Abstract
Purpose
In this study, we compare symptom response and times to response among patients with breast cancer who were assigned to either a cognitive behavioral Nurse-Administered Symptom Management intervention or an Automated Telephone Symptom Management (ATSM) intervention.
Patients and Methods
Patients with breast cancer were identified from a larger trial. Baseline equivalence existed between arms, and there was no differential attrition by arm. Anchor-based definition of response using mild, moderate, and severe categories of symptom severity were used. Responses and times to response for 15 symptoms were investigated in relation to trial arm, comorbid conditions, treatment protocols, and metastatic versus localized disease.
Results
The ATSM arm was more effective among patents with metastatic disease. Compared with patients receiving combination chemotherapy protocols, those treated with single agents had greater response and shorter time to response.
Conclusion
An educational information intervention delivered via an automated voice response system that assesses symptoms and refers patients to a Symptom Management Guide is more effective than a complex cognitive behavioral approach in terms of producing greater symptom responses in shorter time intervals among patients with metastatic disease.
INTRODUCTION
Recent summaries of research indicate that effective symptom management may improve quality of life for patients with cancer.1-3 Behavioral interventions range from complex cognitive behavioral models to educational and information approaches focusing on single symptoms, such as pain, or on arrays of symptoms.4,5 When compared in trials, complex cognitive behavioral models may not produce better outcomes than targeted educational strategies.6-8
The mechanisms of action through which behavioral interventions address a symptom or set of symptoms are frequently moderated by disease status, treatments, comorbid conditions, and total symptom burden of individual patients.9 Because of associations among symptoms, trials directed toward multiple symptoms may not be able to separate a direct effect on symptom(s) or determine whether management is achieved indirectly through reducing severity of other symptoms.
Research on how to measure symptoms and to define the magnitude of their response to interventions continues. Most symptom assessments use 0 to 10–point scales ranging from not present (0) to worst imaginable (10). Recall periods differ from 7 days to past 24 hours, with severity framed for patients as average or worst severity.10,11 The University of Texas M.D. Anderson Cancer Center symptom inventory10 included a six-item measure of interference that related overall symptom severity with patients’ reports of interference with general activities, mood, work, relationship with others, walking, and enjoyment of life. However, this measure describes interference at the level of the patient and does not link interference to each symptom reported by patients. Measures of pain and fatigue are exceptions; their interference scores are associated with levels of severity.12-15
Assessing symptom responses to interventions remains controversial. Response of a single symptom, such as pain, is often based on percentage change. Patients with a 30% decline are viewed as responders and those with 1% to 29% decline as partial responders.16-18 Such approaches fail to differentiate between patients who report comparable percentage declines, but differ according to their initial severity scores. This trial compares the impact of two behavioral interventions on the management of 15 cancer-related symptoms. We use reliable and valid measures of response defined by patients’ transitions among mild, moderate, and severe interference-based severity categories specific to each symptom.19,20
These symptom response outcomes are used to test the following questions. Among breast cancer patients, after adjusting for selected covariates, when compared with a six-contact, 8-week educational intervention delivered by an Automated Telephone Symptom Management (ATSM) system, does a six-contact, 8-week multimodal tailored cognitive behavioral intervention delivered by cancer nurses (Nurse-Administered Symptom Management [NASM]) produce (1) a greater number of symptom responses by the 8-week end point, or (2) shorter times to response?
PATIENTS AND METHODS
This study is part of a larger trial where an intent-to-treat analysis was completed on a sample of patients with solid tumor undergoing chemotherapy. The end point of the initial analysis was a total symptom severity score summed across multiple symptoms and measured at the 10-week patient interview.21 By confining these analyses to patients with breast cancer (41% of the sample), we were able to focus on a single sex, a limited set of treatment protocols, and localized versus metastatic disease.
Sample
Nurses from comprehensive and community oncology centers were trained to follow a recruitment protocol. Patients had to be 21 years of age or older, have a diagnosis of a solid tumor cancer or non-Hodgkin's lymphoma, be undergoing a course of chemotherapy, be able to speak and read English, and have a touchtone telephone. Participating patients signed informed consents, and their sociodemographic information was entered into a web-based tracking system. All patients were screened using an automated voice response version of the M.D. Anderson Cancer Center Symptom Inventory,10 and each patient had to score ≥ 2 in severity on at least one symptom (range, 0 to 10) to be eligible for the trial.22 Eligible patients had an intake interview, received a copy of the Symptom Management Guide (SMG), and were randomly assigned to either the NASM or ATSM arm of the trial using a computer minimization program that balanced the arms with respect to recruitment location and site of cancer.23
Trial
Patients in each intervention arm received weekly telephone calls for the first 4 weeks, were called week 6, and received a final call on week 8. At 10 and 16 weeks, outcome data were obtained. Each arm targeted the same set of 15 symptoms: fatigue, pain, dyspnea, sleep disturbance, depression, nausea/vomiting, difficulty remembering, lack of appetite, dry mouth, peripheral neuropathy, diarrhea, cough, constipation, anxiety, and weakness. Following National Comprehensive Cancer Network guidelines, when patients rated the severity of a symptom at ≥ 4 (threshold) at a contact, then intervention strategies were delivered.17
In the NASM arm, nurses followed the cognitive behavioral model where at each contact, interventions for up to four symptoms above threshold were delivered, supplemented with reference to the SMG. At each subsequent contact, assigned strategies were evaluated: the nurse inquired of the patient if the strategy was tried, and if tried, was it helpful in managing the symptom. Successful interventions were retained; strategies that were not tried or were unsuccessful were evaluated with the patient to determine how they might fit the strategy into their daily activities or different strategies were offered.
The ATSM arm extends past work on automated voice technology by incorporating symptom monitoring with reference to management of specific symptoms from the SMG.24,25 In this system, a prerecorded pleasant female voice queried patients as to the severity of each symptom. For each symptom rated at ≥ 4, patients were asked to read that specific section of the SMG. For each symptom scored at ≥ 4 on a previous contact, at the next contact, patients were queried to learn whether they tried the strategies identified in the SMG and, if so, were they helpful in lowering the severity of that symptom. Patients pressed numbers on their telephone keypad to record all responses. When all symptoms above threshold at the previous contact were evaluated, the system then reviewed the current severity of all symptoms.
Measures
Age, sex, site, and stage of cancer were recorded at enrollment from medical records. Comorbidity was measured by asking patients whether a doctor had ever told them they had conditions such as diabetes, high blood pressure, and other chronic diseases, and the number of “yes” responses was summed.26 The number of comorbid conditions was dichotomized at the median as 0 to one versus two or more. Chemotherapy protocols and dates of administration were obtained from the medical records at the end of the trial and classified into combination protocols versus single agents being administered at the date of the first intervention. Appendix Table A1 (online only) summarizes the protocols and classifications.
Severity of each of the 15 symptoms was reported on a scale from absence (0) to the worst severity possible (10) at each of the six intervention contacts. Patients reporting a severity of a ≥ 1 for any symptom at any contact were then asked to report how that symptom interfered with their enjoyment of life, relationships with others, general daily activities, emotions, and sleep. In prior work, cut points were identified based on increases in the levels of interference associated with successive increases in severity and were different for different symptoms.19 For example, for pain and fatigue, the mild category corresponds to a severity score of 1, the moderate category corresponds to scores of 2 to 4, and scores of 5 to 10 fall into the severe category. For sleep disturbance and peripheral neuropathy, the mild category is 1 to 3, the moderate category is 4 to 6, and severe category is 7 to 10. The cut points for each symptom are included in the first column of Table 1. The cut points consistently differentiated the levels of interference associated with mild, moderate, and severe scores at successive intervention contacts.20
Table 1.
Symptom Severity Cut Points, Onset Time, Severity, and Response by Trial Arm
| Symptom* and Intervention Arm | Onset Time†
|
Onset Severity | No. | % | Nonresponse
|
Response
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Contact
|
Second or Later Contact
|
||||||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| Anxiety, 0-3, 4-5, 6-10 | |||||||||||
| NASM | 17 | 51.5 | 16 | 48.5 | Moderate | 21 | 63.6 | 9 | 42.9 | 12 | 57.1 |
| Severe | 12 | 36.4 | 2 | 16.7 | 10 | 83.3 | |||||
| ATSM | 24 | 80.0 | 6 | 20.0 | Moderate | 19 | 63.3 | 1 | 5.3 | 18 | 94.7 |
| Severe | 11 | 36.7 | 2 | 18.2 | 9 | 81.8 | |||||
| Appetite, 0-3, 4-5, 6-10 | |||||||||||
| NASM | 19 | 63.3 | 11 | 36.7 | Moderate | 18 | 60.0 | 4 | 22.2 | 14 | 77.8 |
| Severe | 12 | 40.0 | 1 | 8.3 | 11 | 91.7 | |||||
| ATSM | 19 | 67.9 | 9 | 32.1 | Moderate | 19 | 67.9 | 2 | 10.5 | 17 | 89.5 |
| Severe | 9 | 32.1 | 0 | 0 | 9 | 100.00 | |||||
| Constipation, 0-3, 4-6, 7-10 | |||||||||||
| NASM | 9 | 60.0 | 6 | 40.0 | Moderate | 12 | 80.0 | 2 | 16.7 | 10 | 83.3 |
| Severe | 3 | 20.0 | 0 | 0 | 3 | 100.0 | |||||
| ATSM | 22 | 81.5 | 5 | 18.5 | Moderate | 17 | 63.0 | 2 | 11.8 | 15 | 88.2 |
| Severe | 10 | 37.0 | 2 | 20.0 | 8 | 80.0 | |||||
| Cough, 0-2, 3-4, 5-10 | |||||||||||
| NASM | 11 | 64.7 | 6 | 35.3 | Moderate | 8 | 47.1 | 4 | 50.0 | 4 | 50.0 |
| Severe | 9 | 52.9 | 2 | 22.2 | 7 | 77.8 | |||||
| ATSM | 9 | 64.3 | 5 | 35.7 | Moderate | 4 | 28.6 | 1 | 25.0 | 3 | 75.0 |
| Severe | 10 | 71.4 | 0 | 0.00 | 10 | 100.0 | |||||
| Depression, 0-1, 2-3, 4-10 | |||||||||||
| NASM | 15 | 62.5 | 9 | 37.5 | Moderate | — | — | — | |||
| Severe | 24 | 100.0 | 10 | 41.7 | 14 | 58.3 | |||||
| ATSM | 20 | 90.9 | 2 | 9.1 | Moderate | — | — | — | |||
| Severe | 22 | 100.0 | 4 | 18.2 | 18 | 81.8 | |||||
| Diarrhea, 0-3, 4-5, 6-10 | |||||||||||
| NASM | 8 | 72.7 | 3 | 27.3 | Moderate | 5 | 45.5 | 1 | 20.0 | 4 | 80.0 |
| Severe | 6 | 54.5 | 1 | 16.7 | 5 | 83.3 | |||||
| ATSM | 7 | 63.6 | 4 | 36.4 | Moderate | 8 | 72.7 | 0 | 0 | 8 | 100.0 |
| Severe | 3 | 27.3 | 0 | 0 | 3 | 100.0 | |||||
| Dry mouth, 0-4, 5-8, 9-10 | |||||||||||
| NASM | 18 | 62.1 | 11 | 37.9 | Moderate | 27 | 93.1 | 5 | 18.5 | 22 | 81.5 |
| Severe | 2 | 6.9 | 0 | 0 | 2 | 100.0 | |||||
| ATSM | 14 | 66.7 | 7 | 33.3 | Moderate | 17 | 81.0 | 4 | 23.5 | 13 | 76.5 |
| Severe | 4 | 19.0 | 1 | 25.0 | 3 | 75.0 | |||||
| Dyspnea, 0-2, 3-6, 7-10 | |||||||||||
| NASM | 7 | 43.8 | 9 | 56.3 | Moderate | 14 | 87.5 | 9 | 64.3 | 5 | 35.7 |
| Severe | 2 | 12.5 | 0 | 0.00 | 2 | 100.0 | |||||
| ATSM | 11 | 64.7 | 6 | 35.3 | Moderate | 14 | 82.4 | 3 | 21.4 | 11 | 78.6 |
| Severe | 3 | 17.7 | 1 | 33.3 | 2 | 66.7 | |||||
| Fatigue, 0-1, 2-4, 5-10 | |||||||||||
| NASM | 43 | 78.2 | 12 | 21.8 | Moderate | 15 | 27.3 | 11 | 73.3 | 4 | 26.7 |
| Severe | 40 | 72.7 | 17 | 42.5 | 23 | 57.5 | |||||
| ATSM | 39 | 73.6 | 14 | 25.4 | Moderate | 14 | 26.4 | 11 | 78.6 | 3 | 21.4 |
| Severe | 39 | 73.6 | 12 | 30.8 | 27 | 69.2 | |||||
| Vomiting, 0-3, 4-6, 7-10 | |||||||||||
| NASM | 12 | 66.7 | 6 | 33.3 | Moderate | 13 | 72.2 | 2 | 15.4 | 11 | 84.6 |
| Severe | 5 | 27.8 | 0 | 0 | 5 | 100.0 | |||||
| ATSM | 14 | 66.7 | 7 | 33.3 | Moderate | 15 | 71.4 | 4 | 26.7 | 11 | 73.3 |
| Severe | 6 | 28.6 | 0 | 0 | 6 | 100.0 | |||||
| Pain, 0-1, 2-4, 5-10 | |||||||||||
| NASM | 22 | 71.0 | 9 | 29.0 | Moderate | 16 | 51.6 | 5 | 31.3 | 11 | 68.7 |
| Severe | 15 | 48.4 | 5 | 33.3 | 10 | 66.7 | |||||
| ATSM | 13 | 72.2 | 5 | 27.8 | Moderate | 3 | 16.7 | 2 | 66.7 | 1 | 33.3 |
| Severe | 15 | 83.3 | 4 | 26.7 | 11 | 73.3 | |||||
| Peripheral neuropathy, 0-3, 4-7, 8-10 | |||||||||||
| NASM | 8 | 66.7 | 4 | 33.3 | Moderate | 12 | 100.0 | 8 | 66.7 | 4 | 33.3 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| ATSM | 13 | 56.5 | 10 | 43.5 | Moderate | 19 | 82.6 | 3 | 15.8 | 16 | 84.2 |
| Severe | 4 | 17.4 | 1 | 25.0 | 3 | 75.0 | |||||
| Difficulty remembering, 0-1, 2-4, 5-10 | |||||||||||
| NASM | 23 | 79.3 | 6 | 20.7 | Moderate | 14 | 48.3 | 8 | 57.1 | 6 | 42.9 |
| Severe | 15 | 51.7 | 4 | 26.7 | 11 | 73.3 | |||||
| ATSM | 15 | 88.2 | 2 | 11.8 | Moderate | 6 | 35.3 | 3 | 50.0 | 3 | 50.0 |
| Severe | 11 | 64.7 | 4 | 36.4 | 7 | 63.6 | |||||
| Sleep disturbance, 0-3, 4-6, 7-10 | |||||||||||
| NASM | 28 | 73.7 | 10 | 26.3 | Moderate | 25 | 65.8 | 9 | 36.0 | 16 | 64.0 |
| Severe | 13 | 34.2 | 1 | 7.7 | 12 | 92.3 | |||||
| ATSM | 21 | 70.0 | 9 | 30.0 | Moderate | 17 | 56.7 | 5 | 29.4 | 12 | 70.6 |
| Severe | 13 | 43.3 | 2 | 15.4 | 11 | 84.6 | |||||
| Weakness, 0-2, 3-4, 5-10 | |||||||||||
| NASM | 26 | 74.3 | 9 | 25.7 | Moderate | 15 | 42.9 | 7 | 46.7 | 8 | 53.3 |
| Severe | 20 | 57.1 | 5 | 25.0 | 15 | 75.0 | |||||
| ATSM | 18 | 69.2 | 8 | 30.8 | Moderate | 4 | 15.4 | 1 | 25.0 | 3 | 75.0 |
| Severe | 22 | 84.6 | 8 | 36.4 | 14 | 63.6 | |||||
Abbreviations: NASM, Nurse-Administered Symptom Management; ATSM, Automated Telephone Symptom Management.
With ranges for none/mild, moderate, severe.
% is for each symptom, of those who ever reached threshold of 4 (or severity ≥ 5 for dry mouth) during intervention.
For each symptom, patients’ reports of severity over the entire trajectory of completed contacts were summarized as either response or nonresponse. Response was defined as transition from severe to moderate, mild, or not present and from moderate to mild or not present between the first contact when a symptom reached threshold of 4 (and was intervened on) and the last completed contact. Nonresponses were defined as remaining severe or moderate or moving from moderate to severe. Patients who never reported a symptom above threshold or who reached threshold for the first time at the last contact were excluded from this analysis (the first because no interventions were delivered for symptoms < 4 and the second because there is no opportunity to assess the impact of interventions delivered). A threshold of 4 falls into a moderate (or severe) category for all symptoms except dry mouth. For this symptom, the definition of response included the first contact at ≥ 5, because 5 is the cut point for the moderate category of this symptom. The intervention protocol, established before the trial, was based on National Comprehensive Cancer Network guidelines17 indicating that symptoms at ≥ 4 require intervention. Interference-based cut points and anchor-based definitions of response represent recent developments and were applied to the data posthoc.
Time to response was defined and measured as the number of days between the contact date when a symptom first reached threshold of 4 (or 5 for dry mouth) and the date of response that was sustained over the remaining intervention contacts. For nonresponders, time to response was treated as censored.
The total number of symptoms ever reaching threshold of 4 (or 5 for dry mouth) was determined and dichotomized at the median as six or fewer versus more than six. Onset time for each symptom was defined as the contact number when symptom first reached threshold (or 5 for dry mouth), classified into two categories: first contact that took place at week 1 versus second or later contact.
Statistical Analysis
Baseline equivalence of the trial arms and an absence of attrition bias for the larger study were established and reported elsewhere, with the results of intent to treat analyses based on summed severity at 10 weeks.21 This analysis is limited to breast cancer patients and was carried out using summary measures of the entire intervention trajectories of multiple symptoms.
Symptom responses were treated as multiple events within patient and analyzed with generalized estimating equations model. Associations among responses to multiple symptoms within patient were accounted for by specifying the exchangeable association structure among symptoms within patient. Odds ratios and their 95% CIs were estimated for trial arm and covariates of interest: onset time, number of symptoms that ever reached threshold, whether disease was metastatic or localized, chemotherapy protocol, and the interaction term of trial arm by presence of metastasis. The generalized estimating equations model was fit using GENMOD procedure in SAS 9.1.27
Time to response analysis was carried out using marginal Cox proportional hazard models implemented in TPHREG procedure in SAS (SAS Institute, Cary, NC). Marginal approach of Lee, Wei, and Amato28 was used for the patient-level analysis that included multiple symptoms and was carried out using maximum partial likelihood estimates of regression parameters and a robust sandwich covariance matrix estimate to account for the dependence among symptoms within patient.28,29 Similar to the response versus nonresponse analyses, the effects of the trial arm and trial by selected covariates interactions were tested. Adjusted hazard ratios and their 95% CIs were obtained.
To illustrate the practical meaning of the observed differences in time to response by levels of covariates, median times in number of days to response for each of 15 symptoms were determined using Kaplan-Meier estimates of the survival function produced by the LIFETEST procedure.
RESULTS
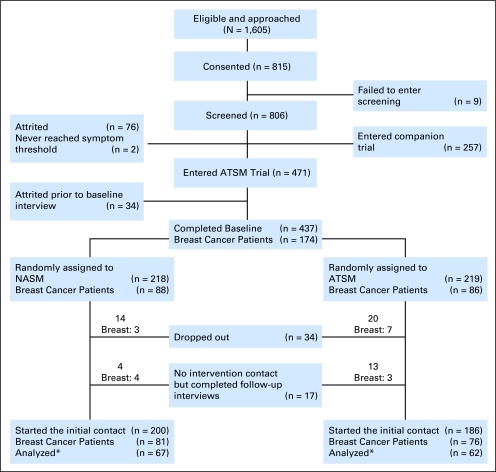
Figure 1 describes the flow and number of patients in both the larger trial and for patients with breast cancer. Eighty-eight patients with breast cancer were randomly assigned to the NASM arm and 86 patients were randomly assigned to the ATSM arm. Table 2 lists patient characteristics and treatment protocols (see Appendix Table A1 for the specific agents). More than 80% of patients completed a total of five or six contacts, with no differences between trial arms. For most patients, contact no. 6 during week 8 was the last one completed (90% in the NASM arm and 86% in the ATSM arm). No differences in attrition by the last contact completed, sociodemographic or disease characteristics, or trial arm were identified.
Fig 1.
Flow chart of the trial. (*) Patients reported at least one symptom above threshold and had a follow-up contact. ATSM, Automated Telephone Symptom Management; NASM, Nurse-Administered Symptom Management.
Table 2.
Characteristics of Patients in the Sample
| Variable | NASM Arm*
|
ATSM Arm*
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Education | ||||
| High school or below | 11 | 16.42 | 14 | 22.58 |
| Some college or technical training | 26 | 38.81 | 17 | 27.42 |
| College | 14 | 20.90 | 14 | 22.58 |
| Graduate professional degree | 16 | 23.88 | 17 | 27.42 |
| Race | ||||
| White | 57 | 85.07 | 55 | 88.70 |
| Nonwhite | 10 | 14.93 | 7 | 11.30 |
| Cancer stage | ||||
| Early | 37 | 55.22 | 28 | 45.16 |
| Late | 30 | 44.78 | 34 | 54.84 |
| Metastatic cancer | ||||
| Yes | 23 | 34.33 | 24 | 38.71 |
| No | 44 | 65.67 | 38 | 61.29 |
| Recurrence | ||||
| Yes | 20 | 29.85 | 18 | 29.03 |
| No | 47 | 70.15 | 44 | 70.97 |
| Comorbid | ||||
| 0 to 1 | 31 | 46.27 | 30 | 48.39 |
| 2+ | 36 | 53.73 | 32 | 51.61 |
| No. of symptoms above threshold | ||||
| ≤ 6 | 40 | 59.70 | 39 | 62.90 |
| > 6 | 27 | 40.30 | 23 | 37.10 |
| Chemotherapy protocol group during intervention | ||||
| Combination agent | 36 | 53.73 | 35 | 56.45 |
| Single agent | 31 | 46.27 | 27 | 43.55 |
| Patient age | ||||
| Mean | 50.12 | 53.27 | ||
| SD | 10.40 | 10.38 | ||
| Range | 26-82 | 34-90 | ||
Abbreviations: NASM, Nurse-Administered Symptom Management; ATSM, Automated Telephone Symptom Management; SD, standard deviation.
No statistically significant differences between arms.
Table 1 describes the number of patients who reached threshold for each symptom at moderate or severe levels. Ranges defining mild, moderate, and severe symptom severity are included in the first column. More than 50% of all symptoms reaching threshold did so on the first contact. The final two columns describe the percent moderate or severe and their response rate. Table 3 lists the results of analysis of response versus nonresponse. Main effects were observed favoring symptoms with onset on the second or later contacts (compared with the first contact that took place during week 1), for fewer comorbid conditions, and for single chemotherapy agents versus combination protocols. The number of symptoms above threshold did not change the estimates of the effects of other covariates in the model, was not significant over and above them, and therefore was not included in the final models.
Table 3.
Analysis of Response Using GEE Model
| Covariate | Level | Reference Level | Adjusted OR | 95% CI | χ2P |
|---|---|---|---|---|---|
| Intervention arm | ATSM | NASM | — | — | |
| Onset time | Second or later contact | First contact | 1.46 | 1.00 to 2.11 | .05 |
| Metastatic | Yes | No | — | — | |
| Chemotherapy protocol group | Single | Combination | 1.79 | 1.18 to 2.73 | < .01 |
| Comorbid conditions | 0-1 | 2+ | 2.03 | 1.31 to 3.15 | < .01 |
| Interaction of intervention arm by metastatic* | Metastatic, ATSM | Metastatic, NASM | 4.40 | 2.25 to 8.60 | < .01 |
| Not metastatic, ATSM | Not metastatic, NASM | 0.87 | 0.51 to 1.51 | .63 |
Abbreviations: GEE, generalized estimating equation; OR, odds ratio; ATSM, Automated Telephone Symptom Management; NASM, Nurse-Administered Symptom Management.
P < .01.
Patients in the ATSM arm with metastatic disease were more likely to achieve a response. As shown in the last two columns of Table 1, for all symptoms except pain and fatigue, those with moderate severity at onset in the ATSM arm were more likely to respond compared with those in the NASM arm. The number of symptoms that reached threshold did not differ by local versus metastatic disease; thus the interaction of trial arm and metastatic status cannot be explained by the difference in the number of symptoms.
Table 4 presents the Cox proportional hazard model for the time to response outcome using the same set of explanatory variables. As expected from response versus nonresponse analysis, onset time at the second or later contact, fewer comorbid conditions, and single-agent chemotherapy protocols were associated with shorter time to response as a result of a smaller proportion of censored (nonresponse) observations. Trial arms had different time to response by disease status: the ATSM arm produced shorter times to response compared with the NASM for patients with metastasis. Finally, Table 5 lists the results by reporting the unadjusted median number of days to response for each symptom by levels of covariates. Fewer than 50% of patients with metastatic disease in the NASM arm achieved responses for fatigue, cough, dyspnea, diarrhea, and peripheral neuropathy; therefore, for these symptoms, only a lower bound for the median is provided.
Table 4.
Analysis of Time in Days to Response of Patients With Breast Cancer Using Marginal Cox Proportional Hazards Model
| Covariate | Level | Reference Level | Adjusted HR | 95% CI | χ2P |
|---|---|---|---|---|---|
| Intervention arm | ATSM | NASM | — | — | |
| Onset time | Second or later contact | First contact | 1.56 | 1.28 to 1.89 | < .01 |
| Metastatic | Yes | No | — | — | |
| Chemotherapy protocol group | Single | Combination | 1.34 | 1.09 to 1.64 | < .01 |
| Comorbid conditions | 0-1 | 2+ | 1.30 | 1.06 to 1.59 | .01 |
| Interaction of intervention arm by metastatic* | Metastatic, ATSM | Metastatic, NASM | 2.45 | 1.81 to 3.30 | < .01 |
| Not metastatic, ATSM | Not metastatic, NASM | 1.08 | 0.88 to 1.34 | .46 |
Abbreviations: HR, hazard ratio; ATSM, Automated Telephone Symptom Management; NASM, Nurse-Administered Symptom Management.
P < .01.
Table 5.
Unadjusted Median Time in Days to Symptom Response by Levels of Covariates
| Symptom | Onset Time
|
Chemotherapy Protocol
|
Comorbid Conditions
|
Nonmetastatic Disease
|
Metastatic Disease
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Contact | Second or Later Contact | Combination | Single Agent | 0-1 | 2+ | NASM | ATSM | NASM | ATSM | |
| Fatigue | 49.00 | 27.00 | 51.00 | 35.00 | 26.00 | 50.00 | 35.00 | 28.00 | > 57.00 | 55.00 |
| Cough | 21.00 | 14.00 | 18.00 | 14.00 | 18.00 | 14.00 | 14.00 | 10.50 | > 49.00 | 9.00 |
| Anxiety | 21.00 | 14.00 | 14.00 | 21.00 | 14.00 | 21.00 | 22.00 | 21.00 | 22.50 | 14.00 |
| Weakness | 27.00 | 17.00 | 27.00 | 20.00 | 14.00 | 29.00 | 21.50 | 20.00 | 55.00 | 24.00 |
| Appetite | 17.00 | 23.00 | 25.00 | 14.00 | 21.00 | 14.50 | 23.00 | 7.50 | 28.00 | 7.00 |
| Pain | 43.00 | 21.00 | 34.00 | 31.00 | 21.00 | 43.00 | 34.00 | 24.00 | 35.00 | 34.00 |
| Dyspnea | 48.00 | 14.00 | 24.00 | 22.00 | 14.00 | 38.00 | 23.50 | 15.00 | > 49.00 | 11.50 |
| Depression | 24.00 | 14.00 | 20.00 | 19.00 | 14.00 | 24.00 | 47.00 | 14.00 | 24.00 | 14.00 |
| Constipation | 15.00 | 14.00 | 15.00 | 7.50 | 13.00 | 15.00 | 11.00 | 8.50 | 51.00 | 15.00 |
| Vomiting | 9.50 | 7.00 | 11.00 | 8.00 | 9.00 | 7.00 | 8.50 | 8.00 | 10.50 | 14.00 |
| Diarrhea | 7.00 | 8.00 | 7.00 | 7.00 | 8.00 | 7.00 | 7.00 | 7.00 | > 53.00 | 7.00 |
| Dry mouth | 14.00 | 8.00 | 19.00 | 7.00 | 7.00 | 16.00 | 11.50 | 14.00 | 7.00 | 29.00 |
| Sleep disturbance | 22.00 | 28.00 | 22.00 | 24.00 | 22.00 | 28.00 | 31.00 | 21.00 | 18.00 | 14.00 |
| Peripheral neuropathy | 41.00 | 17.00 | 48.00 | 28.00 | 21.00 | 35.50 | 36.00 | 14.50 | > 55.00 | 35.00 |
| Difficulty remembering | 51.00 | 25.00 | 25.00 | 53.00 | 19.00 | 51.00 | 15.00 | > 56.00 | 53.00 | 11.00 |
Abbreviations: NASM, Nurse-Administered Symptom Management; ATSM, Automated Telephone Symptom Management.
DISCUSSION
Consistent with other research, complex cognitive behavioral models may not be better than education approaches to assist patients with cancer to implement symptom management strategies.4-6 This research used symptom-specific, interference-based measures of severity, summarized as mild, moderate, and severe. These categories were used to define anchor-based measures of response for each symptom above threshold in each intervention arm. The anchor-based response categories make these findings not only statistically important, but clinically relevant. Clinicians can determine whether a response must move from severe to mild or whether lowering a symptom from severe to moderate is clinically important.30 These analyses extend understanding of the impact of each trial arm beyond summed severity scores. The impact of trial arm and covariates on symptom response and time to response was presented. Using this analytic approach, findings from the breast cancer subsample were consistent with the larger sample and favored the ATSM arm. These analyses combined transitions from severe to moderate or none/mild and transitions from moderate to none/mild as the response outcome. The descriptive statistics suggest that the advantage of the ATSM arm among patients with breast cancer with metastatic disease may come from a greater number of moderate to mild transitions, but further formal testing using larger samples is needed.
The lower rate of responses among patients on combination protocols compared with those receiving single agents may reflect symptom toxicity reactions beyond the capacity of these types of interventions. The ATSM involves less time to implement (approximately 19 minutes per contact v 42 minutes averaged over six contacts) than the NASM and is less costly to implement because the ATSM does not require time from nurses. Limitations of this research include loss of patients during screening who may have disliked the automated system, thereby creating a bias favoring the ATSM arm. The results may not be generalizable to minority patient populations because the sample was primarily highly educated and white. Time to response was analyzed as right-censored but in fact, interval censoring according to scheduled contacts was present.
Although the methodology of assessing responses represents an important advance in the assessment of multiple symptoms, it still must be replicated on other samples of patients (for example, on samples of minority and vulnerable patients) to determine how these responses are interpreted by oncologists and oncology nurses.
In conclusion, in this trial, an automated voice response system that monitored symptom severity and directed patients with cancer to specific strategies contained in an SMG proved more effective than telephone strategies tailored by nurses among patients with metastatic breast cancer. The advantages of the ATSM may be derived from its brevity, lack of intrusiveness, and its reliance on the SMG that directed patients to specific interventions to which they could refer each day. Thus the ATSM is worthy of further research to establish the mechanisms through which patients implement interventions for symptom management.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Charles W. Given, Alla Sikorskii, Barbara Given, Ruth McCorkle, Victoria Champion
Administrative support: Barbara Given
Provision of study materials or patients: Victoria Champion, David Decker
Collection and assembly of data: Charles W. Given, Alla Sikorskii, Barbara Given, Ruth McCorkle
Data analysis and interpretation: Charles W. Given, Alla Sikorskii, Deimante Tamkus, Barbara Given, Mei You, Ruth McCorkle
Manuscript writing: Charles W. Given, Alla Sikorskii, Barbara Given, Ruth McCorkle, Victoria Champion
Final approval of manuscript: Charles W. Given, Alla Sikorskii, Deimante Tamkus, Ruth McCorkle, Victoria Champion
Appendix
Table A1.
Patients With Breast Cancer Chemotherapy Protocol Index Table
| Chemotherapy After Collapse | Chemotherapy Protocol Before Collapse |
|---|---|
| Combination | Doxorubicin, cyclophosphamide |
| Doxorubicin, paclitaxel | |
| Cyclophosphamide, methotrexate, fluorouracil (FU) | |
| Epirubicin, cyclophosphamide | |
| FU, doxorubicin, cyclophosphamide | |
| FU, epirubicin, cyclophosphamide | |
| Docetaxel or paclitaxel, doxorubicin, cyclophosphamide | |
| Paclitaxel, epirubicin, cyclophosphamide | |
| Paclitaxel/gemcitabine | |
| Bevacizumab/docetaxel | |
| Paclitaxel/carboplatin/trastuzumab | |
| Trastuzumab/capecitabine | |
| Trastuzumab/docetaxel | |
| Trastuzumab/gemcitabine/docetaxel | |
| Trastuzumab/paclitaxel/FU/bevacizumab | |
| Trastuzumab/vinorelbine | |
| Trastuzumab/oxaliplatin | |
| Carboplatin/paclitaxel | |
| Oxaliplatin/capecitabine | |
| Docetaxel/estramustine/hydrocortisone | |
| Single agent | Capecitabine |
| Carboplatin | |
| Cyclophosphamide | |
| Docetaxel | |
| Doxorubicin | |
| Gemcitabine | |
| Paclitaxel | |
| Trastuzumab | |
| Vinorelbine |
published online ahead of print at www.jco.org on November 24, 2008
Supported by Grant No. CA030724 from the National Cancer Institute and the Walther Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Buchanan DR, O'Mara AM, Kelaghan JW, et al: Quality-of-life assessment in the symptom management trials of the National Cancer Institute–supported community clinical oncology program. J Clin Oncol 23:591-598, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Sloan JA, Berk L, Roscoe J, et al: Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol 25:5070-5077, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Minasian LM, O'Mara AM, Reeve BB, et al: Health-related quality of life and symptom management research sponsored by the National Cancer Institute. J Clin Oncol 25:5128-5132, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Miaskowski C, Lee KA: Pain, fatigue, and sleep disturbance in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. J Pain Symptom Manage 17:320-332, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Given C, Given B, Rahbar M, et al: Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol 22:507-516, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Newell SA, Sanson-Fisher RW, Savolainen NJ: Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst 94:558-584, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen PB, Meade CD, Stein KD, et al: Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol 20:2851-2862, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Yates P, Aranda S, Hargraves M: Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy. J Clin Oncol 23:6027-6036, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Francoeur RB: The relationship of cancer symptom clusters to depressive affect in the initial phase of palliative radiation. J Pain Symptom Manage 29:130-155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleeland C, Mendoza T, Wang X, et al: Assessing symptom distress in cancer patients. Cancer 89:1634-1646, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Kirkova J, Davis MP, Walsh D, et al: Cancer symptom assessment instruments: A systematic review. J Clin Oncol 24:1459-1473, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Serlin RC, Mendoza TR, Nakamura Y, et al: When is cancer pain mild, moderate, or severe? Grading pain severity by its interference with function. Pain 61:277-284, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Paul SM, Zelman DC, Smith M, et al: Categorizing the severity of cancer pain: Further exploration of the establishment of cut-points. Pain 113:37-44, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Jensen MP, Smith DG, Ehde DM, et al: Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and sever pain. Pain 91:317-322, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Mendoza T, Wang X, Cleeland C, et al: The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 85:1186-1196, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Farrar JT, Young JP Jr, LaMoreaux L, et al: Clinical importance of chances in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94:149-158, 2001 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network: Clinical practice guidelines in oncology: Cancer-related fatigue version 1. 2006. Http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf [DOI] [PubMed]
- 18.Miaskowski C, Dodd M, West C, et al: The use of responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain 129:55-63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Given B, Given C, Sikorskii A, et al: Establishing mild, moderate and severe scores for cancer related symptoms: How consistent and clinically meaningful are interference based severity cut points? J Pain Symptom Manage 35:126-135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon S, Given C, Sikorskii A, et al: Do interference based cut-points differentiate mild, moderate and severe levels of 16 cancer related symptoms over time? J Pain Symptom Manage [epub ahead of print on July 9, 2008] [DOI] [PMC free article] [PubMed]
- 21.Sikorskii A, Given C, Given B, et al: Symptom management for cancer patients: A trial comparing two multimodal interventions. J Pain Symptom Manage 34:253-264, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton AL: How and for whom? Asking questions about the utility of psychosocial interventions for individuals diagnosed with cancer. J Clin Oncol 23:4818-4820, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Taves DR: Minimization: A new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 15:443-453, 1974 [DOI] [PubMed] [Google Scholar]
- 24.Basch E, Artz D, Dulko D, et al: Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 23:3552-3561, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Davis K, Yount S, Del Ciello K, et al: An innovative symptom monitoring tool for people with advanced lung cancer: A pilot demonstration. J Support Oncol 5:381-387, 2007 [PubMed] [Google Scholar]
- 26.Katz JN, Chang LC, Sangha O, et al: Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73-84, 1996 [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute: SAS Help and Document/SAS/STAT /SAS/STAT User Guide. Cary, NC, SAS Institute, 1990
- 28.Lee E, Wei L, Amato D: Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations (237-247). Dordrecht, the Netherlands, Kluwer Academic Publishers, 1992
- 29.Lin DY: Cox regression analysis of multivariate failure time data: The marginal approach. Stat Med 13:2233-2247, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Cleeland CS: Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 37:16-21, 2007 [DOI] [PubMed] [Google Scholar]