Abstract
Purpose
Hot flashes are common and frequently lead to drug discontinuation among women prescribed tamoxifen. We determined whether genetic polymorphisms in estrogen receptors (ESRs) α and β (ESR1 and ESR2, respectively) are associated with tamoxifen-induced hot flashes.
Patients and Methods
We determined ESR1 PvuII and XbaI and ESR2-02 genotypes in 297 women who were initiating tamoxifen. One-week hot flash diaries were collected to calculate a hot flash score (frequency × severity) before and 1, 4, 8, and 12 months after starting tamoxifen.
Results
Approximately 80% of 297 participants reported hot flashes before or during the first year of tamoxifen. After 4 months of tamoxifen, premenopausal women who did not receive adjuvant chemotherapy had a four-fold increase in hot flash score (from 5.9 to 23.6; P = .003) compared with a 1.17-fold increase (from 19.6 to 23; P = .34) in those who received chemotherapy. In premenopausal women, increased number of ESR1 PvuII and XbaI CG alleles was associated with higher baseline hot flash scores compared with those who had other haplotypes (P = .0026). At 4 months, postmenopausal women with ESR1 PvuII CC and ESR2-02 GG genotypes had 4.6 times increases in hot flash scores than other postmenopausal women (56 v 12; P = .0007). Women who had the ESR2-02 AA genotype were significantly less likely to experience tamoxifen-induced hot flashes than women who carried at least one ESR-02 G allele (hazard ratio, 0.26; 95% CI, 0.10 to 0.63; P = .001).
Conclusion
Knowledge of menopausal status, prior chemotherapy, and ESR genotype may help predict which women are most likely to suffer hot flashes during tamoxifen treatment.
INTRODUCTION
In women who have estrogen receptor (ESR)–rich, early-stage breast cancer, tamoxifen administered for 5 years is associated with a 42% reduction in relapse and a 22% reduction in breast cancer-related death.1 Tamoxifen also reduces the risk of a new breast cancer by nearly one half.2 One of the most common and bothersome adverse events associated with tamoxifen is hot flashes. This effect is believed to be secondary to a central nervous system antiestrogenic effect.3 Up to 80% of women prescribed tamoxifen complain of hot flashes, and approximately 30% rate them as severe.4 Tamoxifen-associated symptoms may interfere with quality of life and may result in discontinuation of the agent in more than 40% of women.5 Although tamoxifen may be optional for women at high risk for breast cancer, it is an integral part of adjuvant breast cancer therapy for many women, and completion of the prescribed treatment is optimal to reduce breast cancer recurrence and death.1-3
Selective ESR modulators, like tamoxifen, exert their pharmacologic effect via interaction with the ESRs. Two primary ESRs have been identified in humans: ESR α (ESR1) and ESR β (ESR2). Both are genetically polymorphic in the germline. In the ESR1 gene, two single nucleotide polymorphisms (SNPs), PvuII (c.454-397T>C, rs#2234693) and XbaI (c.454-351A>G, rs#9340799), have been associated with clinical phenotypes and have influenced high-density lipoprotein cholesterol response to estrogen in menopausal women6 and bone density.7 The same SNPs have been correlated with breast cancer risk8 and survival.9 The haplotypes of these two SNPs have been associated with risk of myocardial infarction.10 Several polymorphisms in ESR2 also are associated with clinical outcomes. We have reported differential changes in total cholesterol and triglycerides according to ESR2-02 genotype.11 These findings suggest that SNPs in ESR1 and ESR2 may be associated with differential risk of tamoxifen-induced effects.
We hypothesized that genetic variants in the ESRs may play a role in susceptibility to tamoxifen-induced hot flashes. We investigated the association between ESR1 and ESR2 genotypes and hot flashes in a prospective study of a cohort of tamoxifen-treated women.
PATIENTS AND METHODS
Patients and Study Design
We designed an open-label, prospective observational trial to test associations between polymorphisms in candidate genes and tamoxifen pharmacokinetics, adverse effects, and secondary benefits. The study design and results related to pharmacogenetic influence on tamoxifen pharmacokinetics and associations between SNPs in ESR1 and ESR2 with lipid levels have been previously published.11,12
Participants included women 18 years or older who were recommended tamoxifen for adjuvant treatment or for the prevention of breast cancer. The women were recruited from three academic cancer centers (Georgetown University, University of Michigan, and Indiana University). Women were excluded if they had concurrent adjuvant chemotherapy, radiation therapy, other endocrine therapy except ovarian suppression, or chronic corticosteroid therapy; or if they used clonidine, bellargal, or megestrol acetate for the treatment of hot flashes. Vitamin E, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or herbal use was allowed, provided that the participant had been taking the agent for at least 4 weeks before study entry and intended to continue taking the agent for at least the first study month. The protocol was approved by the institutional review boards and general clinical research centers of all three sites. Each participant provided written informed consent.
Baseline history and physical, a comprehensive medication list, and clinical laboratory tests were obtained for each participant. Menopausal status was determined based on menstrual history. Women who were age 60 years or older or those who had no menses during the prior 12 months or who had a prior bilateral oophorectomy were defined as postmenopausal; women who had regular menses before adjuvant chemotherapy or tamoxifen were defined as premenopausal; and the rest were defined as perimenopausal. Participants received open-label tamoxifen 20 mg orally each day. Medical history and current medications were recorded 1, 4, 8, and 12 months after initiating tamoxifen.
Genotyping Analysis
At baseline, a 10-mL blood sample in a heparinized Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) was collected from each participant. Samples were stored in a −80°C freezer. Genomic DNA was extracted by using a QIAmp DNA Mini Kit (Qiagen, Valencia, CA). ESR1 PvuII and XbaI genotypes were determined according to the method of Herrington et al,6 with minor modifications.11 Genotyping for ESR2-02 was performed by Taqman assays, as previously described by the National Cancer Institute Cancer Genome Anatomy Project (snp500cancer.nci.nih.gov). The amplification and analysis were performed by using the iCycler Real-Time Thermocycler (Bio-Rad Life Science Research Group, Hercules, CA).
Hot Flash Diaries
Participants completed validated hot flash daily diaries for 7 days at baseline and 1, 4, 8, and 12 months after starting tamoxifen.13 During each 24 hour period, the women recorded the number of hot flashes that they had experienced and how many were mild, moderate, severe, or very severe. A composite score was generated by multiplying the number of mild, moderate, severe, or very severe hot flashes by 1, 2, 3, and 4, respectively, and summing the values into one score.
Statistical Analysis
The primary end point of the clinical protocol was to assess the relationship between pharmacogenetics, tamoxifen metabolism, and frequency and severity of hot flashes associated with tamoxifen. The women were categorized into seven different groups determined by menopausal status, presence of hot flashes at baseline (0 to 2 or > 2), and prior adjuvant chemotherapy, to allow for heterogeneity. An enrollment target of 43 participants per group (301 participants overall) was chosen to have an 85% power to detect a .5 effect size in the difference of hot flash score change between two genotype groups, considering a 15% homozygous variant genotype frequency and a 15% drop-out rate. Given that the standard deviation of the hot flash score is 9 units, this .5 effect size is translated to the 4.5-unit difference in hot flash score change from baseline to month 4.
Follow-up weekly hot flash scores were compared with baseline scores by using linear regression with repeated measures. Associations between ESR genotypes and baseline weekly hot flash scores were examined in each menopausal group. The comparisons were performed by using linear regression within each menopausal status. Associations between ESR genotypes and the changes in hot flash score from baseline to month 4 were assessed by the interactions between genotypes and time by using linear regression with repeated measures.
The ESR1 haplotypes were constructed from ESR1PvuII and ESR1XbaI SNPs by using the PHASE2 online software (http://www.stat.washington.edu/stephens/software.html). Its association with hot flash score was tested with a generalized estimating equation approach,14 through its online implementation (http://www.mayo.edu/statgene).
The association with genetic and clinical predictors was tested through survival analyses. Time to hot flash was treated as a time-to-event outcome. Kaplan-Meier plots are displayed, and P values were calculated from log-rank tests. Odds ratios (ORs) for the presence of hot flashes predicted from ESR genotypes are reported; their P values were based on χ2 tests.
Most of the analyses, including χ2 tests, Wilcoxon tests, survival analyses, and linear regression with repeated measures, were conducted in SAS 9.1(SAS Institute Inc, Cary, NC). P values were confirmed by bootstrap algorithms. Haplotype and haplotype-phenotype analyses were performed with prescribed online software.14,15
RESULTS
Demographic Characteristics of Subjects
Two hundred ninety-seven participants were enrolled on the study. Baseline patient characteristics were compared among the three menopausal groups (Table 1). Baseline mean hot flash frequency and hot flash composite scores were significantly lower in the premenopausal group compared with the post- and perimenopausal groups (P = .02).
Table 1.
Patient Characteristics at Baseline
| Characteristic | Overall (N = 297)*
|
Menopausal Status
|
P† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Premenopausal (n = 93)
|
Perimenopausal (n = 37)
|
Postmenopausal (n = 164)
|
|||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age, years | < .0001 | ||||||||
| Mean | 52.1 | 43.2 | 49.1 | 57.9 | |||||
| Range | 29-87 | 29-57 | 40-59 | 35-87 | |||||
| BMI, kg/m2 | 28.0 ± 0.4 | 27.2 ± 0.7 | 28.1 ± 1.1 | 28.4 ± 0.5 | .29 | ||||
| Ethnicity | |||||||||
| White | 275 | 92.6 | 87 | 93.8 | 34 | 91.9 | 154 | 93.6 | .70 |
| Other | 19 | 6.5 | 6 | 6.2 | 3 | 8.1 | 10 | 6.4 | |
| Prior chemotherapy | 142 | 47.8‡ | 44 | 46.8 | 19 | 51.4 | 78 | 47.6 | .89 |
| Prior ET | |||||||||
| Yes | 102 | 34.3 | 4 | 4.3 | 8 | 21.6 | 90 | 55.5 | < .001 |
| No | 147 | 49.5 | 72 | 77.7 | 26 | 70.3 | 48 | 29.3 | |
| Unknown | 48 | 16.7 | 17 | 18.1 | 3 | 8.1 | 26 | 15.9 | |
| SSRI/SNRI | |||||||||
| Yes | 55 | 14.1 | 13 | 14.0 | 9 | 24.3 | 33 | 20.1 | .18 |
| No | 230 | 81.8 | 78 | 84 | 24 | 64.9 | 128 | 78.0 | |
| Unknown | 12 | 4 | 2 | 2.2 | 4 | 10.8 | 3 | 1.8 | |
| Hot flash frequency | 12.7 ± 1.1 | 8.6 ± 1.6 | 13.9 ± 2.7 | 15.0 ± 1.7 | .03 | ||||
| Hot flash score§ | 20.9 ± 2.2 | 12.5 ± 2.8 | 22.7 ± 5.9 | 25.3 ± 3.2 | .02 | ||||
Abbreviations: BMI, body mass index; ET, estrogen therapy; SSRI/SNRI, selective serotonin or serotonin-noradrenergic reuptake inhibitor.
Menopausal status was unknown in three women who are, therefore, not included in the subgroup comparison.
Comparison among different menopausal status stages.
One patient who had prior chemotherapy did not provide information regarding her menopausal status.
Hot flash score equals hot flash frequency times hot flash severity.
ESR Genotype
ESR1 PvuII, ESRI XbaI, and ESR 2-02 genotypes were determined in 289, 287, 254 participants, respectively (Appendix Table A1, online only). Eight, 10, and 43 samples were not assessable for ESR1 PvuII, ESRI XbaI, and ESR 2-02 genotypes, respectively. Genotype distributions were in Hardy-Weinberg equilibrium for the entire cohort. We observed four possible haplotypes in the ESR1 gene on the basis of the ESR1 PvuII and XbaI genotype: T-A (frequency, 48.8%), C-G (frequency, 31.8%), C-A (frequency, 16.0%), and T-G (frequency, 3.4%).
Changes in Hot Flash Prevalence Rate and Weekly Composite Score During the First Year of Tamoxifen Treatment
Among the 297 participants, 286 returned their baseline hot flash diaries, and 250, 238, 213, and 212 diaries were available 1, 4, 8, and 12 months after tamoxifen treatment, respectively. Diaries were not available on all patients because of discontinuation of tamoxifen during the first year of treatment (n = 41) or because of failure to return diaries.
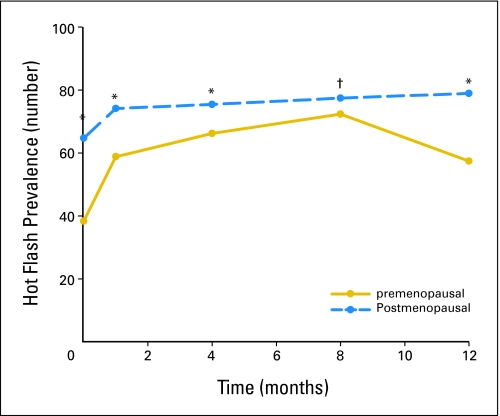
A higher proportion of perimenopausal and postmenopausal patients reported hot flashes compared with premenopausal women at all the time points. Before tamoxifen treatment, 36% of premenopausal, 65% of perimenopausal, and 65% of postmenopausal women reported symptoms of hot flashes (P < .001). Four months after initiation of tamoxifen therapy, premenopausal hot flash prevalence increased from 36% to 61% (P = .001), whereas it increased in postmenopausal women from 65% to 78% (P = .02; Appendix Figure A1, online only).
As expected, the mean weekly hot flash score was significantly lower in premenopausal women before tamoxifen treatment (12.5 ± 2.8) compared with postmenopausal women (25.3 ± 3.2, P = .02). This difference disappeared after the first month of treatment (Table 2). Hot flash scores remained elevated throughout the first year of tamoxifen treatment regardless of baseline menopausal status.
Table 2.
Hot Flash Score During the First Year of Tamoxifen Treatment
| Time, Months | Score by Treatment Group
|
P* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall
|
Premenopausal
|
Perimenopausal
|
Postmenopausal
|
||||||
| No. | Mean ± SE | No. | Mean ± SE | No. | Mean ± SE | No. | Mean ± SE | ||
| Baseline† | 286 | 20.9 ± 2.1 | 94 | 13.9 ± 2.9 | 36 | 22.7 ± 4.9 | 156 | 24.7 ± 3.2 | 0.02 |
| 1‡ | 250 | 32.5 ± 3.1 | 80 | 30.2 ± 6.3 | 30 | 32.0 ± 7.9 | 139 | 34.0 ± 3.9 | 0.58 |
| 4§ | 238 | 32.5 ± 3.4 | 77 | 27.5 ± 5.5 | 26 | 52.2 ± 10.7 | 134 | 38.9 ± 4.8 | 0.14 |
| 8§ | 213 | 37.6 ± 3.8 | 65 | 30.0 ± 6.7 | 23 | 48.0 ± 9.5 | 124 | 39.4 ± 5.1 | 0.27 |
| 12§ | 212 | 37.9 ± 4.4 | 67 | 36.2 ± 10.3 | 21 | 45.9 ± 11.1 | 123 | 37.3 ± 4.8 | 0.90 |
Abbreviation: SE, standard error.
Comparison of premenopausal v postmenopausal patients.
All patients who returned their hot flash diaries provided information regarding their menopausal status.
One participant who did not provide information regarding her menopausal status returned hot flash diaries on months 1, 4, 8, and 12.
Comparison among different menopausal status stages.
Baseline Hot Flash Score and ESR Genotype
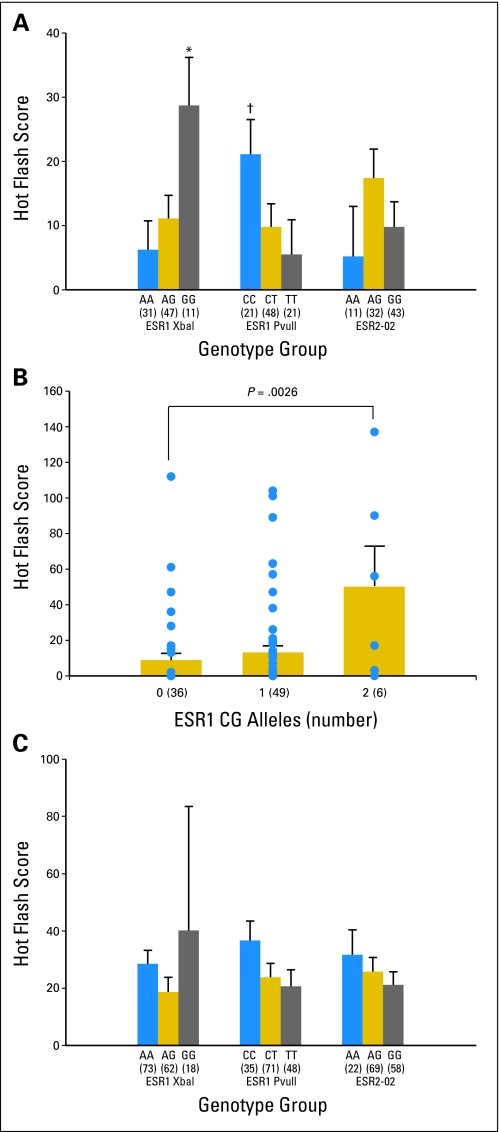
In premenopausal women before tamoxifen treatment, a statistically significant association was observed between baseline weekly hot flash score and the ESR1 XbaI and PvuII genotypes. Participants who had the ESR1 XbaI GG genotype had significantly higher hot flash scores than those who had AG and AA genotypes (Appendix Figure A2, panel A, online only). Similarly, participants who had the ESR1 PvuII CC genotype had statistically significantly higher hot flash scores than carriers of the CT and TT genotypes. Therefore, ESR1 CG haplotype was associated with higher hot flash scores in premenopausal women. One copy more of the CG ESR1 haplotype is associated with a 2.5-fold higher hot flash score (P = .0026 for gene-dose effect; Appendix Figure A2, panel B, online only). A similar trend was maintained in premenopausal women after 1 and 4 months of tamoxifen treatment, although the P values were not statistically significant (data not shown).
In postmenopausal women, neither history of prior chemotherapy, SSRI/SNRI use, prior hormone therapy use, nor the ESR genotypes (Appendix Figure A2, panel C, online only) predicted baseline hot flash score. However, the sample size is too small to reach firm conclusions. The effects of these factors on the hot flash score in perimenopausal women could not be tested because of the small sample size.
Tamoxifen-Induced Changes in Weekly Hot Flash Composite Score
After initiation of tamoxifen, hot flash scores reached a plateau 4 months after the initiation of tamoxifen treatment in all women and did not change significantly after that time (Table 2 and online only Appendix Figure A4). Because many women were prescribed SSRI/SNRI after 4 months of tamoxifen, and because the number of patients without diaries increased at later time points, we used the comparison between baseline and 4 month values as the best indicator of tamoxifen-induced hot flashes.
In the 36 premenopausal women who had previously received adjuvant chemotherapy, hot flash scores increased from 19.6 ± 4.9 at baseline to 23.0 ± 6.8 at 4 months after tamoxifen treatment, a change that was not statistically significant (P = .57). In contrast, in 36 premenopausal women who did not receive chemotherapy, we observed a significant increase in hot flash scores from 5.9 ± 2.5 at baseline to 23.6 ± 9.4 after 4 months of tamoxifen treatment (P = .0015).
Tamoxifen-Induced Changes in Weekly Hot Flash Score and Genotype
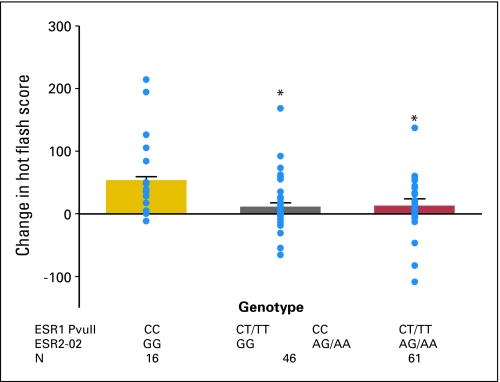
The effects of ESR genotypes on tamoxifen-induced hot flashes at 4 months were observed only in the postmenopausal group. In premenopausal women, ESR genotype did not predict changes in hot flash score. Among postmenopausal women, no obvious association was observed between tamoxifen-induced changes in hot flash score and ESR1 or ESR2 genotype. When interactions between ESR1 and ESR2-02 genotypes were considered (Fig 1), carriers of ESR1 PvuII CC and ESR2-02 GG genotype had significantly more increases in hot flash scores after tamoxifen treatment than the other genotype combinations (P = .0005). Because women who were homozygotes for both ESR1 PvuII CC and ESR2-02 GG genotype had the highest increases in hot flash composite scores after tamoxifen treatment, and because the women who were not homozygotes for either of these genotypes had the least increase, a significant gene-dose relationship was also observed (P = .0007).
Fig 1.
Effect of estrogen receptor (ESR) genotype on changes in hot flash composite scores after 4 months of tamoxifen treatment. ESR1 and ESR2 gene interactions and tamoxifen-associated change in hot flash score. (•) Individual data points; *P = .0005 for ESR1 PvuII CC and ESR2-02 GG group compared with other haplotypes.
Risk of Developing Hot Flashes During the First Year of Tamoxifen Treatment
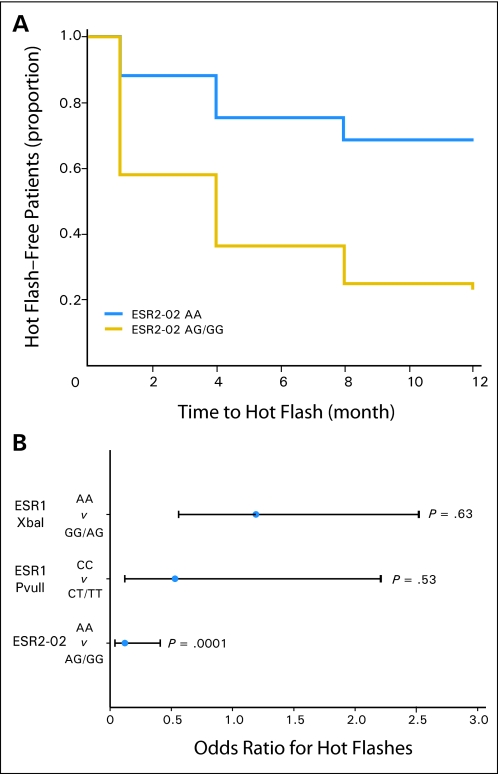
Among 297 participants, 242 (81%) reported hot flashes at least once, either before or during the study period, and 27 (9%) did not report any hot flashes in their returned diaries. The other 28 participants did not report hot flashes in their diaries, but they failed to follow-up at some point during the study; therefore, the hot flash status was censored at the last follow-up time point for these participants. The risk of developing hot flashes during the study period was analyzed with a Cox regression model. Menopausal status, history of chemotherapy, and the number of ESR1 GC haplotype alleles or ESR2-02 genotypes were included in the Cox regression model, which excluded participants who reported hot flashes before tamoxifen treatment. Women who carried a ESR2-02 AA genotype were significantly less likely to develop hot flashes compared with participants who had the AG/GG genotype (hazard ratio, 0.26; 95% CI, 0.10 to 0.63; P = .001; Fig 2A). If we included participants who reported hot flashes at baseline, those who had the ESR2-02 AA genotype were also significantly less likely to report hot flashes compared with participants who had the AG or GG genotype (OR, 0.12; 95% CI, 0.04-0.41; P = .0001; Fig 2B).
Fig 2.
Effect of estrogen receptor (ESR) genotypes on risk of developing hot flashes during the first year of tamoxifen treatment. (A) Kaplan-Meier curves for the effect of ESR2-02 AA genotype on hot flash–free survival during the first year of tamoxifen treatment (n = 115). Patients who had baseline hot flashes were excluded. (B) Odds ratio of experiencing hot flashes before and during the first year of tamoxifen treatment according to genotype. Error bars represent 95% CI (n = 122).
DISCUSSION
As previously documented in cross-sectional surveys,4,16,17 we observed that premenopausal women had a greater increase in tamoxifen-induced hot flashes compared with peri- or postmenopausal women. Prior history of chemotherapy may alter the risk of tamoxifen-induced hot-flashes in premenopausal women. In premenopausal women, an increase in the number of baseline ESR1 CG haplotypes was associated with higher hot flash scores. After initiation of tamoxifen, postmenopausal women homozygotes of the ESR1 PvuII CC and ESR2-02 GG genotype had the greatest increase (55.6 to v 11.9 to 12.5; Figure 1) in hot flash scores. Most notably, women who had the ESR2-02 AA genotype had a significantly lower risk for developing tamoxifen-induced hot flashes compared with women who had AG or GG genotypes regardless of menopausal status (OR, 0.12).
Women who report hot flashes during menopause are more likely to complain of tamoxifen-associated symptoms compared with other women.18 This observation suggests that genetic factors in the ESR signaling pathway may influence the risk for experiencing hot flashes. Several small cross-sectional studies have reported associations between hot flashes during natural menopause and polymorphisms in the ESR1 and ESR2 genes. In a study that evaluated the influence of ESR1 genotypes PvuII and XbaI on prevalence of hot flashes in 177 postmenopausal women, women in the ESR1 PvuII TT group were less likely to develop hot flashes compared with those in the ESR1 Pvull CT group,19 which is consistent with data in this study. In a study of 51 postmenopausal Japanese women, a short number of CA repeats of the ESR2 gene was associated with a 7.0-fold (range, 1.25- to 35.9-fold) increased the risk of hot flashes compared with women who had extremely short or long variants.20 Although intriguing, the sample size is small and the confidence interval wide. While we did not determine the CA repeat genotype for participants in this study, it is possible that both a short CA repeat genotype and the ESR2 associations observed in our study may result in a change in the expression or functionality of the estrogen receptor beta protein, and both factors may influence the risk for and severity of tamoxifen-induced hot flashes.
Genetic polymorphisms of ESR1 and ESR2 are extremely complex. Greater than 1,000 SNPs have been reported in ESR1, and there are hundreds of assumed haplotypes. The functional consequences of these variants are not well understood. As a result, we chose to assess the effects of the ESR SNPs that have already been shown to have functional impact. The role of other important SNPs in ESR1 and ESR2 genes will be studied in the future, when more data become available on the molecular functionality of SNPs in either of the estrogen receptors.
Other potential candidate genes may explain tamoxifen-induced hot flashes more specifically.21 We have previously demonstrated that polymorphisms in cytochrome P450 2D6 (CYP2D6) are associated with decreased conversion of the parent drug tamoxifen to its most active and abundant metabolite, endoxifen.12 Our group is currently developing bioinformatic tools to stratify participants by a CYP2D6 score that considers both genotype and concomitant medication.
In this study, we used subjective criteria to determine menopausal status. Future studies that include serum estrogen and pituitary hormone concentrations may help better correlate tamoxifen-induced changes in neuro-hormonal concentrations and menopausal symptoms. We used highly validated but subjective diaries to assess hot flash frequency and severity. Although it is possible that diary data may underestimate the frequency of hot flashes, we would expect a similar degree of underestimation in all genotype groups.22 We have also allowed SSRI/SNRI use, but these are agents that may reduce the frequency or severity of hot flashes and may, therefore, influence our results. However, most women who initiated new SSRI/SNRI prescriptions received a prescription after the 4-month visit, which somewhat reduced the bias. Finally, 20% and 40% of women were lost to follow-up 4 and 12 months after tamoxifen treatment, respectively, a rate that is comparable with that of the Breast Cancer Prevention Trial P-1 trial and with studies that evaluated treatments for hot flashes.4,23 The impact of these lost data points may also influence the observed associations between ESR1 and ESR2 and hot flashes, because patients who experienced worse hot flashes may have dropped out at a greater rate. If specific genotypes predispose women to hot flashes and lead to drug discontinuation, it is possible that such genes are over-represented in a drop-out group and under-represented in the studied group. Thus, we may actually underestimate the genotype effects on hot flashes.
In conclusion, we have demonstrated that specific ESR genotypes may also influence hot flash risk and score in breast cancer patients before and after tamoxifen treatment. If our findings are confirmed in other datasets or prospective studies, evaluation of risk factors for hot flashes may enable clinicians to provide prospective counseling and symptom management in women with breast cancer who are recommended tamoxifen.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Yan Jin, Eli Lilly (C); Daniel F. Hayes, Eli Lilly (C); Anna Maria Storniolo, Eli Lilly (C) Consultant or Advisory Role: Todd C. Skaar, Roche Diagnostics (C); Anna Maria Storniolo, Eli Lilly (C); David A. Flockhart, LabCorp (C), Roche Diagnostics (C); Vered Stearns, Wyeth (C), Concert Pharmaceuticals (C), JDS Pharmaceuticals (C) Stock Ownership: None Honoraria: Todd C Skaar, Roche Diagnostics; Anna Maria Storniolo, GlaxoSmithKline Research Funding: Daniel F. Hayes, AstraZeneca, GlaxoSmithKline, Novartis, Pfizer; Anna Maria Storniolo, GlaxoSmithKline; David A. Flockhart, Novartis, Pfizer; Vered Stearns, GlaxoSmithKline, Novartis, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Yan Jin, Daniel F. Hayes, Lang Li, Anne Nguyen, David A. Flockhart, Vered Stearns
Financial support: Daniel F. Hayes, David A. Flockhart, Vered Stearns
Administrative support: Daniel F. Hayes, Anne Nguyen, Jill Hayden, Suzanne Lemler, David A. Flockhart, Vered Stearns
Provision of study materials or patients: Daniel F. Hayes, Anne Schott, Jill Hayden, Suzanne Lemler, Anna Maria Storniolo, David A. Flockhart, Vered Stearns
Collection and assembly of data: Yan Jin, Daniel F. Hayes, Lang Li, Jason D. Robarge, Todd C. Skaar, Santosh Philips, Anne Nguyen, Jill Hayden, Suzanne Lemler, Anna Maria Storniolo, David A. Flockhart, Vered Stearns
Data analysis and interpretation: Yan Jin, Daniel F. Hayes, Lang Li, Jason D. Robarge, Todd C. Skaar, Santosh Philips, Anne Nguyen, Anne Schott, Anna Maria Storniolo, David A. Flockhart, Vered Stearns
Manuscript writing: Yan Jin, Daniel F. Hayes, Lang Li, Jason D. Robarge, Todd C. Skaar, Santosh Philips, Anne Nguyen, Anne Schott, Anna Maria Storniolo, David A. Flockhart, Vered Stearns
Final approval of manuscript: Yan Jin, Daniel F. Hayes, Lang Li, Jason D. Robarge, Todd C. Skaar, Santosh Philips, Anne Nguyen, Anne Schott, Jill Hayden, Suzanne Lemler, Anna Maria Storniolo, David A. Flockhart, Vered Stearns
Acknowledgments
We thank Claudine Isaacs, MD, Lynda Ullmer, and Ann Gallagher for their invaluable help in the enrollement and monitoring of study participants.
Appendix
Fig A1.
Hot flash prevalence rate before and during the first year of tamoxifen. †P < .01 compared with premenopausal group; *P = .015 compared with premenopausal group.
Fig A2.
Effects of estrogen receptor (ESR) genotype on hot flash score before tamoxifen. The number of participants in each genotype is in parentheses. (Error bars) Standard errors of the mean. (A) Effect of ESR genotype on hot flash score before tamoxifen treatment in premenopausal women. *P = .015 when ESR1 XbaI GG group was compared with AG and AA groups (P = .025 for gene-dose effect.); #P = .025 when ESR1 PvuII CC group was compared with CT and TT groups (P = .032 for gene-dose effect). (B) Effect of ESR1 CG haplotype on hot flash score before tamoxifen treatment in premenopausal women. (•) Individual data points; P = .0026 for gene-dose effect. (C) Hot flash score by ESR genotypes before tamoxifen treatment in postmenopausal women. None of the differences was statistically significant.
Table A1.
ESR Allelic Frequency
| Genotype | Frequency (%)
|
P* | |||
|---|---|---|---|---|---|
| Overall | Menopausal Status
|
||||
| Premenopausal | Perimenopausal | Postmenopausal | |||
| ESR1 PvuII | |||||
| C | 48.3 | 51.1 | 47.1 | 46.5 | .75 |
| T | 51.7 | 48.9 | 52.9 | 53.5 | |
| ESR1 XbaI | |||||
| G | 34.6 | 40.1 | 33.8 | 32.9 | .39 |
| A | 65.4 | 59.9 | 66.2 | 67.1 | |
| ESR 2-02 | |||||
| A | 35.6 | 31.3 | 31.1 | 37.9 | .57 |
| G | 64.4 | 68.7 | 68.9 | 62.1 | |
Abbreviation: ESR, estrogen receptor.
P value is for a comparison between different menopausal groups.
published online ahead of print at www.jco.org on November 17, 2008
Supported in part by Pharmacogenetics Research Network Grants No. U-01 GM61373 (D.F.) and R-01 GM56898 (D.F.); Clinical Pharmacology Training Grant No. 5T32-GM-08425 (D.F.) from the National Institute of General Medical Sciences; Damon Runyon-Lilly Clinical Investigator award CI-3 from the Damon Runyon Cancer Research Foundation (V.S.); Fashion Footwear Foundation/QVC Presents Shoes on Sale (D.F.H.); and the General Clinical Research Centers at the University of Michigan (Grant No. M01-00042 from the National Institutes of Health), Georgetown University (Grant No. M01-RR13297 from the National Institutes of Health), and Indiana University (Grant No. M01-RR00750 from the National Institutes of Health).
Presented in part at the 108th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, March 21-24, 2007, Anaheim, CA; and at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00228930
REFERENCES
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Powles T, Veronesi U, et al: Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296-300, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Stearns V, Ullmer L, Lopez JF, et al. Hot flushes. Lancet 360:1851-1861, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Day R: Quality of life and tamoxifen in a breast cancer prevention trial: A summary of findings from the NSABP P-1 study. Ann NY Acad Sci 949:143-150, 2001 [PubMed] [Google Scholar]
- 5.Fallowfield L: Acceptance of adjuvant therapy and quality of life issues. The Breast 14:612-616, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Herrington DM, Howard TD, Hawkins GA, et al: Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med 346:967-974, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Kurabayashi T, Matsushita H, Tomita M, et al: Association of vitamin D and estrogen receptor gene polymorphism with the effects of longterm hormone replacement therapy on bone mineral density. J Bone Miner Metab 22:241-247, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cai Q, Shu XO, Jin F, et al: Genetic polymorphisms in the estrogen receptor α gene and risk of breast cancer: Results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev 12:853-859, 2003 [PubMed] [Google Scholar]
- 9.Boyapati SM, Shu XO, Ruan ZX, et al: Polymorphisms in ER-α gene interact with estrogen receptor status in breast cancer survival. Clin Cancer Res 11:1093-1098, 2005 [PubMed] [Google Scholar]
- 10.Schuit SC, Oei HH, Witteman JC, et al: Estrogen receptor α gene polymorphisms and risk of myocardial infarction. JAMA 291:2969-2977, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ntukidem NI, Nguyen AT, Stearns V, et al: Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen. Clin Pharmacol Ther 83:702-710, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Desta Z, Stearns V, et al: CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30-39, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Sloan JA, Loprinzi CL, Novotny PJ, et al: Methodologic lessons learned from hot flash studies. J Clin Oncol 19:4280-4290, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Schaid DJ, Rowland CM, Tines DE, et al: Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425-434, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens M, Smith NJ, Donnelly P: A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978-989, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandall C, Petersen L, Ganz PA, et al: Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11:519-530, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Biglia N, Cozzarella M, Cacciari F, et al: Menopause after breast cancer: A survey on breast cancer survivors. Maturitas 45:29-38, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi CL, Zahasky KM, Sloan JA, et al: Tamoxifen-induced hot flashes. Clin Breast Cancer 1:52-56, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Malacara JM, Perez-Luque EL, Martinez-Garza S, et al: The relationship of estrogen receptor-α polymorphism with symptoms and other characteristics in post-menopausal women. Maturitas 49:163-169, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Takeo C, Negishi E, Nakajima A, et al: Association of cytosine-adenine repeat polymorphism of the estrogen receptor-beta gene with menopausal symptoms. Gend Med 2:96-105, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Goetz MP, Rae JM, Suman VJ, et al: Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312-9318, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Carpenter JS, Monahan PO, Azzouz F: Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol 104:1322-1326, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Stearns V, Slack R, Greep N, et al: Paroxetine is an effective treatment for hot flashes: Results from a prospective randomized clinical trial. J Clin Oncol 23:6919-6930, 2005 [DOI] [PubMed] [Google Scholar]