Abstract
Purpose
CA 19-9 is an important tumor marker in patients with pancreatic adenocarcinoma. A secondary end point of Radiation Therapy Oncology Group trial 9704 was prospective evaluation of the ability of postresectional CA 19-9 to predict survival.
Methods
CA 19-9 expression was analyzed as a dichotomized variable (< 180 v ≥ 180) or (≤ 90 v > 90). Cox proportional hazards models were utilized to identify the impact of CA 19-9 expression on overall survival (OS). Actuarial estimates for OS were calculated using Kaplan-Meier methods.
Results
Three hundred eighty-five patients patients had assessable CA 19-9 levels. The majority had a CA 19-9 level lower than 180 or ≤ 90 (n = 220 and 200, respectively), while 34% were Lewis Antigen negative and 33 (9%) and 53 (14%) patients had levels higher than 180 and higher than 90. When CA 19-9 was analyzed as a dichotomized variable, there was a significant survival difference favoring patients with CA 19-9 lower than 180 (hazard ratio [HR], 3.53; P < .0001). This corresponds to a 72% reduction in the risk of death for patients with a CA 19-9 lower than 180. This was also true for patients with CA 19-9 ≤ 90 (HR, 3.4; P < .0001). Multivariate analyses confirmed that CA 19-9, when analyzed as both a continuous and a dichotomized variable, is a highly significant predictor of OS in patients with resected pancreatic cancer.
Conclusion
To our knowledge, this is the first phase III trial to perform prospective analysis of CA 19-9 levels in patients treated with adjuvant chemoradiotherapy. It definitively confirms the prognostic importance of postresectional CA 19-9 levels after surgery with curative intent in patients with pancreatic cancer.
INTRODUCTION
Pancreatic cancer is a highly lethal malignancy. In 2007, it was estimated that more than 90% of the 37,170 patients who will be diagnosed with this disease will die from it.1 Attempts to improve survival have been made by adding chemotherapy and radiation in the adjuvant setting for those patients who undergo curative resection. Numerous studies have shown that the addition of chemotherapy (in the form of gemcitabine or fluorouracil [FU]) with or without external-beam radiation will improve survival compared with surgery alone.2-5 Multiple investigators have attempted to determine what the most important prognostic factors are for patients with pancreatic cancer who undergo curative resection. Some of these factors include presence of positive lymph nodes (LNs), LN ratio, and lymphovascular invasion.
One factor demonstrated to have an important prognostic effect is the postoperative CA 19-9 level. CA 19-9 is the most common and important tumor marker used in the United States for patients with pancreatic cancer. CA 19-9 is a tumor-associated antigen that requires the presence of sialylated Lewis (Le)a blood group antigen to be expressed. Individuals with a Lea-b- phenotype (ie, lacking the Lewis antigen glycosyl-transferase) are unable to synthesize CA 19-9.6 In a retrospective study by Montgomery et al, 7 the postoperative CA 19-9 level was one of the most significant prognostic factors. Patients whose levels normalized by 3 to 6 months had significantly longer median survival. Patients with postoperative levels lower than 180 U/mL at 1 to 3 months had significantly improved survival compared with those with a CA 19-9 higher than 180 and similar survival to those with a normalized CA 19-9.7
Radiation Therapy Oncology Group (RTOG) trial 9704 was a randomized phase III trial comparing the use of either continuous infusion FU or gemcitabine before and after adjuvant chemoradiotherapy with FU in patients with resected pancreatic adenocarcinoma. The primary end point of this study was overall survival and has been recently reported.8 A secondary end point of this study was to examine prospectively the importance of postoperative CA 19-9 level in this group of patients. This article examines cutoff points of 90 U/mL and 180 U/mL in patients with resected pancreatic cancer, all of whom received adjuvant chemoradiotherapy.
METHODS
The methodological details of RTOG 9704 have been previously detailed.8 Briefly, patients were eligible for the trial if they had histologic proof of adenocarcinoma of the pancreas and had undergone a potentially curative resection. Patients were staged according to the American Joint Committee on Cancer (AJCC) staging system (fifth edition).
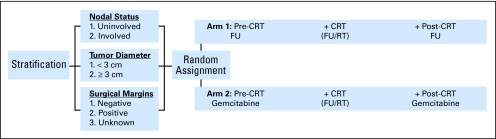
Patients were entered onto the study and randomly assigned to their treatment arm between 3 and 8 weeks postoperatively. Patients were stratified according to the following three factors—nodal status (involved v uninvolved), tumor diameter (< 3 v ≥ 3 cm), and surgical margins (negative v positive v unknown). All patients underwent potentially curative resection of their primary tumor. Postoperatively, patients were randomly assigned to one of two treatment arms depicted in Figure 1.
Fig 1.
Treatment schema. CRT, chemoradiotherapy; FU, fluorouracil; RT, radiation therapy.
CA 19-9 Testing
Because Lewis antigen expression is essential for expression of CA 19-9, red cell phenotyping for Lewis A and Lewis B antigens was required for study eligibility and was obtained at each institution's laboratory. If patients were negative for both Lewis A and B antigens, they were considered to be CA 19-9 nonexpressers. For patients that expressed either antigen, blood was drawn no more than 3 weeks before random assignment or after random assignment but before the start of protocol treatment, serum was prepared, frozen at −20C, and shipped (frozen) to the RTOG tissue bank at LDS hospital. Centralized determination of CA 19-9 was done using enzyme-linked immunosorbent assay GI-MA kits provided by Diagnostic Products Corporation (Gwynedd, United Kingdom), a Siemens Company. CA 19-9 nonexpressing patients (Lewis antigen A and B negative) were assigned values of zero because by definition, they do not have the ability to secrete CA 19-9 into their serum.6
Statistical Methods
The protocol specific secondary end point analysis was to prospectively evaluate the ability of postresectional CA19-9 to predict survival among adjuvantly treated patients who have undergone a potentially curative resection for adenocarcinoma of the pancreas. The failure event for overall survival was defined as death due to any cause. Survival time was measured from the date of random assignment to the date of death or last follow-up. The following baseline characteristics were dichotomized; pathological t-stage (T1, T2 v T3, T4), AJCC stage (I, II v III, IV), primary tumor location (head v everything else). Race was categorized as white, African American, and other. Statistical comparisons to assess potential associations between baseline characteristics and (1) missing CA19-9 data and (2) CA19-9 levels were carried out using the χ2 test. Lewis Antigen–negative patients were assigned a CA19-9 value of 0. CA19-9 baseline expression was analyzed ungrouped (Lewis Antigen negative v < 180 v ≥ 180) with two dummy variables with a value of lower than 180 as the reference level based on previous data from Fox-Chase Cancer Center (FCCC).7
A cut point of lower than 90 versus higher than 90 was also analyzed in light of the recently published adjuvant chemotherapy trial from Germany (CONKO-001).5 Cox proportional hazards models9 were utilized to identify the impact of CA19-9 expression on overall survival. Per the statistical analysis plan, in addition to treatment, the following variables were included in the multivariate analyses: nodal involvement (no v yes), tumor diameter (< 3 v ≥ 3 cm), and surgical margin status (negative v positive and negative v unknown). As there were three possible responses for surgical margin status (negative, positive, or unknown), this variable was broken into two dummy variables with a value of negative as the reference level. For figure presentations, actuarial estimates for overall survival were calculated using Kaplan-Meier methods.10
RESULTS
The primary end point results for this study were initially presented at the Annual Meeting of the American Society of Clinical Oncology in 2006 and recently published.8 A total of 538 patients were accrued to the study from RTOG (n = 370), Eastern Cooperative Oncology Group (n = 86), and Southwest Oncology Group (n = 82); of these, 85 were ineligible, two patients withdrew their consent, and another 66 patients did not have a specimen available for analysis of CA 19-9. The remaining 385 patients were eligible with analyzable CA 19-9 levels and are the basis of this report. This subset of patients was representative of the entire cohort. Of the 385 patients, 335 had tumors of the head (87%), while 50 patients had nonhead tumors. There were 225 males and 160 females (58% v 42%). The majority of patients were white (88%) and had a Karnofsky performance status of 90 or 100 (64%). The most frequent T stage was T3 (68%) followed by T2, T4, and T1 in decreasing frequency (15%, 9%, and 8%, respectively). Sixty-seven percent of patients had positive lymph nodes. In terms of AJCC staging, 233 were stage III (61%), 71 were stage II, 47 were classified as stage I, and 34 were staged as IVA. Surgical margin status was unknown in 98 of patients (25%), negative in 40%, and positive in 35% of patients. Finally, there were 192 analyzable patients in the gemcitabine arm and 193 analyzable patients in the FU arm. The median time from surgery to beginning treatment was 48 days (range, 22 to 58).
CA 19-9 levels were lower than 180 U/mL in 220 patients (57%), and ≥ 180 in 33 patients (8.6%). The Lewis antigen was reported as negative in 132 patients (34%). CA 19-9 levels were ≤ 90 U/mL in 200 patients and higher than 90 in 53 patients. There was no difference in CA 19-9 by treatment group. Table 1 details baseline characteristics of the patient population in RTOG 9704 with analyzable CA 19-9 level. Patients with CA 19-9 levels ≥ 180 were associated with larger tumors (P = .048). The median time from surgery to the blood draw for CA 19-9 determination was 45 days (range, 11 to 57).
Table 1.
Baseline Demographics
| Demographic | Lewis Ag Negative
|
CA 19-9
|
P | ||||
|---|---|---|---|---|---|---|---|
| < 180
|
≥ 180
|
||||||
| No. | % | No. | % | No. | % | ||
| Median age, years | 60 | 64 | 63 | ||||
| Range | 37-83 | 35-84 | 41-77 | ||||
| Sex | .36 | ||||||
| Male | 74 | 56 | 128 | 58 | 23 | 70 | |
| Female | 58 | 44 | 92 | 41 | 10 | 30 | |
| T stage | .16 | ||||||
| T1, T2 | 23 | 17 | 57 | 26 | 9 | 27 | |
| T3, T4 | 109 | 83 | 163 | 74 | 24 | 73 | |
| N stage | .26 | ||||||
| N0 | 37 | 28 | 80 | 36 | 10 | 30 | |
| N1 | 95 | 72 | 140 | 64 | 23 | 70 | |
| AJCC stage | .08 | ||||||
| I, II | 101 | 77 | 143 | 65 | 23 | 70 | |
| III, IVA | 31 | 23 | 77 | 35 | 10 | 30 | |
| Surgical margin | .35 | ||||||
| R0 | 50 | 38 | 87 | 40 | 17 | 52 | |
| R1 | 51 | 39 | 71 | 32 | 11 | 33 | |
| Unknown | 31 | 23 | 62 | 28 | 5 | 15 | |
| Tumor size, cm | .048 | ||||||
| < 3 | 56 | 42 | 96 | 44 | 7 | 21 | |
| ≥ 3 | 76 | 57 | 124 | 56 | 26 | 79 | |
Abbreviation: AJCC, American Joint Committee on Cancer.
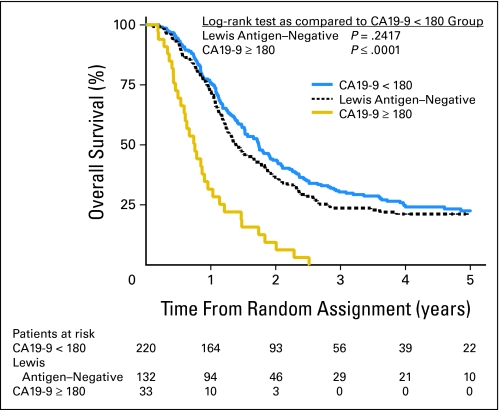
At last follow-up, all patients with a CA 19-9 ≥ 180 were dead, while only 74% patients (162 of total 220) with a CA 19-9 lower than 180 had died. This corresponds to a median survival of 9 months for patients with CA 19-9 ≥ 180 compared with 21 months for those with CA 19-9 lower than 180 (P < .0001; Fig 2). There were no significant differences in overall survival between the Lewis antigen–negative patients and those with CA 19-9 levels lower than 180. We also looked at 3-year overall survival. In patients with undetectable CA 19-9 (Lewis antigen negative), 3-year survival was 24%. For patients with CA 19-9 ≥ 180, survival was 0%, while those with CA 19-9 lower than 180 had a 3-year survival of 30%. When patients were examined by whether they had tumors of the pancreatic head (n = 335) or body/tail (n = 50), these significant differences were maintained.
Fig 2.
Kaplan-Meier survival curve by CA 19-9 with 180 cutoff.
A multivariate model was developed to examine the impact of the following factors—treatment, CA 19-9 cutoff (<180 v ≥180), nodal involvement, tumor diameter, and surgical margin status. As presented in Table 2, two factors remained specific in this model: nodal involvement and CA 19-9 level. Patients with CA 19-9 ≥ 180 had a significantly increased risk of death compared with their counterparts with CA 19-9 lower than 180 (P < .0001; HR, 3.58). The magnitude of this impact was far greater than that seen with lymph node involvement (P = .004; HR, 1.46).
Table 2.
Multivariate Analysis: Lewis Antigen Negative v CA 19-9 ≤ 180 v CA 19-9 > 180
| Adjustment Variable | Adjusted HR | 95% CI | P* |
|---|---|---|---|
| Gemcitabine v FU | 1.23 | 0.97 to 1.54 | .08 |
| CA 19-9 | |||
| < 180 | — | — | — |
| ≥ 180 | 3.58 | 2.40 to 5.34 | < .0001 |
| Lewis antigen negative | 1.12 | 0.87 to 1.44 | .37 |
| Nodal involvement | |||
| No v yes | 1.46 | 1.13 to 1.89 | .004 |
| Tumor diameter, cm | |||
| < 3 v ≥ 3 | 1.07 | 0.83 to 1.37 | .60 |
| Surgical margin status | |||
| Negative | — | — | — |
| Positive | 1.24 | 0.94 to 1.63 | .13 |
| Unknown | 1.96 | 0.71 to 1.30 | .78 |
Abbreviations: HR, hazards ratio; FU, fluorouracil.
P from χ2 test using the Cox proportional hazards model.
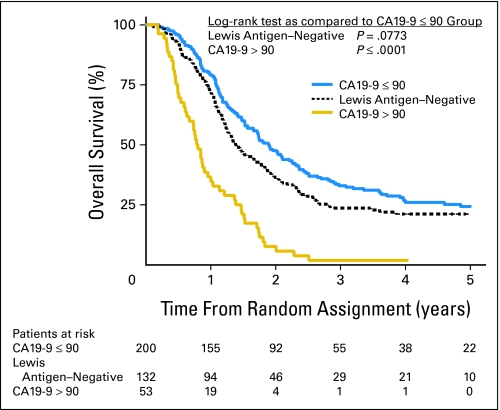
Using the cutoff of 90 U/mL, all but two patients (96% of n = 42) were dead at last follow-up and 73% (128 of total 176) of patients with CA 19-9 lower than 90 had died. The median survival time for patients with CA 19-9 ≤ 90 was 23 months while for patients with CA 19-9 higher than 90 had a median survival of 10.4 months (P < .0001; Fig 3). Three-year survival was 2% for patients with CA 19-9 higher than 90% and 32% for patients with CA 19-9 ≤ 90.
Fig 3.
Kaplan-Meier curve for overall survival by CA 19-9 with 90 cutoff.
In the multivariate model, with CA 19-9 ≤ 90 versus higher than 90 substituted (Table 3), the independent predictors of survival were again CA 19-9 level, and lymph node involvement. With a postresection CA 19-9 higher than 90 U/mL, patients had a highly significant increased risk of death (HR, 3.34; P < .0001) compared with those with a value less than or equal to that cutoff. Again, this was the most important predictor of death in this cohort of patients.
Table 3.
Multivariate Analysis: Lewis Antigen Negative v CA 19-9 ≤ 90 v CA 19-9 > 90
| Adjustment Variable | Adjusted HR | 95% CI | P* |
|---|---|---|---|
| Gemcitabine v FU | 1.21 | 0.96 to 1.52 | .11 |
| CA 19-9 | |||
| ≤ 90 | — | — | — |
| > 90 | 3.34 | 2.4 to 4.64 | < .0001 |
| Lewis antigen negative | 1.22 | 0.94 to 1.58 | .13 |
| Nodal involvement | |||
| No v yes | 1.46 | 1.12 to 1.94 | .004 |
| Tumor diameter, cm | |||
| < 3 v ≥ 3 | 1.11 | 0.87 to 1.42 | .39 |
| Surgical margin status | |||
| Negative | — | — | — |
| Positive | 1.12 | 0.87 to 1.47 | .40 |
| Unknown | 0.92 | 0.68 to 1.24 | .56 |
Abbreviations: HR, hazards ratio; FU, fluorouracil.
P from χ2 test using the Cox proportional hazards model.
DISCUSSION
CA 19-9 was first described by Koprowski et al11 in 1979; since that time, it has become the most important tumor marker for pancreatic cancer. It is a carbohydrate tumor-associated antigen which was actually first isolated from a human colorectal cancer cell line. It is a derivative of lacto-N-fucopenteose II (sialyl-Lewis[a], hapten of human Lewis[a] blood-group determinant).12 Because of this, CA 19-9 levels detected by conventional antibody tests may be influenced by Lewis blood group phenotypes. In fact, people with a Lewis negative phenotype will have an undetectable CA 19-9 level—this is present in 5% to 10% of the population.13 In a previous study from FCCC, this population comprised 5% of patients over a 12-year period.14
Several hundred reports worldwide have attested to the clinical usefulness of CA 19-9 in the diagnosis, prognosis, and monitoring of patients with pancreatic cancer. In particular, a few studies have demonstrated that serum CA 19-9 is an independent predictor of survival after resection. In a small study by Beretta et al,15 the authors found that in seven patients whose postresection CA 19-9 never returned to normal, no patient survived longer than 7 months (median, 4.8). However, in seven patients who had a normal postoperative CA 19-9 level, median survival was 17.3 months (P < .005). In another study from the Surgery Branch of the National Cancer Institute, Glenn et al16 also demonstrated that a return to normal of the serum CA 19-9 level after surgery was associated with a significantly longer survival than those who never returned to normal range (P < .005).
This observation was taken further by Montgomery et al from FCCC. These authors studied 32 patients who underwent resection of pancreatic cancer over an 8-year period. They confirmed earlier findings by demonstrating that patients whose CA 19-9 values returned to normal between 3 and 6 months after surgery had a longer survival compared with those whose CA 19-9 did not (34 v 13 months; P < .04).7 They then looked at the best threshold value for the 1- to 3-month time period postoperatively because this is when the majority of patients will begin their adjuvant therapy. This analysis revealed patients with CA 19-9 values lower than 180 U/mL in this time period had similar disease-free and overall survival to that of patients with normal CA 19-9 values measured between 3 and 6 months postoperatively.7 In another analysis of postoperative CA 19-9 levels, Ferrone et al from the Massachusetts General Hospital demonstrated that patients with a postoperative CA 19-9 higher than 200 U/mL had a significantly worse survival by univariate and multivariate analysis than those with CA 19-9 ≤ 200 U/mL.17 These same authors demonstrated that the strongest univariate predictor of overall survival was whether a patient's CA 19-9 decreased after surgery—this was also an independent predictor of improved survival.17 In this study, we did not evaluate preoperative CA 19-9 levels nor does the current analysis evaluate trends in CA 19-9 during the follow-up period. Based on the availability of these data, a future analysis may be performed.
While the significance of an elevated postoperative CA 19-9 is clear, the pathophysiology is not. These elevated levels could be due to tumor burden, spread of disease, or differences in the biologic behavior of tumors. In addition, there may be postoperative pancreatic inflammation or ductal irritation—indeed, Montgomery et al found that the time period in which CA 19-9 values returned to normal ranged from 2 months to 1 year. Finally, other factors related to the production, secretion, and metabolism of CA 19-9 probably play a role in these variations because a significant overlap between individual CA 19-9 values has been demonstrated.18
Another interesting observation from this trial was the unexpectedly high percentage of patients who were determined to be Lewis antigen negative. All patients who enrolled on RTOG 9704 had their RBC phenotype for Lewis A and Lewis B antigens determined by the laboratory/blood bank of the enrolling institution. If patients were determined to be positive at either the A or B locus, their serum was sent to the RTOG tissue bank (LDS Hospital in Utah) to have enzyme-linked immunosorbent assay performed for CA 19-9. If testing for A and B were both negative, the patient was determined to be Lewis antigen negative and thus have an undetectable CA 19-9.6 In this trial, the rate of Lewis Antigen–negative patients was 34% which is much higher than has ever been seen in any previous retrospective analysis. It is unclear whether this is a phenomenon of variations in testing for the Lewis A and B antigens in local laboratories (likely) or the percentage of Lewis Antigen patients is really close to 30% (less likely). For the purposes of data collection, patients were designated as either Lewis antigen positive or negative. We do not have data available as to the percentages of Lewis A and B positive, Lewis A negative B positive, and Lewis A positive B negative. The survival analysis shows that these patients had similar survival to those patients with CA 19-9 lower than 180 U/mL. This is contrary to the findings of Berger et al;14 however, this does represent a much larger and prospective cohort unlike the retrospective evaluation of seven patients. It is clear that future prospective trials that examine CA 19-9 as an end point should probably use a central laboratory for both determination of Lewis antigen status as well as CA 19-9 level.
When RTOG 9704 was conceived in the mid-1990s, the principal investigators made a conscious decision to make the evaluation of postoperative CA 19-9 as a secondary end point based on the previous work at FCCC. In January 2007, the results of an adjuvant chemotherapy trial from Germany were published. In this trial (CONKO-001), patients with a CA 19-9 level higher than 2.5 times the upper limit of normal (≈90) were deemed ineligible.5 With the results of this positive trial, an analysis of CA 19-9 less than 90 versus ≥ 90 U/mL was also undertaken. Briefly, the CONKO-001 trial was a randomized controlled phase III trial from Germany in which patients who had undergone curative resection for pancreatic cancer were randomly assigned to adjuvant gemcitabine (weekly, 1,000 mg/m2, every 3 of 4 weeks for six cycles) or observation.5 The primary end point for this trial was disease-free survival, and this end point was met with a significant benefit in the treatment group (13.4 months) versus the control arm (6.9 months; P < .001). There was not a significant difference in overall survival between the treatment (median, 22.1 months; 3 years, 34%) and control (median, 20.2 months; 3 years, 20.5%).5 Interestingly, the median and 3-year overall survival for patients on 9704 with CA 19-9 ≤ 90 U/mL was 23 months and 32% which compares favorably with these results.
The results of this analysis of postoperative CA 19-9 level are important because they clearly identify a subgroup of patients who have a much higher risk of death. When a CA 19-9 cutoff of 180 U/mL is used, there were 33 patients with CA 19-9 higher than 180, of whom, none survived 3 years. These patients were 3.52 times more likely to die of pancreatic cancer than patients with CA 19-9 lower than 180 U/mL. Using this cutoff point defines a population of patients with stage I and II disease (by the current staging system) who will need an intensification of their postoperative treatment to improve survival. Future adjuvant chemoradiotherapy trials should stratify patients by CA 19-9 level and/or possibly exclude patients with CA 19-9 ≥ 90 U/mL.
In summary, serum CA 19-9 is an important tumor marker for patients with pancreatic adenocarcinoma. In the postoperative setting, the CA 19-9 level can be used as a predictor of overall survival. Patients with a postoperative CA 19-9 level ≥ 180 U/mL have a significantly worse survival than those patients with CA 19 lower than 180 U/mL. These patients should be considered for alternative systemic therapy or chemoradiotherapy protocols. In addition, future phase III adjuvant trials should stratify patients by CA 19-9 values.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John P. Hoffman, William Regine, Ross A. Abrams, Howard Safran, John MacDonald, Christopher Willett
Administrative support: Al B. Benson, John MacDonald, Christopher Willett
Provision of study materials or patients: John P. Hoffman, William Regine, Ross A. Abrams, Howard Safran, Andre Konski, Al B. Benson, Christopher Willett
Collection and assembly of data: Miguel Garcia
Data analysis and interpretation: Adam Berger, Miguel Garcia
Manuscript writing: Adam Berger, Miguel Garcia, William Regine
Final approval of manuscript: Adam Berger, John P. Hoffman, William Regine, Ross A. Abrams, Howard Safran, Andre Konski, Al B. Benson, John MacDonald, Christopher Willett
published online ahead of print at www.jco.org on November 24, 2009.
Supported by Radiation Therapy Oncology Group Grants No. U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115, and by grants from the National Cancer Institute.
Presented in part in poster format at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
This manuscript's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E: Cancer Statistics, 2007. CA Cancer J Clin 57:43-66, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kalser MH, Ellenberg SS: Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899-903, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Klinkenbijl JH, Jeekel J, Sahmoud T, et al: Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776-782, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Dunn JA, Stocken DD, et al: Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 358:1576-1585, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, et al: Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 297:267-277, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA, Uchida E, Takasaki H, et al: Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 47:5501-5503, 1987 [PubMed] [Google Scholar]
- 7.Montgomery RC, Hoffman JP, Riley LB, et al: Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol 4:551-556, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Regine WF, Winter KA, Abrams RA, et al: Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 299:1019-1026, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cox, DR: Regression models and life-tables. Journal of the Royal Statistical Society, Series B 34:187-202, 1972 [Google Scholar]
- 10.Kaplan EL, Meier P: Nonparameteric estimation from incomplete observations. J Amer Statist Assoc 53:457-481, 1958 [Google Scholar]
- 11.Koprowski H, Steplewski Z, Mitchell K, et al: Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 5:957-971, 1979 [DOI] [PubMed] [Google Scholar]
- 12.Magnani JL, Nilsson B, Brockhaus M, et al: A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem 257:14365-14369, 1982 [PubMed] [Google Scholar]
- 13.Lamerz R: Role of tumour markers, cytogenetics. Ann Oncol 10:145-149, 1999. (suppl 4) [PubMed] [Google Scholar]
- 14.Berger AC, Meszoely IM, Ross EA, et al: Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol 11:644-649, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Beretta E, Malesci A, Zerbi A, et al: Serum CA 19-9 in the postsurgical follow-up of patients with pancreatic cancer. Cancer 60:2428-2431, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Glenn J, Steinberg WM, Kurtzman SH, et al: Evaluation of the utility of a radioimmunoassay for serum CA 19-9 levels in patients before and after treatment of carcinoma of the pancreas. J Clin Oncol 6:462-468, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Ferrone CR, Finkelstein DM, Thayer SP, et al: Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 24:2897-2902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundin J, Roberts PJ, Kuusela P, et al: Prognostic significance of serum CA 242 in pancreatic cancer: A comparison with CA 19-9. Anticancer Res 15:2181-2186, 1995 [PubMed] [Google Scholar]