Abstract
Purpose
Endometrial carcinoma in the lower uterine segment (LUS) is a poorly described cancer that can be clinically confused with endocervical carcinoma. We performed a case-comparison study to document the clinicopathologic characteristics of LUS tumors and their association with risk factors for endometrial cancer.
Patients and Methods
The clinical records and pathology reports from women who underwent hysterectomy at our institution for endometrial or endocervical adenocarcinoma over an 11-year interval were reviewed. The LUS group consisted of women with endometrial tumors that clearly originated between the lower uterine corpus and the upper endocervix. Immunohistochemistry and microsatellite instability and MLH1 methylation assays were performed.
Results
Thirty-five (3.5%) of 1,009 women had endometrial carcinoma of the LUS. Compared with patients with corpus tumors, LUS patients were younger, had higher stage tumors, and had more invasive tumors. Preoperative diagnosis of the LUS tumors more frequently included the possibility of endocervical adenocarcinoma. Seventy-three percent of the LUS tumors had an immunohistochemical expression pattern typical of conventional endometrioid adenocarcinoma. Ten (29%) of 35 women with LUS tumors were confirmed to have Lynch syndrome or were strongly suspected to have Lynch syndrome on the basis of tissue-based molecular assays.
Conclusion
The prevalence of Lynch syndrome in patients with LUS endometrial carcinoma (29%) is much greater than that of the general endometrial cancer patient population (1.8%) or in endometrial cancer patients younger than age 50 years (8% to 9%). On the basis of our results, the possibility of Lynch syndrome should be considered in women with LUS tumors.
INTRODUCTION
Uterine corpus cancer is the most common gynecologic cancer in the United States and the fourth most common cancer in women. The American Cancer Society estimated that there will be 40,100 new cases of endometrial carcinoma in 2008 and that 7,470 women will die from this disease.1 Pathologically, the endometrium comprises two distinct areas, the uterine corpus proper (UC) and the lower uterine segment (isthmus; LUS).2 Endometrial carcinoma most commonly arises in the mucosa of the UC, which includes the body and fundus of the uterus. The mucosal layer of the lower uterine segment (LUS) is thin compared with the corpus mucosa and is much less responsive to hormonal stimulation.3 Because the LUS is located between the corpus proper and the endocervix, the glands and stroma of the LUS have a hybrid appearance that incorporates features from both anatomic areas.2
Clinically, LUS tumors may be confused with endocervical adenocarcinomas because of similarities in presentation such as an enlarged cervix, tumor protruding through the cervix, and vaginal bleeding. This is particularly important from a treatment perspective, as an enlarged endocervical tumor is often treated with primary chemoradiotherapy, including external-beam radiation therapy. In contrast, an endometrial tumor with possible cervical involvement can be treated with surgery alone, with the addition of adjuvant vaginal brachytherapy if cervical involvement is confirmed.4
Immunohistochemistry is a powerful tool to help distinguish between endometrial and endocervical tumors. It is well established in the literature that endometrial adenocarcinomas express estrogen receptor (ER) and vimentin and are negative for carcinoembryonic antigen (CEA).5,6 In contrast, endocervical adenocarcinomas are typically negative for ER and vimentin but do express cytoplasmic CEA.5,6 The use of p16 immunohistochemistry and human papillomavirus (HPV) in situ hybridization has also proven useful in confirming diagnosis of endocervical adenocarcinoma.7-9 This battery of immunohistochemical and in situ hybridization tests are part of standard pathology practice. However, they have not been systematically applied to a large series of LUS carcinomas.
A few reports have described endometrial carcinomas that arise solely from the LUS.10-14 These studies are plagued by small sample sizes and an inconsistent definition of an LUS tumor. The largest of these four studies included 18 so-called isthmic tumors but provided no definition about this phenotype and lacked a central pathologic review.10 We hypothesized that tumors that arose in the LUS would have a unique set of clinical and pathologic characteristics. Thus, we performed a retrospective analysis to compare endometrial carcinomas arising in the LUS with endometrial carcinomas of the UC, and we described these tumor types, with particular focus on clinical characteristics and immunohistochemical expression patterns. As the study unfolded, we uncovered a significant and previously unreported association of LUS tumors with Lynch syndrome.
PATIENTS AND METHODS
Patient Population and Study Design
After obtaining institutional review board approval, we identified all women diagnosed with endometrial adenocarcinoma or endocervical adenocarcinoma between January 1996 and October 2007, from The University of Texas M. D. Anderson Cancer Center (MDACC) Tumor Registry Database. This list was cross-referenced with a list of all hysterectomy specimens submitted to the MDACC Department of Pathology during this same period.
Possible LUS tumors were identified through detailed review of pathologic records of all patients who underwent hysterectomy for endometrial or endocervical adenocarcinoma. The criterion for this diagnosis was stringent and included only patients with tumors specifically described as originating or arising in the LUS. Patients were not included if there was tumor present in any other part of the uterus. Microscopically, the absence of endocervical adenocarcinoma in situ and the presence of adjacent endometrial hyperplasia were used to accurately classify tumors arising in the LUS as endometrial carcinomas rather than endocervical adenocarcinomas. All diagnoses were verified by a gynecologic pathologist (R.R.B.) via microscopic examination of slides stained with hematoxylin and eosin.
The group of patients with endometrial carcinoma arising in the LUS was compared with a group of patients with endometrial carcinoma arising in the UC. Large tumors that involve the entire endometrial cavity, including the LUS, are relatively common. Thus, 22 (28%) of 79 of the tumors in the UC group also involved the LUS. The UC comparison group comprised all women identified through Tumor Registry and Pathology Databases as having undergone hysterectomy for treatment of uterine carcinoma in 2001. The year 2001 was selected arbitrarily from the time span of our study and contained a number of cases comparable with that of an average year in our institution. The choice of this group by year of diagnosis allowed for a reasonable comparison group without inadvertently eliminating the ability to detect differences in variables between the two groups. Only patients who did not undergo hysterectomy at our institution were excluded.
Demographic, clinical, and pathologic data were collected. Stage was determined using the criteria established by the International Federation of Gynecology and Obstetrics.15 Amsterdam II criteria (ie, personal and family history of cancer) were used to identify Lynch syndrome patients.16
Molecular Analyses
We performed immunohistochemistry using formalin-fixed, paraffin-embedded sections of LUS carcinoma, if it had not been previously performed during the diagnostic work-up. Immunohistochemistry was performed using standard techniques for ER (6F11, 1:35; Novacastra/Leica Microsystems, Chicago, IL), vimentin (V9[1], 1:900; Dako, Carpenteria, CA), CEA (AB-2, 1:200; Labvision/Neomarkers, Freemont, CA), and p16INK4a (16P07, 1:40; Labvision/Neomarkers). Protein expression was scored as present if ≥ 10% of tumor cells demonstrated strong expression. To test for exposure to HPV, we performed in situ hybridization using established techniques (HPV111 Family 16, Predilute; Ventana, Tuscon, AZ).
In addition, immunohistochemistry was performed for DNA mismatch repair gene products MLH1 (G168-15, 1:25; BD Biosciences Pharmingen, San Diego, CA), MSH2 (FE11, 1:100; Calbiochem, La Jolla, CA), MSH6 (44, 1:300; BD Biosciences Pharmingen), and PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen). Stromal and normal tissues within the tumor served as internal controls.
Tumors that demonstrated a loss of DNA mismatch repair gene product expression underwent microsatellite instability (MSI) analysis using a panel of six National Cancer Institute-recommended microsatellites as previously described.17 Tumors were designated as MSI high if they demonstrated allelic shift at two or more markers and MSI low with allelic shift in one marker. Tumors without allelic shift were considered microsatellite stable. A methylation-specific polymerase chain reaction MLH1 promoter methylation assay was performed on MSI-high tumors, which demonstrated loss of MLH1 protein expression as described previously.18
Statistical Analysis
Demographic, clinical, and pathologic variables were compared between the two groups using Fisher's exact, χ2, Mann-Whitney, and t tests. Where appropriate, 95% confidence intervals (CI) for point estimates were obtained using the adjusted Wald method. A P value of < .05 was considered statistically significant. Statistical analysis was performed using SPSS 15.0 (SPSS Inc, Chicago, IL) software.
RESULTS
Clinical and Pathologic Features
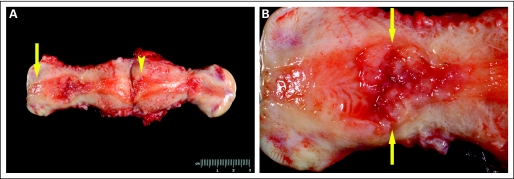
There were 3,892 patients identified with endometrial or endocervical adenocarcinoma at MDACC from January 1996 through October 2007. Of those, 1,009 underwent hysterectomy at MDACC or an affiliated hospital. After pathologic review, 35 patients (3.5%; 95% CI, 2% to 4%) were identified as having endometrial tumors that arose solely in the LUS. Figure 1 illustrates a typical LUS endometrial carcinoma.
Fig 1.
(A) Uterus with endometrial carcinoma arising in the lower uterine segment (LUS). The cervix is designated by an arrow. Note the absence of tumor in the uterine fundus (arrowhead). (B) LUS tumor at higher resolution. Note the location of the tumor (arrows) between the corpus and endocervix.
Table 1 summarizes the clinical features of LUS tumors compared with those of UC tumors. Patients with LUS tumors were significantly younger (54.2 v 62.9 years, P = .001). In addition, there was more preoperative clinical uncertainty in patients with LUS tumors. Of note, nine patients had a preoperative diagnosis that included the possibility of endocervical adenocarcinoma. Four of these patients underwent radiation therapy before definitive surgical management.
Table 1.
Clinical Characteristics of LUS Tumors and UC Tumors
| Characteristic | LUS Tumor
|
UC Tumor
|
|||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | P | |
| Median age at diagnosis, years | 54.2 | 62.9 | .001 | ||
| Median body mass index, kg/m2 | 31.1 | 33.6 | .334 | ||
| Median parity | 2.0 | 2.0 | .583 | ||
| Cesarean section | |||||
| No | 29 | 85.3 | 76 | 96.2 | .052 |
| Yes | 5 | 14.7 | 3 | 3.8 | |
| Preoperative diagnosis | |||||
| Endometrial carcinoma | 24 | 68.6 | 78 | 98.7 | |
| Endometrial carcinoma | |||||
| v endocervical carcinoma | 9 | 25.7 | 0 | 0.0 | < .001 |
| No carcinoma present* | 2 | 5.7 | 1 | 1.3 | |
Abbreviations: LUS, lower uterine segment; UC, uterine corpus.
Two hysterectomies were performed for benign indications, including adnexal mass and leiomyomas. One hysterectomy was performed prophylactically secondary to known Lynch syndrome.
Magnetic resonance imaging (MRI) can potentially be used to clinically distinguish between endometrial and endocervical adenocarcinoma.19 Although the LUS group had a higher proportion of preoperative MRIs compared with the UC group (28.6% v 12.7%; P = .039), this study was often not helpful in distinguishing between the two tumor types. Of the nine patients with uncertain preoperative diagnoses, five had MRIs from which we were not able to definitively diagnose the cancer origin. Furthermore, two of the patients with LUS tumors were given the radiologic diagnosis of probable endocervical adenocarcinoma after MRI.
Pathologically, the LUS tumors demonstrated a higher stage at diagnosis compared with UC tumors (Table 2), which may be attributed to the higher proportion of stage II tumors in the LUS group compared with the UC group (33.3% v 3.8%). Interestingly, the median depth of myometrial wall invasion for the LUS group was significantly greater than that for the UC group (48.0% v 15.0%; P = .001). However, there was no difference in median thickness of the uterine wall at the deepest point of invasion between LUS tumors and UC tumors (17.5 v 19.0 mm; P = .881). LUS tumors were also significantly smaller in greatest median diameter compared with UC tumors (18.5 v 40.0 mm; P < .001).
Table 2.
Pathologic Characteristics of LUS Tumors and UC Tumors
| Characteristic | LUS Tumor
|
UC Tumor
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Presence of hyperplasia | |||||
| Yes | 12 | 34.3 | 24 | 30.4 | .670 |
| No | 23 | 65.7 | 55 | 69.6 | |
| Histology | |||||
| Endometrioid | 27 | 77.1 | 56 | 70.9 | .649 |
| Nonendometrioid | 8 | 22.9 | 23 | 29.1 | |
| Grade of endometrioid | |||||
| 1 | 4 | 14.8 | 20 | 36.4 | .131 |
| 2 | 17 | 63.0 | 26 | 47.3 | |
| 3 | 6 | 22.2 | 9 | 16.4 | |
| Lymphovascular space invasion | |||||
| Positive | 5 | 14.3 | 32 | 40.5 | .009 |
| Negative | 30 | 85.7 | 47 | 59.5 | |
| Stage | |||||
| I | 17 | 51.5 | 60 | 76.9 | < .001 |
| II | 11 | 33.3 | 3 | 3.8 | |
| III | 4 | 12.1 | 10 | 12.8 | |
| IV | 1 | 3.0 | 5 | 6.4 | |
| Median myometrial wall invasion, % | 48.0 | 15.0 | .001 | ||
| Median tumor size, mm | 18.5 | 40.0 | < .001 | ||
Abbreviations: LUS, lower uterine segment; UC, uterine corpus.
There was no difference in histology or grade of endometrioid type tumors between the two groups. In contrast to the results of previous, smaller studies of LUS tumors,11,12 UC tumors were found to have a higher proportion of lymphovascular space invasion when compared with LUS tumors (40.5% v 14.3%; P = .008).
Table 3 summarizes data for immunohistochemical and in situ hybridization properties of the LUS tumors. Tumors that had a component of endometrioid-type histology were included in immunohistochemical testing analysis. Of 31 endometrioid-type LUS tumors, 26 had sufficient tissue for immunohistochemical testing. Of those, 92% had positive ER expression, and 96% had positive vimentin expression. CEA expression was absent in 80% of the LUS tumors, and HPV in situ hybridization was uniformly negative (100%). Generally, LUS tumors in this study had a similar protein expression pattern compared with conventional endometrial carcinoma.5,6 Nineteen (73%) of 26 LUS tumors had the typical endometrial endometrioid carcinoma immunohistochemical profile: ER positive, vimentin positive, and CEA negative. Five additional tumors were positive for ER, vimentin, and CEA. The final two cases had expression patterns that differed significantly from that of typical endometrial carcinoma. One undifferentiated carcinoma had a profile of ER negative, vimentin positive, and CEA positive. The final tumor was of mixed histology, including undifferentiated and clear cell carcinoma, and was negative for ER, vimentin, and CEA. Eighty-three percent of LUS tumors demonstrated some level of p16 protein expression, and 46% had strong p16 expression in at least 50% of tumor cells.
Table 3.
Immunohistochemical Features of LUS Tumors
| % Tumor Cells Positive | Level of Expression (%)
|
||||
|---|---|---|---|---|---|
| ER | Vimentin | CEA | HPV | p16 | |
| 0 | 8.0 | 4.5 | 61.5 | 100.0 | 16.7 |
| 1-9 | 0.0 | 0.0 | 19.2 | 0.0 | 0.0 |
| 10-50 | 20.0 | 54.5 | 15.4 | 0.0 | 50.0 |
| 51-75 | 40.0 | 13.6 | 0.0 | 0.0 | 8.3 |
| 76-100 | 32.0 | 27.3 | 3.8 | 0.0 | 25.0 |
NOTE: Typical pattern for endometrioid endometrial carcinoma is ER positive, vimentin positive, CEA negative, and HPV negative. Typical pattern for endocervical adenocarcinoma is ER negative, vimentin negative, CEA positive, and HPV positive. Typically, p16 is variably positive in both endocervical and endometrial adenocarcinoma, with more diffuse expression in endocervical tumors. Expression was scored as present if > 10% of tumor cells demonstrated strong marker expression.
Abbreviations: LUS, lower uterine segment; ER, estrogen receptor; CEA, carcinoembryonic antigen; HPV, human papillomavirus.
Lynch Syndrome and LUS Tumors
The significantly younger age of the LUS group prompted us to consider whether women with an LUS tumor had a hereditary disposition. Therefore, to evaluate for the possibility of Lynch syndrome in the LUS group, we performed a medical record review for personal and family history of cancer in accordance with Amsterdam II criteria. Five patients in this cohort met criteria, and Lynch syndrome diagnosis was confirmed in the medical record. All five had a germline mutation in MSH2 and had MSI-high LUS tumors with immunohistochemical loss of MSH2 (Table 4 and Appendix). Of note, there were no patients in the UC tumor group who met Amsterdam II criteria.
Table 4.
Summary of LUS Patients With Germline Mutations or Abnormal IHC or MSI Results
| Pt | IHC
|
MSI Status | MLH1Hypermethylation | Mutation | Age at Diagnosis | Amsterdam II Criteria | First-Degree Relative With LS-Associated Cancer | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | PMS2 | |||||||
| 1 | + | − | − | + | MSI-H | NP | MSH2 | 39 | Positive | Yes |
| 2 | + | − | − | + | MSI-H | NP | MSH2 | 41 | Positive | Yes |
| 3 | + | − | − | + | MSI-H | NP | MSH2 | 48 | Positive | Yes |
| 4 | + | − | − | + | MSI-H | NP | MSH2 | 49 | Positive | Yes |
| 5 | + | − | − | + | MSI-H | NP | MSH2 | 57 | Positive | Yes |
| 6 | + | − | − | + | MSI-H | NP | Neg | 39 | Negative | No |
| 7 | + | − | − | + | MSI-H | NP | NP | 48 | Negative | No |
| 8 | + | − | − | + | MSI-H | NP | NP | 59 | Negative | No |
| 9 | + | − | − | + | MSI-H | NP | NP | 81 | Negative | No |
| 10 | − | + | + | − | MSI-H | Negative | NP | 44 | Negative | No |
| 11 | − | + | + | − | MSI-H | Present | NP | 62 | Negative | No |
| 12 | − | + | + | − | MSI-H | Present | NP | 90 | Unavailable | Unavailable |
Abbreviations: LUS, lower uterine segment; IHC, immunohistochemistry; MSI, microsatellite instability; LS, Lynch syndrome; MSI-H, MSI high; NP, not performed; Neg, negative.
For the remainder of the LUS cohort (n = 25), we performed immunohistochemistry for DNA mismatch repair gene products MLH1, MSH2, MSH6, and PMS2, and when sufficient tissue was present, we also performed MSI analysis. We performed MLH1 promoter hypermethylation analysis for those tumors with immunohistochemical loss of MLH1 expression. We found four additional women with a probable diagnosis of Lynch syndrome, as demonstrated by loss of MSH2 and MSH6 protein expression identified by immunohistochemistry. This protein expression pattern is virtually pathonogmonic for a mutation in MSH2. In addition, in all four of these women, tumors were noted to be MSI high. One of these women underwent germline testing, with a negative result. However, given the results of her molecular studies, she received a presumptive diagnosis of Lynch syndrome. We are attempting to contact the remaining three women to request that they undergo testing. Interestingly, although two of the four women were relatively young (39 and 49 years), none had a first-degree relative with a Lynch syndrome–associated cancer (Table 4).
There were three women with loss of MLH1 protein expression in their tumor (patients 10 through 12; Table 4). Loss of this gene product can be associated with sporadic carcinoma caused b MLH1 methylation; therefore, we performed MSI analysis and MLH1 promoter hypermethylation testing. All three tumors were noted to be MSI high, but only two (patients 11 and 12) had MLH1 promoter hypermethylation. Thus, patient 10 received the likely diagnosis of Lynch syndrome. The tumors from the 12 patients summarized in Table 4 did not have unique immunohistochemical expression patterns with respect to ER, CEA, vimentin, p16, and HPV (data not shown).
The final prevalence of Lynch syndrome in our LUS population is 10 of 35 (29%; 95% CI, 16.0% to 45.0%). Among patients in the LUS tumor group, those with Lynch syndrome were significantly more likely to have a first-degree relative with a Lynch syndrome–associated cancer (50.0% v 4.8%; P = .007; Table 5 and Appendix). Other than family history, none of the clinical or pathological variables reliably distinguished Lynch syndrome–associated LUS tumors from sporadic LUS tumors.
Table 5.
Clinical and Pathologic Characteristics of LUS Patients With Lynch Syndrome Compared With LUS Patients Without Lynch Syndrome
| Characteristic | LUS With Lynch Syndrome
|
LUS Without Lynch Syndrome
|
P | 95% CI | ||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |||
| Mean age at diagnosis, years | 50.9 | 55.6 | .330 | −4.56 to +13.88 | ||
| Mean body mass index, kg/m2 | 30.3 | 35.1 | .194 | −3.08 to +12.64 | ||
| First-degree relative with Lynch syndrome–associated cancer | −0.76 to −0.10 | |||||
| Yes | 5 | 50.0 | 1 | 4.8 | .007 | |
| No | 5 | 50.0 | 20 | 95.2 | ||
| Histology | −0.08 to +0.58 | |||||
| Endometrioid | 6 | 60.0 | 21 | 84.0 | .186 | |
| Nonendometrioid | 4 | 40.0 | 4 | 16.0 | ||
| Grade of endometrioid | ||||||
| 1 | 1 | 16.7 | 3 | 14.3 | .807 | 1 v 2: −0.61 to +0.31 |
| 2 | 3 | 50.0 | 14 | 66.7 | ||
| 3 | 2 | 33.3 | 4 | 19.0 | 1 v 3: −0.59 to +0.65 | |
| 2 v 3: −0.26 to +0.65 | ||||||
Abbreviation: LUS, lower uterine segment.
DISCUSSION
In the present study, we found that the prevalence of LUS endometrial carcinomas was 3.5% among women who had undergone hysterectomy for endometrial carcinoma or endocervical adenocarcinoma. Our case-comparison study is the largest assessment of LUS tumors in the literature and provides a compelling association between these tumors and Lynch syndrome. To our knowledge, this is the first study to report an association between LUS tumors and Lynch syndrome. Seiden et al recently reported the case of a 46-year-old woman with three primary tumors in the endometrium, ovary, and descending colon. Interestingly, the endometrial tumor was located in the LUS, and the patient was subsequently diagnosed with Lynch syndrome, with a mutation in MSH2.20
The 29% prevalence of Lynch syndrome among women diagnosed with LUS tumors in the present study is impressive when compared with the 1% to 2% prevalence in the general population.21-23 In a study by our group and in a Dutch study, the prevalence of Lynch syndrome in women younger than 50 years was 9%.17,24 Thus, our data suggest that the association between LUS tumors and the presence of Lynch syndrome is the strongest reported association of any tumor type with Lynch syndrome.
The reason for the relatively high occurrence of Lynch syndrome among women with LUS tumors Lynchis not known. As noted previously, the glands and stroma of the LUS have a unique microscopic appearance compared with the remainder of the endometrium. Perhaps the LUS epithelium is more susceptible to mismatch repair errors, just as there is a predilection for right-sided colon tumors in Lynch syndrome.25
Among the patients with LUS tumors, those with a first-degree relative with a Lynch syndrome–associated cancer (ie, endometrial, colorectal, small bowel, ureter, or renal pelvis) were more likely to be diagnosed with Lynch syndrome. This finding is supported by other population-based studies which indicate that in the absence of full Amsterdam II criteria, the presence of a first-degree relative with a Lynch syndrome–associated cancer has predictive value.17,24
With regard to overall prevalence of LUS tumors in patients with endometrial carcinoma, our finding of 3.5% is concordant with the reported frequency in the literature, which ranges from 3% to 8%.10-12 The first description of LUS tumors in a prospective study found that 18 (8.1%) of 222 patients possessed a tumor that originated in the isthmus/cervix region.10 Another large descriptive study of 204 surgical cases of endometrial cancer in a Japanese university hospital reported that eight (3%) originated in the LUS.11 Although our prevalence findings are in agreement with these studies, we were unable to confirm others’ findings that LUS tumors were associated with more aggressive features, such as high-grade histology,11,12,14 lymphovascular space invasion,11,12 and adnexal involvement.10
Young age at diagnosis among patients with LUS tumors has been reported in several studies, including the present one.11,14 Interestingly, in a Japanese study of 88 patients younger than < 50 years diagnosed with endometrial carcinoma, 18% had LUS tumors.14 In our study, 151 patients younger than 50 years were diagnosed with endometrial carcinoma . Of those, only 13 (9%) had tumors arising in the LUS. Given that the present study and the Japanese study used identical definitions of LUS tumor, we surmise that this disparity in prevalence may be related to inherent differences in the epidemiology and genetics of endometrial cancer between the Japanese and Western populations. This topic has yet to be explored with regard to endometrial carcinoma.
We feel confident that the LUS tumors in our study were appropriately included, given the rigorous case definition for inclusion in our cohort. It is important to contrast this type of tumor with tumors that involve, but do not arise from, the LUS. These carcinomas do not arise solely in the isthmus, but rather include tumors that originate in the UC and extend to the LUS. Recently, substantial data regarding this clinical entity have confused the literature on this subject, suggesting that tumors that involve the LUS and those that arise solely in the LUS are equivalent.26,27 Our findings indicate that tumors that arise in the LUS are a subtype of endometrial cancer with clinical features and risk factors.
MRI has been advocated as a method to distinguish between endometrial and endocervical adenocarcinoma preoperatively. A study of 56 patients who presented with a cervical mass of unclear origin indicated that certain findings, such as endometrial cavity expansion from a mass, could be used to distinguish endometrial tumors.19 In our study, MRI was not helpful in distinguishing LUS tumors from endocervical tumors; however, we did find immunohistochemistry useful for this distinction.
We found that a majority of LUS tumors had protein expression patterns similar to the pattern established for UC endometrial tumors. The use of a panel of ER, vimentin, and CEA immunohistochemistry along with HPV in situ hybridization is a reasonable method to classify LUS tumors pathologically. Use of this panel, in concert with thorough light microscopic analysis to detect cervical endometrial hyperplasia or endocervical adenocarcinoma in situ, can help to accurately classify a tumor that arises in the LUS. Accurate diagnosis has the potential to reduce unnecessary preoperative treatment and guide decision making in the evaluation of Lynch syndrome.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shannon N. Westin, Robin A. Lacour, Diana L. Urbauer, Karen H. Lu, Russell R. Broaddus
Financial support: Russell R. Broaddus
Administrative support: Shannon N. Westin
Provision of study materials or patients: Shannon N. Westin, Rajyalakshmi Luthra, Karen H. Lu, Russell R. Broaddus
Collection and assembly of data: Shannon N. Westin, Robin A. Lacour, Russell R. Broaddus
Data analysis and interpretation: Shannon N. Westin, Robin A. Lacour, Diana L. Urbauer, Rajyalakshmi Luthra, Diane C. Bodurka, Karen H. Lu, Russell R. Broaddus
Manuscript writing: Shannon N. Westin, Robin A. Lacour, Diane C. Bodurka, Karen H. Lu, Russell R. Broaddus
Final approval of manuscript: Shannon N. Westin, Robin A. Lacour, Diana L. Urbauer, Rajyalakshmi Luthra, Diane C. Bodurka, Karen H. Lu, Russell R. Broaddus
Acknowledgments
We thank Matthew B. Schabath, PhD and Mary Sarah Baraniuk, PhD from the University of Texas School of Public Health, Houston, TX for their assistance in the design and analysis of this project.
Appendix
Of the overall endometrial carcinoma patient population at M. D. Anderson Cancer Center, 78% had endometrioid carcinomas and 22% had nonendometrioid carcinomas. This is a proportion similar to that often quoted in the endometrial cancer literature.
We have previously shown that MSI analysis by itself is a relatively weak predictor of subsequent detection of a Lynch syndrome mutation, with a positive predictive value of only 38%. This is due in large part to sporadic MSI-high endometrial carcinomas with MLH1 methylation. However, immunohistochemical loss of MSH2 protein in an endometrial carcinoma is associated with 64% probability of detecting a Lynch syndrome mutation.18
MSH2/MSH6 and MLH1/PMS2 form heterodimer pairs to function in DNA mismatch repair. It should be noted in Table 4 that immunohistochemical loss of MLH1 protein is usually associated with concomitant loss of PMS2, whereas loss of MSH2 protein is usually associated with loss of MSH6. Interestingly, mutation of MSH6 or PMS2 does not appear to result in loss of MSH2 or MLH1 protein, respectively (Chang DK, Ricciardiello L, Goel A, et al. J Biol Chem 275: 18424-18431, 20002).
The negative statistical comparisons for age at diagnosis, body mass index, and histology in Table 5 should be interpreted with caution. Because of the low patient numbers in this subgroup analysis, these comparative analyses were likely underpowered. Despite these low numbers, it is clear that family history can be used as a distinguishing feature.
Of the 10 patients with Lynch syndrome–associated LUS endometrial carcinomas, nine had likely/confirmed mutations in MSH2, and only one had a defect in MLH1. Previously, in two different studies, we also detected a preponderance of MSH2 mutation carriers.1,3 The majority of the Lynch syndrome patients in these studies were young. It is possible that younger age at endometrial diagnosis favors MSH2 mutations. Accordingly, in the population-based study of Hampel et al, the MSH6 mutation carriers tended to be older than 60 years.23
published online ahead of print at www.jco.org on November 10, 2008.
Supported by: National Institutes of Health (NIH) T32 Training Grant No. 5 T32 CA101642 02 and NIH Specialized Programs of Research Excellence for Uterine Cancer P50 CA098258 (S.W., K.L., R.B.).
Presented in part at the 97th United States and Canadian Academy of Pathology Annual Meeting, March 1-7, 2008, Denver, CO, and at the 39th Society of Gynecologic Oncologists Annual Meeting on Women's Cancer, March 9-12, 2008, Tampa, FL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58:71-96, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson MR, Kempson RL: Uterus and fallopian tubes, in Sternberg SS (ed): Histology for Pathologists. New York, NY, Raven Press, 1992
- 3.Sorvari TE, Laakso L: Histochemical investigation of epithelial mucosubstances in the uterine isthmus. Obstet Gynecol 36:76-81, 1970 [PubMed] [Google Scholar]
- 4.Kehoe S: Treatments for gynaecological cancers. Best Pract Res Clin Obstet Gynaecol 20:985-1000, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dabbs DJ, Sturtz K, Zaino RJ: The immunohistochemical discrimination of endometrioid adenocarcinomas. Hum Pathol 27:172-177, 1996 [DOI] [PubMed] [Google Scholar]
- 6.McCluggage WG, Sumathi VP, McBride HA, et al: A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol 21:11-15, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Ansari-Lari MA, Staebler A, Zaino RJ, et al: Distinction of endocervical and endometrial adenocarcinomas: Immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol 28:160-167, 2004 [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG, Jenkins D: P16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol 22:231-235, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Staebler A, Sherman ME, Zaino RJ, et al: Hormone receptor immunohistochemistry and human papillomavirus in situ hybridization are useful for distinguishing endocervical and endometrial adenocarcinomas. Am J Surg Pathol 26:998-1006, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Boronow RC, Morrow CP, Creasman WT, et al: Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obstet Gynecol 63:825-832, 1984 [PubMed] [Google Scholar]
- 11.Hachisuga T, Kaku T, Enjoji M: Carcinoma of the lower uterine segment. Clinicopathologic analysis of 12 cases. Int J Gynecol Pathol 8:26-35, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Jacques SM, Qureshi F, Ramirez NC, et al: Tumors of the uterine isthmus: Clinicopathologic features and immunohistochemical characterization of p53 expression and hormone receptors. Int J Gynecol Pathol 16:38-44, 1997 [PubMed] [Google Scholar]
- 13.Masuda K, Yutani C, Akutagawa K, et al: Cytopathological observations in a 27-year-old female patient with endometrioid adenocarcinoma arising in the lower uterine segment of the uterus. Diagn Cytopathol 21:117-121, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Hachisuga T, Fukuda K, Iwasaka T, et al: Endometrioid adenocarcinomas of the uterine corpus in women younger than 50 years of age can be divided into two distinct clinical and pathologic entities based on anatomic location. Cancer 92:2578-2584, 2001 [DOI] [PubMed] [Google Scholar]
- 15.International Federation of Gynecology and Obstetrics: Announcements: FIGO staging. Gynecol Oncol 35:125-127, 1989 [Google Scholar]
- 16.Vasen HF, Watson P, Mecklin JP, et al: New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116:1453-1456, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Broaddus RR, Lynch HT, Chen LM, et al: Pathologic features of endometrial carcinoma associated with HNPCC: A comparison with sporadic endometrial carcinoma. Cancer 106:87-94, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lu KH, Schorge JO, Rodabaugh KJ, et al: Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol 25:5158-5164, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Haider MA, Patlas M, Jhaveri K, et al: Adenocarcinoma involving the uterine cervix: Magnetic resonance imaging findings in tumours of endometrial, compared with cervical, origin. Can Assoc Radiol J 57:43-48, 2006 [PubMed] [Google Scholar]
- 20.Seiden MV, Patel D, O’Neill MJ, et al: Case records of the Massachusetts General Hospital: Case 13-2007—A 46-year-old woman with gynecologic and intestinal cancers. N Engl J Med 356:1760-1769, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Goodfellow PJ, Buttin BM, Herzog TJ, et al: Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A 100:5908-5913, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollikainen M, Abdel-Rahman WM, Moisio AL, et al: Molecular analysis of familial endometrial carcinoma: A manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J Clin Oncol 23:4609-4616, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hampel H, Frankel W, Panescu J, et al: Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66:7810-7817, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Berends MJ, Wu Y, Sijmons RH, et al: Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 21:4364-4370, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lynch HT, Watson P, Lanspa SJ, et al: Natural history of colorectal cancer in hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II). Dis Colon Rectum 31:439-444, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Brown AK, Madom L, Moore R, et al: The prognostic significance of lower uterine segment involvement in surgically staged endometrial cancer patients with negative nodes. Gynecol Oncol 105:55-58, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Phelan C, Montag AG, Rotmensch J, et al: Outcome and management of pathological stage I endometrial carcinoma patients with involvement of the lower uterine segment. Gynecol Oncol 83:513-517, 2001 [DOI] [PubMed] [Google Scholar]