conclusion, the use of sirolimus for GVHD prophylaxis after RIC HSCT in patients with lymphoma seems to be associated with a significantly decreased risk of disease progression, as well as with increased PFS and OS. We are planning to confirm this finding in a prospective trial. This could be one of the first examples of the use of dual-activity agents that may impact both GVHD and disease recurrence. Other possible examples of such agents are alemtuzumab and rituximab, which both may have anti-GVHD activity30,31 and could turn out to particularly benefit patients who received transplantation for CD52- and CD20-positive malignancies, respectively.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Philippe Armand, Haesook T. Kim, Robert J. Soiffer, Joseph H. Antin
Provision of study materials or patients: Philippe Armand, Corey S. Cutler, Vincent T. Ho, John Koreth, Edwin P. Alyea, Ann S. LaCasce, Eric D. Jacobsen, David C. Fisher, Jennifer R. Brown, George P. Canellos, Arnold S. Freedman, Robert J. Soiffer, Joseph H. Antin
Collection and assembly of data: Philippe Armand, Supriya Gannamaneni, Haesook T. Kim, Jennifer R. Brown
Data analysis and interpretation: Philippe Armand, Haesook T. Kim, Robert J. Soiffer, Joseph H. Antin
Manuscript writing: Philippe Armand, Haesook T. Kim
Final approval of manuscript: Philippe Armand, Supriya Gannamaneni, Haesook T. Kim, Corey S. Cutler, Vincent T. Ho, John Koreth, Edwin P. Alyea, Ann S. LaCasce, Eric D. Jacobsen, David C. Fisher, Jennifer R. Brown, George P. Canellos, Arnold S. Freedman, Robert J. Soiffer, Joseph H. Antin
published online ahead of print at www.jco.org on November 10, 2008.
Supported in part by Grant No. P01 HL070149 from the National Heart, Lung, and Blood Institute. P.A. is a recipient of a career development award from the Leukemia and Lymphoma Society.
Presented in part at the American Society of Blood and Marrow Transplantation/Center for International Blood and Marrow Transplant Research Tandem Meeting, February 13-17, 2008, San Diego, CA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Sehgal SN: Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant Proc 35:7S–14S, 2003. (suppl) [DOI] [PubMed] [Google Scholar]
- 2.Sehgal SN: Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem 31:335-340, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi A, Fonouni H, Kashfi A, et al: The role and value of sirolimus administration in kidney and liver transplantation. Clin Transplant 20:30-43, 2006. (suppl 17) [DOI] [PubMed] [Google Scholar]
- 4.Benito AI, Furlong T, Martin PJ, et al: Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation 72:1924-1929, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Johnston LJ, Brown J, Shizuru JA, et al: Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 11:47-55, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Couriel DR, Saliba R, Escalon MP, et al: Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol 130:409-417, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Jurado M, Vallejo C, Perez-Simon JA, et al: Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant 13:701-706, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Antin JH, Kim HT, Cutler C, et al: Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 102:1601-1605, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cutler C, Li S, Ho VT, et al: Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 109:3108-3114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler C, Kim HT, Hochberg E, et al: Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 10:328-336, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Costa LJ: Aspects of mTOR biology and the use of mTOR inhibitors in non-Hodgkin's lymphoma. Cancer Treat Rev 33:78-84, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Decker T, Hipp S, Ringshausen I, et al: Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood 101:278-285, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Jundt F, Raetzel N, Muller C, et al: A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood 106:1801-1807, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Wanner K, Hipp S, Oelsner M, et al: Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol 134:475-484, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Sin SH, Roy D, Wang L, et al: Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 109:2165-2173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaysberg M, Balatoni CE, Nepomuceno RR, et al: Rapamycin inhibits proliferation of Epstein-Barr virus-positive B-cell lymphomas through modulation of cell-cycle protein expression. Transplantation 83:1114-1121, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Brown VI, Fang J, Alcorn K, et al: Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A 100:15113-15118, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teachey DT, Obzut DA, Cooperman J, et al: The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood 107:1149-1155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haritunians T, Mori A, O'Kelly J, et al: Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia 21:333-339, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Witzig TE, Geyer SM, Ghobrial I, et al: Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 23:5347-5356, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Reeder CB, Gornet MK, Habermann TM, et al: A phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed aggressive non-Hodgkin lymphoma (NHL). Blood 110:121a, 2007. (abstr) [Google Scholar]
- 22.Johnston PB, Ansell SM, Colgan JP, et al: MTOR inhibition for relapsed or refractory Hodgkin lymphoma: Promising single agent activity with everolimus (RAD001). Blood 110:2555a, 2007. (abstr) [Google Scholar]
- 23.Ghobrial IM, Leduc R, Nelson M, et al: Phase II trial of the oral mTOR inhibitor RAD001 (everolimus) in relapsed and/or refractory Waldenstrom macroglobulinemia: Preliminary results. Blood 110:4496a, 2007. (abstr) [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al: 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825-828, 1995 [PubMed] [Google Scholar]
- 25.Gray R: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1140-1154, 1988 [Google Scholar]
- 26.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 27.Marty FM, Bryar J, Browne SK, et al: Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: A cohort analysis. Blood 110:490-500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sureda A, Robinson S, Canals C, et al: Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 26:455-462, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Sorror ML, Storer BE, Maloney DG, et al: Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood 111:446-452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler C, Miklos D, Kim HT, et al: Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 108:756-762, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, et al: Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 14:10-15, 2008 [DOI] [PubMed] [Google Scholar]