Abstract
Purpose
Phase II trials with biochemotherapy (BCT) have shown encouraging response rates in metastatic melanoma, and meta-analyses and one phase III trial have suggested a survival benefit. In an effort to determine the relative efficacy of BCT compared with chemotherapy alone, a phase III trial was performed within the United States Intergroup.
Patients and Methods
Patients were randomly assigned to receive cisplatin, vinblastine, and dacarbazine (CVD) either alone or concurrent with interleukin-2 and interferon alfa-2b (BCT). Treatment cycles were repeated at 21-day intervals for a maximum of four cycles. Tumor response was assessed after cycles 2 and 4, then every 3 months.
Results
Four hundred fifteen patients were enrolled, and 395 patients (CVD, n = 195; BCT, n = 200) were deemed eligible and assessable. The two study arms were well balanced for stratification factors and other prognostic factors. Response rate was 19.5% for BCT and 13.8% for CVD (P = .140). Median progression-free survival was significantly longer for BCT than for CVD (4.8 v 2.9 months; P = .015), although this did not translate into an advantage in either median overall survival (9.0 v 8.7 months) or the percentage of patients alive at 1 year (41% v 36.9%). More patients experienced grade 3 or worse toxic events with BCT than CVD (95% v 73%; P = .001).
Conclusion
Although BCT produced slightly higher response rates and longer median progression-free survival than CVD alone, this was not associated with either improved overall survival or durable responses. Considering the extra toxicity and complexity, this concurrent BCT regimen cannot be recommended for patients with metastatic melanoma.
INTRODUCTION
Many investigators have studied combinations of interleukin-2 (IL-2)–based immunotherapy and cisplatin- and dacarbazine-based chemotherapy (so-called biochemotherapy [BCT]) in patients with metastatic melanoma.1-7 Composite results from a variety of inpatient regimens show a response rate near 50%, with 10% to 20% complete responses and a median survival of 11 to 12 months.3 Two meta-analyses performed in the mid-1990s suggested that BCT produced a higher response rate than either chemotherapy or IL-2–based immunotherapy alone and a potentially longer median survival.8,9 Furthermore, a single-institution phase III trial comparing cisplatin, vinblastine, and dacarbazine (CVD) chemotherapy with sequentially administered CVD and IL-2 plus interferon alfa-2b (IFN-α) showed that the BCT regimen produced a doubling of the response rate and median time to progression and a 3-month prolongation in median overall survival that was of borderline significance (P = .06).10 Although both a National Cancer Institute (NCI) Surgery Branch phase III trial comparing cisplatin, dacarbazine, and tamoxifen with or without high-dose IL-2 and IFN-α and a European Organization for Research and Treatment of Cancer phase III trial comparing IL-2 and IFN-α with or without cisplatin produced higher response rates for the BCT arms, no overall survival benefit was observed in either study.5,11
In 1995, Legha et al12 reported on a BCT regimen involving administration of CVD chemotherapy concurrent with IL-2 and IFN-α, necessitating only a 5-day inpatient hospitalization for each 3-week cycle. This regimen was shown to be tolerable and to have activity roughly equivalent to the more intensive sequential regimen. This regimen was modified slightly by McDermott et al13 in an effort to further moderate toxicity and enhance convenience. Modifications included reducing the vinblastine dose, the use of antibiotic and granulocyte colony-stimulating factor prophylaxis and more aggressive antiemetics, the prohibition of long-term central venous access, and the restriction to a maximum of four cycles of therapy. A phase II pilot trial of this modified regimen confirmed its activity and enhanced tolerability. Significant toxicities necessitating a dose reduction were limited primarily to nausea and vomiting and myelosuppression. Hypotension and renal insufficiency were uncommon, and significant cardiopulmonary toxicity and treatment-related deaths were not observed. Tumor response was seen in 19 (48%) of 40 patients, including 20% complete responses, with a median response duration of 7 months. These encouraging results prompted the United States Intergroup to conduct a randomized phase III trial comparing this regimen with CVD chemotherapy alone.
PATIENTS AND METHODS
Patient Eligibility Criteria
All patients entered onto this study had histologically confirmed, bidimensionally measurable, and clearly progressive metastatic melanoma; an Eastern Cooperative Oncology Group performance status of 0 or 1; and adequate organ function as defined by WBC count more than 4,000/μL, platelet count more than 100,000/μL, serum bilirubin ≤ 1.5 mg/dL, and serum creatinine less than 1.5 mg/dL or calculated creatinine clearance ≥ 75 mL/min. Patients were required to have a forced expiratory volume in one second of more than 2.0 L or ≥ 75% of predicted value. Patients older than 50 years or with history of cardiac disease were required to have a normal cardiac stress test. Patients with active brain metastases, medical conditions requiring systemic corticosteroids, organ allografts, contraindications to the use of vasopressor agents, active infections, or a history of second malignancy were also excluded. Patients who received prior cytotoxic chemotherapy or IL-2 therapy were also excluded. Prior immunotherapy with agents other than IL-2 in the adjuvant or metastatic setting was permitted. The protocol was approved by the human investigation review boards at the participating institutions, and all patients provided voluntary written informed consent before enrollment.
Study Design
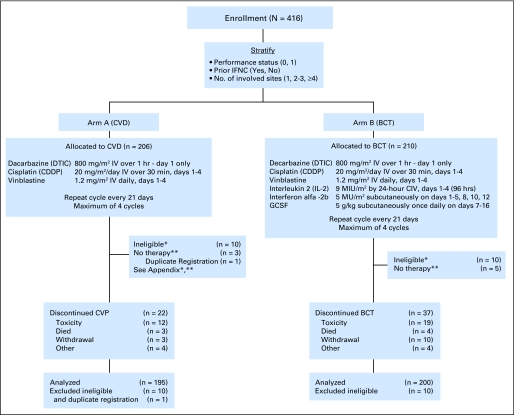
The schema for this study, including stratification factors and treatment doses, is shown in Figure 1. Treatment assignments were obtained from the Central Randomization Desk at the Eastern Cooperative Oncology Group Coordinating Center and were based on permuted blocks within strata, with dynamic balancing within main institutions and their affiliate networks.
Fig 1.
CONSORT flow diagram and study schema displays the study design and treatment regimens as well as the proportion of eligible and assessable patients on each treatment arm. IFNC, interferon alfa chemotherapy; CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy; IV, intravenously; CIV, continuous intravenous infusion; GCSF, granulocyte colony-stimulating factor. (*,**) Refer to Appendix.
Treatments
CVD chemotherapy.
Cisplatin was administered over 30 minutes on days 1 through 4. Vinblastine was delivered as an intravenous push immediately after cisplatin. Dacarbazine was administered intravenously over 1 hour after vinblastine on day 1 only. Treatment was generally administered in the outpatient setting, with serotonin 3 (S-HT3) receptor antagonist antiemetics and dexamethasone as premedication.
Biochemotherapy.
Patients were admitted to the hospital for the first 5 days of each treatment cycle. Therapy was generally administered on a regular oncology ward with specialized patient monitoring. Patients received cisplatin, vinblastine, and dacarbazine at doses and schedules identical to the CVD alone arm. In addition, IL-2 was administered as a continuous intravenous infusion over 24 hours daily on days 1 through 4 (96 hours), and IFN-α was administered subcutaneously days 1 through 5 and on an outpatient basis on days 8, 10, and 12.
Patients discontinued antihypertensive therapy 24 hours before beginning each treatment cycle. A triple-lumen central venous catheter was inserted at the beginning of each treatment cycle and typically was removed at the time of discharge. Patients received cephalexin 250 mg twice daily days 1 to 14 and granulocyte colony-stimulating factor 5 μg/kg/d subcutaneously on days 7 through 16 (or until ANC exceeded 10,000/dL). Aggressive intravenous hydration and antiemetics (ondansetron 32 mg administered intravenously or equivalent) were administered during therapy and continued for several days after discharge in patients with persistent nausea and vomiting. Prophylactic acetaminophen and ranitidine were provided, and antipruritics, antidiarrheal agents, and anxiolytics were administered as needed.
Treatment Duration
Cycles were repeated at 3-week intervals for both treatment arms. Further therapy was withheld until laboratory values and functional status returned to within eligibility criteria. Tumor response was assessed after cycles 2 and 4. Patients with stable or responding disease continued on therapy until disease progression, unacceptable toxicity, or until they received a maximum of four cycles. Patients completing four cycles of therapy without evidence of disease progression underwent computed tomography scans at 3-month intervals and head computed tomography/magnetic resonance imaging at month 6 and then at 6-month intervals until documented disease progression.
Dose Modification Criteria
CVD arm.
Patients experiencing grade 3 toxicity as described in the NCI Common Toxicity Criteria while receiving day 1 through 4 therapy had cisplatin and vinblastine held until toxicity returned to grade 2 or less. Therapy was then restarted at full doses. Specific modifications in subsequent CVD therapy for nausea, vomiting, renal insufficiency, peripheral neuropathy, and hematologic toxicity were as described for the BCT arm (see Appendix, online only).
BCT arm.
Dose modifications for BCT were performed largely according to the criteria developed in the phase II pilot of CVD/IL-2 + IFN-α BCT.13 In general, patients experiencing grade 3 toxicity as described in the NCI Common Toxicity Criteria while receiving inpatient therapy (days 1 through 5) had treatment held until toxicity returned to grade 2 or less. Therapy was then restarted at full doses of chemotherapy and a 50% dose reduction for both IL-2 and IFN-α. If a portion of an IL-2 infusion or a dose of IFN-α was held, it was not readministered. All dose reductions were permanent. If grade 3 or 4 toxicity recurred despite a 50% dose reduction in IL-2 and IFN-α, no further IL-2 or IFN-α was administered in that or subsequent cycles. If a grade 3 toxicity was encountered during week 2 of any cycle, the remaining IFN-α injections were held for the rest of that cycle. Subsequent IFN-α was given at full dose. Exceptions to this approach included the management of hypotension, nephrotoxicity, hematologic toxicity, nausea and vomiting, and neurotoxicity (see Appendix).
Response Criteria
Standard WHO tumor response criteria were used. Specifics are described in the Appendix. Response durations were measured from the date of partial response or complete response and were updated through October 2006.
Statistical Methods
The purpose of this study was to determine whether CVD + IL-2/IFN-α was superior to CVD alone with respect to overall survival, progression-free survival, response rate, and response duration. The study had a group sequential design with one-sided O'Brien-Fleming type boundaries that allowed for early termination of the trial. The original study design was constructed to detect a 50% relative increase in median overall survival from 8 months on the CVD arm to 12 months on BCT with 90% power. This required a sample size of 264 patients based on a one-sided log-rank test using an overall significance level of .05. Interim analyses were conducted at 30% and 51% information, and no boundaries were crossed in either analysis. In March 2001, when results of the University of Texas M.D. Anderson Cancer Center phase III trial became available,10 the statistical design was revised to have 85% power to detect a 33% improvement in median overall survival from 9 months on the CVD arm to 12 months on the BCT. The sample size required to detect this difference using a one-sided significance level of .05 was determined to be 468 patients. Allowing for 3% ineligibility, the total accrual goal was set for 482 patients. Two more interim analyses were conducted at 47% and 63% information based on the revised accrual goal. The lower boundary was crossed at the fourth interim analysis, closing the study before achieving the total accrual goal.
The distributions of overall survival, progression-free survival, and response duration were estimated using the method of Kaplan and Meier. Stratified log-rank tests using the stratification factors indicated previously were conducted to test for differences in treatment effect. Response data and toxicity data were analyzed as binomial proportions and tested using Fisher's exact test. All reported P values are for two-sided tests unless otherwise specified.
RESULTS
Study Population
Between October 1997 and April 2002, 416 patients with metastatic melanoma were entered into this study. Two hundred six patients were randomly assigned to CVD, and 210 patients were randomly assigned to BCT. There was one duplicate registration, and 20 patients were deemed ineligible (see Appendix and Fig 1) resulting in 395 analyzable patients (195 patients on the CVD arm and 200 patients on the BCT arm). Patient characteristics are displayed in Table 1. The treatment arms were well balanced for age, sex, and various disease-related factors, including the stratification factors: performance status, prior IFN therapy, and number of disease sites. Seventy-four percent of patients on each arm were American Joint Committee on Cancer classification M1C, and serum lactate dehydrogenase levels were elevated at baseline in 35% of patients on the CVD arm and 39% on the BCT arm.
Table 1.
Patient Characteristics
| Characteristic | CVD
|
BCT
|
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| No. of patients | 195 | 200 | ||
| Male sex | 147 | 75 | 133 | 67 |
| Age | ||||
| Median | 50.5 | 50.7 | ||
| Range | 19.5-80.4 | 21.0-78.5 | ||
| ECOG performance status | ||||
| 0 | 128 | 66 | 130 | 65 |
| 1 | 67 | 34 | 70 | 35 |
| Prior interferon therapy | ||||
| Yes | 74 | 38 | 81 | 40 |
| No | 121 | 62 | 140 | 60 |
| No. of metastatic sites | ||||
| 1 | 46 | 24 | 47 | 24 |
| 2-3 | 113 | 58 | 111 | 55 |
| ≥ 4 | 36 | 18 | 42 | 21 |
| AJCC stage | ||||
| M1a | 23 | 12 | 15 | 8 |
| M1b | 26 | 14 | 34 | 18 |
| M1c | 141 | 74 | 144 | 74 |
| Serum LDH | ||||
| Normal | 126 | 65 | 122 | 61 |
| Above normal | 69 | 35 | 78 | 39 |
| Prior therapy | ||||
| Radiation | 28 | 15 | 23 | 12 |
| Other immunotherapy | 9 | 5 | 5 | 43 |
Abbreviations: CVD, cisplatin, vinblastine, dacarbazine; BCT, biochemotherapy; ECOG, Eastern Cooperative Oncology; AJCC, American Joint Committee on Cancer; LDH, lactate dehydrogenase.
Treatment Characteristics
Within the 395 analyzable cases, eight patients never started their assigned therapy (Fig 1; Appendix Table A1, online only). A significantly smaller proportion of patients treated with BCT received full doses of therapeutic agents throughout treatment (P values ranging from < .001 to .01) than those receiving CVD (Table 2). In particular, the percentage of patients receiving the full amount of IL-2 or IFN-α per cycle was 87%, 73%, 67%, and 57%, and 60%, 57%, 40%, and 37% for cycles 1 to 4, respectively. In addition, 10 patients (six achieving response) treated with CVD (compared with none treated with BCT) received more than the protocol prescribed four cycles of therapy.
Table 2.
Treatment Characteristics: Percentage of Patients per Cycle Who Received Full Therapy
| Characteristic | % of Patients per Cycle Who Received Full Therapy
|
|
|---|---|---|
| CVD Arm | BCT Arm | |
| Cycle 1 | n = 187 | n = 177 |
| Cisplatin | 99 | 93 |
| Vinblastine | 98 | 95 |
| Dacarbazine | 99 | 98 |
| IL-2 | 87 | |
| IFN | 60 | |
| Cycle 2 | n = 159 | n = 154 |
| Cisplatin | 97 | 84 |
| Vinblastine | 93 | 82 |
| Dacarbazine | 94 | 84 |
| IL-2 | 73 | |
| IFN | 57 | |
| Cycle 3 | n = 93 | n = 96 |
| Cisplatin | 97 | 80 |
| Vinblastine | 92 | 71 |
| Dacarbazine | 91 | 74 |
| IL-2 | 67 | |
| IFN | 40 | |
| Cycle 4 | n = 81 | n = 82 |
| Cisplatin | 94 | 72 |
| Vinblastine | 80 | 59 |
| Dacarbazine | 81 | 60 |
| IL-2 | 57 | |
| IFN | 37 | |
Abbreviations: CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy; IL-2, interleukin-2; IFN, interferon alfa-2b.
Toxicity
Toxicity was assessed on all patients receiving treatment regardless of eligibility. Toxicity data were available for 388 patients (199 patients on the CVD arm and 189 patients on the BCT arm). Grade 3 or worse toxicity was seen in 73% of patients on CVD and 95% of patients on biochemotherapy (P = .001; Table 3). The most common toxicities included leukopenia, granulocytopenia, thrombocytopenia, anemia, infection, nausea, vomiting, hepatic and metabolic abnormalities, hypotension, and fatigue, with all except granulocytopenia and infection being significantly more frequent on the BCT arm. There were five treatment-related toxic deaths: three on CVD therapy (myocardial infarction, hypotension, and infection) and two on BCT (infection and renal failure).
Table 3.
Grade 3, 4, and 5 Toxicity Results
| Toxicity | % of Patients
|
|
|---|---|---|
| CVD Arm (n = 199) | BCT Arm (n = 189) | |
| Leukopenia | 28 | 78 |
| Granulocytopenia | 50 | 46 |
| Thrombocytopenia | 13 | 57 |
| Anemia | 9 | 24 |
| Infection | 5 | 10 |
| Nausea | 11 | 26 |
| Vomiting | 10 | 20 |
| Liver | 4 | 15 |
| Hypotension | 3 | 15 |
| Metabolic | 3 | 22 |
| Fatigue | 4 | 13 |
| Worst degree | ||
| Grade 3 | 34 | 29 |
| Grade 4 | 37 | 64 |
| Grade 5* | 2 | 1 |
| Total | 73 | 95 |
Abbreviations: CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy.
Three toxic deaths occurred on the CVD arm, and two toxic deaths occurred on the BCT arm.
Efficacy
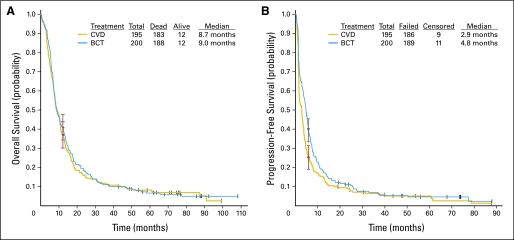
The response rates, response duration, median progression-free survival, and median overall survival by treatment arm are listed in Table 4. Ninety-four percent (371 of 395) of analyzable patients had died by the time of analysis. Median overall survival was 8.7 months for CVD (95% CI, 7.9 to 10.6 months) and 9.0 months for BCT (95% CI, 7.7 to 10.8 months; Fig 2A); hazard ratio = 0.95 (95% CI, 0.78 to 1.17; P = .639). The percentage of patients alive at 1 year was 36.9% (95% CI, 30.1% to 43.7%) for CVD and 41% (95% CI, 34.2% to 47.8%) for BCT. No treatment-related survival benefit was observed for any of the stratified categories (Table 5).
Table 4.
Summary of Major Efficacy End Points
| Treatment Arm | Assessable Patients | Response Rate (%)
|
Response Duration (months)
|
PFS (months)
|
OS (months)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||
| CVD | 195 | 13.8 | 9.3 to 19.5 | 9.4 | 4.2 to 22.6 | 2.9 | 1.8 to 3.4 | 8.7 | 7.9 to 10.6 |
| BCT | 200 | 19.5 | 14.2 to 25.7 | 6.1 | 4.4 to 7.7 | 4.8 | 3.9 to 5.5 | 9.0 | 7.7 to 10.8 |
Abbreviations: PFS, progression-free survival; OS, overall survival; CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy.
Fig 2.
Kaplan-Meier plots for (A) overall survival and (B) progression-free survival by treatment arm. The (A) 1-year overall survival and (B) 6-month progression-free survival points (with 95% CIs) are overlaid for each treatment arm. CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy.
Table 5.
Response and Survival Correlates
| Characteristic | CVD
|
BCT
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. | RR (%) | PFS (months) | OS (months) | No. | RR (%) | PFS (months) | OS (months) | |
| Performance status | ||||||||
| 0 | 131 | 12.2 | 3.0 | 10.5 | 132 | 21.2 | 5.3* | 11.1 |
| 1 | 64 | 17.2 | 2.4 | 5.8 | 68 | 16.2 | 3.6 | 6.9 |
| Sex | ||||||||
| Male | 147 | 15.0 | 3.0 | 9.7 | 133 | 21.8 | 5.0* | 10.2 |
| Female | 48 | 10.4 | 2.0 | 8.4 | 67 | 14.9 | 4.6 | 7.8 |
| No. of sites | ||||||||
| 1 | 44 | 15.9 | 3.0 | 11.5 | 48 | 18.8 | 5.9 | 14.0 |
| 2-3 | 115 | 14.8 | 2.9 | 8.6 | 111 | 18.9 | 4.7 | 8.9 |
| ≥ 4 | 36 | 8.3 | 1.7 | 5.1 | 41 | 22.0 | 3.6 | 6.0 |
| AJCC stage | ||||||||
| M1a | 23 | 26.1 | 3.2 | 13.1 | 15 | 20.0 | 5.5 | 13.0 |
| M1b | 26 | 26.9 | 5.6 | 16.2 | 34 | 23.5 | 5.8 | 13.3 |
| M1c | 141 | 9.9 | 1.7 | 7.9 | 144 | 17.4 | 4.0* | 7.6 |
| Prior IFN | ||||||||
| No | 120 | 14.2 | 3.0 | 8.9 | 120 | 21.7 | 4.7* | 8.0 |
| Yes | 75 | 13.3 | 1.7 | 8.6 | 80 | 16.3 | 4.8 | 10.8 |
Abbreviations: CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy; RR, response rate; PFS, progression-free survival; OS, overall survival; AJCC, American Joint Committee on Cancer; IFN, interferon alfa-2b.
P < .05.
The median progression-free survival was 2.9 months (95% CI, 1.8 to 3.4 months) for CVD and 4.8 months (95% CI, 3.9 to 5.5 months) for BCT (Fig 2B); hazard ratio = 0.77 (95% CI, 0.63 to 0.94; P = .015). The percentage of patients progression free at 6 months was 25.0% (95% CI, 18.9% to 31.1%) for CVD and 38.9% (95% CI, 32.1% to 45.7%) for BCT. Significant progression-free survival benefit was observed for BCT in several stratified categories (Table 5).
Forty-nine patients were unassessable for response, including nine patients (4.6%) on the CVD arm and 40 patients (20%) on the BCT arm. The majority of unassessable patients on the BCT arm (25 of 40 patients) were unassessable because of treatment-limiting toxicity (Appendix Table A2, online only). For the purpose of response assessment, these patients were counted as nonresponders. The response rate on the CVD arm was 13.8% (27 of 195 patients; 95% CI, 9.3% to 19.5%), compared with 19.5% (39 of 200 patients; 95% CI, 14.2% to 25.7%) on BCT (P = .140). The complete response rate was 4.6% for CVD and 2.5% for BCT, with durable complete responses (> 2 years) occurring in six patients on CVD and only two patients on BCT.
Response duration was analyzed for the 66 patients who experienced either complete or partial response. The median response duration for CVD was 9.4 months (95% CI, 4.2 to 22.6 months) and for BCT was 6.1 months (95% CI, 4.4 to 7.7 months); hazard ratio = 1.47 (95% CI, 0.83 to 2.60; P = .181).
DISCUSSION
BCT has been extensively investigated in patients with metastatic melanoma over the past two decades.3 Despite promising antitumor activity reported in initial studies,8,9 BCT regimens have consistently failed to produce statistically significant benefits in overall survival in randomized phase III trials. Of six previously reported phase III trials involving a spectrum of BCT combinations,5,10,11,14-16 only a single-institution trial comparing sequential administration of CVD followed by IL-2 and IFN with CVD reported an increase in overall survival that was even of borderline significance (11.9 v 9.2 months; P = .06).10 Furthermore, two systematic reviews of the literature encompassing 18 trials and more than 2,600 patients in which BCT (including IFN, IL-2, or IL-2 plus IFN regimens) was compared with chemotherapy alone reported higher response rates, but no survival advantage, for the BCT regimens.17,18
The current report represents the largest phase III trial and most definitive test of BCT conducted within the United States Cooperative Group network. In this study, the concurrent administration of CVD, IL-2, and IFN-α produced a slightly higher response rate and significantly longer median progression-free survival than CVD alone, but this once again failed to translate into any meaningful benefit in median overall survival. Furthermore, the BCT regimen, despite being modified to facilitate its use in a Cooperative Group setting, produced significantly more toxicity than the chemotherapy alone regimen. This toxicity was evident by the fact that 95% of patients on the BCT arm experienced grade 3 or worse toxicity, and 20% of patients were unassessable for response assessment, largely because of an inability to complete even two cycles of therapy. These results clearly indicate that BCT cannot be considered the standard of care for patients with advanced melanoma.
In contrast to previous studies with sequential BCT regimens and treatment with high-dose IL-2 alone,10,19 the concurrent BCT regimen used in this trial failed to produce durable responses. This suggests that the concurrent administration of chemotherapy and IL-2–based immunotherapy may have negated the durable immunotherapeutic effect of the IL-2 component. This notion is supported by the report of complete responses to high-dose IL-2 in 15% of patients who had failed to respond to this BCT regimen20 and the impressive median progression-free survival (9 months) and overall survival (14 months) with the use of maintenance IL-2 and granulocyte-macrophage colony-stimulating factor immunotherapy after four to six cycles of a similar concurrent BCT regimen.21,22 Furthermore, the finding that the median response duration was longer in patients treated with CVD than those treated with BCT suggests that the curtailing of treatment at four cycles, even in responding patients, may have also truncated the effectiveness of the chemotherapy component of BCT in some patients. The fact that many responding patients on the CVD arm actually received more than four cycles of therapy supports this conjecture. In addition, the toxicity of the BCT regimen was such that not only was treatment limited to four cycles, but many patients were required to receive treatment with considerably reduced doses of immunotherapy, particularly in cycles 3 and 4 (Table 2), perhaps obscuring somewhat any potential distinction between the two treatment regimens in terms of response duration. Taken together, these observations suggest that the choice of a concurrent BCT regimen in this study, the omission of a maintenance component, and the various modifications necessitated by the toxicity of the regimen all may have limited the ability of BCT to produce more durable responses.
Korn et al23 recently reported a meta-analysis of phase II Cooperative Group trials, suggesting that when prognostic factors such as performance status, visceral disease, and sex were controlled for, clinical trials produced a consistent outcome in terms of 1-year overall survival. More variability was seen, however, in 6-month progression-free survival rates among the trials. The failure of the progression-free survival benefit associated with BCT (either median or at 6 months) in this study to translate into a benefit in overall survival supports the notion that progression-free survival end points may not always be accurate surrogates of clinical benefit in patients with advanced melanoma, especially those receiving complex treatment strategies.
The treatment of patients with advanced melanoma remains disappointing. Despite encouraging data from phase II trials, no agent or regimen has yet shown improvement in overall survival in phase III trials. The experience with BCT must now be added to this legacy. Many current opportunities exist for improving the treatment of patients with melanoma, including novel immunotherapy approaches, molecularly targeted and antiangiogenic therapies, and efforts to select treatments for patients based on tumor and host molecular and genetic features. In the context of these promising investigations, the experience with BCT suggests that the empiric combination of immunotherapy and chemotherapy approaches in the hope of producing additive or synergistic effects may be naïve. Instead, efforts should focus on enhancing the individual treatment strategies and defining the subsets of patients and tumors most likely to benefit.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Michael B. Atkins, Schering-Plough (C), Chiron/Novartis (C); Lawrence E. Flaherty, Schering-Plough (C), Chiron/Novartis (C); Jeffrey A. Sosman, Schering-Plough (C), Chiron/Novartis (C); Vernon K. Sondak, Schering-Plough (U); John M. Kirkwood, Schering-Plough (C) Stock Ownership: None Honoraria: Michael B. Atkins, Chiron/Novartis, Schering-Plough; Lawrence E. Flaherty, Schering-Plough, Chiron/Novartis; Jeffrey A. Sosman, Schering-Plough, Chiron/Novartis; Vernon K. Sondak, Schering-Plough; John M. Kirkwood, Schering-Plough Research Funding: Michael B. Atkins, Schering-Plough, Chiron/Novartis; Lawrence E. Flaherty, Schering-Plough, Chiron/Novartis; Jeffrey A. Sosman, Chiron/Novartis; John M. Kirkwood, Schering-Plough, Chiron/Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael B. Atkins, Gary I. Cohen, Lawrence E. Flaherty, Jeffrey A. Sosman, Vernon K. Sondak, John M. Kirkwood
Provision of study materials or patients: Michael B. Atkins, Gary I. Cohen, Lawrence E. Flaherty, Jeffrey A. Sosman, John M. Kirkwood
Collection and assembly of data: Michael B. Atkins, Lawrence E. Flaherty
Data analysis and interpretation: Michael B. Atkins, Jessie Hsu, Sandra Lee, Lawrence E. Flaherty, Vernon K. Sondak, John M. Kirkwood
Manuscript writing: Michael B. Atkins, Jessie Hsu, Sandra Lee, Lawrence E. Flaherty, Jeffrey A. Sosman, Vernon K. Sondak, John M. Kirkwood
Final approval of manuscript: Michael B. Atkins, Jessie Hsu, Sandra Lee, Gary I. Cohen, Lawrence E. Flaherty, Jeffrey A. Sosman, Vernon K. Sondak, John M. Kirkwood
Acknowledgments
We thank Joe Ibrahim for providing the original statistical design; Frank Haluska, MD, for his role as Chair of the Cancer and Leukemia Group B Melanoma Committee; and Caroline Stone, RN, Karishma Shah, Christabel Kwabi, Jonathan Atkins, and Kai Nalipinski for help in compiling and cleaning the study data.
Appendix
Additional Toxicity Management Information
Hypotension.
Patients discontinued antihypertensive therapy at least 24 hours before initiating each cycle of biochemotherapy (BCT). Vital signs were monitored every 4 hours for stable patients receiving interleukin-2 (IL-2). Target minimum systolic blood pressure (SBP) was 85 mmHg for patients younger than 40 years with no prior history of cardiac disease or hypertension and 90 mmHg for the remainder of patients. Patients experiencing a decrease in SBP below target SBP received a 250 mL normal saline fluid bolus administered over 15 minutes. This was repeated once for persistent hypotension. If the SBP did not increase to more than the target SBP with fluids, the IL-2 infusion was interrupted and other therapy held until the SBP recovered to over the target, at which time IL-2 and interferon alfa-2b (IFN-α) were restarted at 50% of the baseline dose. If the SBP decreased to less than 85 mmHg (80 mmHg for patients younger than 40 years with no history of cardiac disease or hypertension) regardless of response to fluid boluses, IL-2 infusion was interrupted and IFN-α administration was held. Both agents were restarted at 50% of their original doses when the SBP recovered to more than the target SBP. If the SBP remained less than 85 mmHg (80 mmHg for patients younger than 40 years with no history of cardiac disease or hypertension), despite fluid boluses and interruption of the IL-2 infusion, dopamine was started at 2 μg/kg/min and increased up to a maximum of 6 μg/kg/min to keep the SBP greater than the target. IL-2 and IFN-α were restarted at 50% of their original doses once the SBP was greater than the target SBP after cessation of all vasopressor agents. Patients who did not respond sufficiently to dopamine received phenylephrine beginning at 0.2 μg/kg/min and increased as necessary to keep SBP greater than the target value. Patients requiring both dopamine and phenylephrine for blood pressure support did not receive additional IL-2 therapy during that cycle but received IFN-α at 50% dose reduction once their SBP recovered. Such patients received a 50% dose reduction of both IL-2 and IFN-α in subsequent cycles. Cisplatin and vinblastine were also held until the patient's SBP recovered to greater than the target value without vasopressor support. If SBP failed to recover within 6 hours of the scheduled time of cisplatin, vinblastine, and IFN-α administration, therapy was omitted for that day. Missed time of the IL-2 infusion and missed doses of IFN-α, cisplatin, or vinblastine were not replaced. All dose reductions in IL-2 and IFN-α applied to all subsequent cycles.
Nephrotoxicity.
Patients had a serum creatinine checked before cisplatin administration on days 1 through 4. If serum creatinine was more than 1.6 mg/dL, cisplatin was held and a 500-mL normal saline fluid bolus was administered. If the serum creatinine improved to ≤ 1.6 mg/dL within 4 hours, scheduled cisplatin chemotherapy was administered. If creatinine remained more than 1.6 mg/dL, cisplatin was held for that day. If creatinine was more than 2.0 mg/dL, further cisplatin during that cycle was withheld. Missed doses of cisplatin were not replaced. Patients continued treatment with vinblastine, IL-2, and IFN-α unless grade 3 nephrotoxicity (creatinine > 3.0 mg/dL) developed. Subsequent cycles included full-dose cisplatin as long as the creatinine returned to less than 1.5 mg/dL. Grade 3 nephrotoxicity occurring during weeks 2 or 3 of each cycle necessitated a 25% reduction in cisplatin dose in subsequent cycles. Patients experiencing grade 3 nephrotoxicity despite a 50% reduction in cisplatin dose were removed from study treatment.
Hematologic toxicity.
Second and subsequent cycles of therapy were delayed until the WBC count, hemoglobin, and platelets returned to the levels required for protocol eligibility. If the next cycle was delayed more than 2 weeks, the patient was removed from study. Patients experiencing grade 4 hematologic toxicity, grade 3 neutropenia with fever, or grade 3 thrombocytopenia with bleeding had a 25% dose reduction of vinblastine and dacarbazine on subsequent cycles. Patients with recurrent hematologic toxicity, as described above, despite dose reduction had a second 25% dose reduction in these agents in subsequent cycles. Patients who developed hematologic toxicity as described above despite a 50% dose reduction in vinblastine and dacarbazine had treatment with these agents held. Patients experiencing grade 3 or worse hematologic toxicity during the week 2 IFN-α therapy (days 8, 10, or 12) had IFN-α held for the remainder of the week. IFN-α was then administered at full dose in subsequent cycles.
Nausea and vomiting.
Patients experiencing grade 3 nausea and vomiting during days 1 through 4 had cisplatin and vinblastine held and additional antiemetics administered. If nausea or vomiting resolved to grade 2 or less within 6 hours of scheduled therapy, treatment proceeded without modification. Patients whose hospital discharge was delayed because of persistent nausea and vomiting or who experienced grade 3 nausea and vomiting after discharge had their IFN-α doses for week 2 withheld and had 25% dose reductions in both cisplatin and dacarbazine in subsequent treatment cycles.
Neurotoxicity.
Patients underwent a thorough neurologic examination before each cycle of therapy. If a patient developed a grade 2 peripheral neuropathy, cisplatin administration was discontinued. Patients experiencing grade 2 neuropsychiatric or neurocortical toxicity during therapy had IL-2 and IFN-α held until toxicity returned to grade 1. IL-2 and IFN-α were then restarted at a 50% dose reduction for the remainder of therapy.
Response Criteria
Standard WHO response criteria were used. Complete response was defined as the complete disappearance of all clinical and radiographic evidence of malignant disease for at least two determinations separated by a minimum of 4 weeks; partial response was defined as a greater than 50% decrease in the sum of the products of the perpendicular diameters of all measurable lesions for at least two determinations separated by a minimum of 4 weeks, with no new lesions or progression of existing lesions; minor response was defined as greater than 25% but less than 50% decrease in the sums of the areas of all lesions on at least two determinations separated by a minimum of 4 weeks; progressive disease was defined as a greater than 25% increase in the sum of the areas of all lesions or the appearance of any new lesion.
Table A1.
Reasons for Excluding Patients
| Reason | No. of Patients
|
||
|---|---|---|---|
| CVD | BCT | Total | |
| Ineligible patients | |||
| No histologic documentation | 1 | 1 | |
| Missing baseline tests | 5 | 2 | 7 |
| Baseline brain metastases | 1 | 1 | |
| Baseline measurements > 4 weeks before randomization | 3 | 3 | |
| Inadequate PFTs | 2 | 1 | 3 |
| Low PS | 1 | 1 | |
| Low baseline hemoglobin | 1 | 1 | |
| Prior IL-2 | 1 | 1 | |
| Prior chemotherapy | 1 | 1 | |
| Immunotherapy within 4 wks | 1 | 1 | |
| Total | 10 | 10 | 20 |
| Failure to start assigned therapy | |||
| Decrease in PS | 2 | 2 | |
| Treatment refusal | 3 | 1 | 4 |
| No insurance coverage | 2 | 2 | |
| Total | 3 | 5 | 8 |
Abbreviations: CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy; PFTs, pulmonary function tests; PS, performance status; IL-2, interleukin-2.
Table A2.
Maximum Clinical Response by Treatment Arm
| Response | CVD (n = 195)
|
Biochemotherapy (n = 200)
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete response | 9 | 4.6 | 5 | 2.5 |
| Partial response | 18 | 9.2 | 34 | 17 |
| No change | 63 | 31.8 | 67 | 33.5 |
| Progressive disease | 9 | 49.8 | 54 | 27 |
| Unassessable | 9 | 4.6 | 40 | 20 |
| No subsequent measurements provided | 4 | 25 | ||
| Death before assessment | 1 | 6 | ||
| Early nonprotocol therapy | 1 | 4 | ||
| Did not start assigned therapy | 3 | 5 | ||
Abbreviations: CVD, cisplatin, vinblastine, and dacarbazine; BCT, biochemotherapy.
published online ahead of print at www.jco.org on November 10, 2008.
Supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA16116, CA80775, CA39229, CA14028, CA74811, CA32102, CA27057, and CA38926 and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Legha SS, Buzaid AC: Role of recombinant interleukin-2 in combination with interferon-alfa and chemotherapy in the treatment of advanced melanoma. Semin Oncol 20:27-32, 1993 [PubMed] [Google Scholar]
- 2.Legha SS, Ring S, Eton O, et al: Development and results of biochemotherapy in metastatic melanoma: The University of Texas M.D. Anderson Cancer Center experience. Cancer J Sci Am 3:S9–S15, 1997. (suppl 1) [PubMed] [Google Scholar]
- 3.Atkins MB: Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res 12:2353s-2358s, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, O'Boyle KR, Sosman JA, et al: Multiinstitutional phase II trial of intensive combination chemoimmunotherapy for metastatic melanoma. J Clin Oncol 12:1553-1560, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Keilholz U, Goey SH, Punt CJ, et al: Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: A randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol 15:2579-2588, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Richards JM, Gale D, Mehta N, et al: Combination of chemotherapy with interleukin-2 and interferon alfa for the treatment of metastatic melanoma. J Clin Oncol 17:651-657, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Antoine EC, Benhammouda A, Bernard A, et al: Salpetriere Hospital experience with biochemotherapy in metastatic melanoma. Cancer J Sci Am 3:S16-21, 1997. (suppl 1) [PubMed] [Google Scholar]
- 8.Keilholz U, Conradt C, Legha SS, et al: Results of interleukin-2-based treatment in advanced melanoma: A case record-based analysis of 631 patients. J Clin Oncol 16:2921-2929, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Allen IE, Kupelnick B, Kumashiro M, et al: Efficacy of interleukin-2 in the tretment of metastatic melanoma: Systematic review and meta-analysis. Sel Cancer Ther 1:168, 2000 [Google Scholar]
- 10.Eton O, Legha SS, Bedikian AY, et al: Sequential biochemotherapy versus chemotherapy for metastatic melanoma: Results from a phase III randomized trial. J Clin Oncol 20:2045-2052, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al: Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol 17:968-975, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Legha SS, Ring S, Eton O, et al: Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol 16:1752-1759, 1998 [DOI] [PubMed] [Google Scholar]
- 13.McDermott DF, Mier JW, Lawrence DP, et al: A phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin 2, and interferon alpha-2B in patients with metastatic melanoma. Clin Cancer Res 6:2201-2208, 2000 [PubMed] [Google Scholar]
- 14.Keilholz U, Punt CJ, Gore M, et al: Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: A randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol 23:6747-6755, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ridolfi R, Chiarion-Sileni V, Guida M, et al: Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: Results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol 20:1600-1607, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Bajetta E, Del Vecchio M, Nova P, et al: Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol 17:571-577, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sasse AD, Sasse EC, Clark LG, et al: Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev CD005413, 2007 [DOI] [PubMed]
- 18.Ives NJ, Stowe RL, Lorigan P, et al: Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: A meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol 25:5426-5434, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB, Kunkel L, Sznol M, et al: High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J Sci Am 6:S11-S14, 2000. (suppl 1) [PubMed] [Google Scholar]
- 20.Tarhini AA, Kirkwood JM, Gooding WE, et al: Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol 25:3802-3807, 2007 [DOI] [PubMed] [Google Scholar]
- 21.O'Day SJ, Boasberg PD, Piro L, et al: Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin Cancer Res 8:2775-2781, 2002 [PubMed] [Google Scholar]
- 22.O'Day SJ, Atkins MB, Weber J, et al: A phase II multi-center trial of maintenance biotherapy after induction concurrent biochemotherapy for patinets with metastatic melanoma. J Clin Oncol 23:710s, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Korn EL, Liu PY, Lee SJ, et al: Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26:527-534, 2008 [DOI] [PubMed] [Google Scholar]