Abstract
Purpose
Patients with myelodysplastic syndromes (MDS) often require treatment with growth factors (GFs) or non-GF therapies. One non-GF drug, lenalidomide, is particularly effective at achieving transfusion independence (TI) in patients with lower-risk MDS with the del(5q) cytogenetic abnormality. However, approximately half of del(5q) patients and one quarter of non–del(5q) patients treated with lenalidomide experience significant cytopenias. Lenalidomide-induced cytopenias occurring early in treatment may serve as a surrogate marker of clonal suppression and, therefore, may be predictive of a TI response.
Patients and Methods
We analyzed 362 low-risk, transfusion-dependent patients with MDS, with or without the del(5q) abnormality, enrolled in two phase II studies (MDS-003 and MDS-002) to determine whether treatment-related cytopenias are correlated with lenalidomide response. Cytopenias were assessed during the first 8 weeks of therapy, and response was defined as TI; response predictors were explored in univariate and multivariate analyses.
Results
Among patients with del(5q), 70% of those whose platelet count decreased by ≥ 50% achieved TI, as compared with 42% of those whose platelet count remained stable or declined by less than 50% (P = .01). Among patients without baseline neutropenia, 82% of those whose absolute neutrophil count (ANC) decreased by ≥ 75% achieved TI, as compared with 51% whose ANC remained stable or decreased by less than 75% (P = .02). These relationships were consistent in multivariate analyses. No relationship between the development of cytopenias and response could be established for lower-risk patients with MDS without del(5q).
Conclusion
These findings support the hypothesis that a direct cytotoxic effect of lenalidomide specific to the del(5q) clone may be indicative of a TI response.
INTRODUCTION
The myelodysplastic syndromes (MDS) comprise a spectrum of pathologically and cytogenetically distinct bone marrow disorders that are associated with cytopenias and, as a consequence, increased risk of bleeding and infection.1,2 MDS affects more than 10,000 people annually in the United States.3 The age-adjusted annual incidence is 4.5 per 100,000 in men and 2.7 per 100,000 in women.3,4 The sole curative therapy is bone marrow transplantation, which is given to a minority of affected patients because the median age at diagnosis is between 70 and 72 years.1,5 Most patients with MDS are treated with supportive measures, including blood transfusions, growth factor (GF) therapies (eg, erythropoiesis-stimulating agents), and non-GF therapies.6
Three non-GF drugs have been approved by the US Food and Drug Administration: the hypomethylating agents 5-azacytidine and decitabine, and lenalidomide.7,8-10 Using International Working Group response criteria, overall response rates to the hypomethylating agents range from 35% to 47%.11-13 Lenalidomide, an immunomodulatory agent that also works through antiangiogenic and direct cytotoxic mechanisms, is particularly effective in patients with the del(5q) cytogenetic abnormality, yielding transfusion independence (TI) in 67% of patients in the MDS-003 registration trial.10,14-20 However, approximately half of MDS patients with del(5q) treated with lenalidomide experienced National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 or 4 neutropenia or thrombocytopenia early in the course of treatment, with 84% requiring a dose reduction for myelosuppression.10 In a second multicenter phase II study (MDS-002) in which lenalidomide was administered to transfusion-dependent patients with MDS without the del(5q) cytogenetic abnormality, TI was achieved in 26% of patients, and the corresponding frequency of grade 3 or 4 cytopenias was lower, occurring in 25% to 30% of patients.21 Given the concordance between suppression of the MDS clone and TI response in patients with the del(5q) abnormality, we investigated whether lenalidomide-induced cytopenias that occur early in the treatment course serve as a surrogate marker of clonal suppression and, therefore, may be predictive of TI response. We analyzed data from the MDS-002 and MDS-003 studies to determine the relationship between treatment-related cytopenias and response to lenalidomide.
PATIENTS AND METHODS
Patients with lower-risk MDS (International Prognostic Scoring System [IPSS] classified low or intermediate-1 risk [IPSS score ≤ 1])7 who were transfusion-dependent were treated with lenalidomide in two phase II clinical trials. These trials included patients with the del(5q) cytogenetic abnormality with or without other cytogenetic abnormalities (MDS-003)10 and patients without del(5q) (MDS-002).21 Study eligibility as well as definition of TI as the primary end point have been reported previously and are summarized in the Appendix (online only).10,21
Definition of Terms
Baseline neutropenia and thrombocytopenia were defined as values that would qualify as NCI-CTC version 3.0 grades 1 to 4. For neutropenia, this included an absolute neutrophil count (ANC) of less than 2,000/μL and, for thrombocytopenia, this included a platelet count of less than 150,000/μL. Treatment-related cytopenias were given functional definitions of percent change from baseline that were clinically meaningful, rather than absolute, empiric values that would not consider variance in baseline laboratory values. For example, arbitrarily defining treatment-related thrombocytopenia as a platelet count of less than 50,000/μL would represent significant thrombocytopenia in a patient whose baseline platelet count was 500,000/μL but only mild thrombocytopenia in a patient who initiated therapy with a platelet count of 60,000/μL. Thus analyses were performed to determine the appropriate cutoff point in establishing a relationship between a platelet or ANC decline of 25%, 50%, or 75% and treatment outcome. On the basis of these analyses, for patients with the del(5q) lesion treated in the MDS-003 study, treatment-related thrombocytopenia was defined as a decline in platelet count by ≥ 50%, whereas treatment-related neutropenia was defined as an ANC decline of ≥ 75%. For patients without the del(5q) abnormality treated in the MDS-002 study, treatment-related thrombocytopenia was defined as a platelet count decrease of ≥ 50%, whereas treatment-related neutropenia was defined as an ANC decline of ≥ 50%. The functional difference in definition of percent change in cytopenias in the MDS-002 trial was selected on the basis of the paucity of patients experiencing an ANC decline of ≥ 75%, which would have prevented informative univariate analyses. Cytopenias were assessed during the initial 8 weeks of treatment with lenalidomide, as the majority of dose adjustments for cytopenias occurred early in the treatment course. Additional information about assessment of cytopenia cutoffs is available in the Appendix.
Statistical Analysis
Investigation of the relationship between early lenalidomide-induced cytopenias to TI was conducted using univariate and multivariate analyses. For univariate analyses, percent decline in neutrophil and platelet counts was dichotomized using cutoff points of 25%, 50%, and 75%. Cross-tabulations of these dichotomized variables with TI response (yes, no) were constructed and examined for trends. Fisher's exact P values were provided from these analyses as an indication of the potential correlative effect of early lenalidomide-induced cytopenias to erythroid response.
Multivariate analyses were performed to investigate the relationship of percent platelet and ANC declines to erythroid response in the presence of other prognostic factors. Stepwise logistic regression techniques were used, where the criteria for entrance into the predictive model was P = .25 and the criterion for variables to remain in the model was P = .10. Modeling using maximum percent decline in ANC and platelet count and similarly continuous variables for all other applicable prognostic variables was performed to maximize the information and the power of the analyses. Further analyses with categorization of clinically relevant continuous variables were conducted for more practical interpretations of results. Details of variables considered in this analysis can be found in the Appendix. The three percentage decline cutoff points were explored in the univariate setting to determine which display of the data to provide for ease of clinical interpretation, with knowledge of the significant relationship demonstrated in the multivariate analysis using continuous variables. TI response rate was also assessed by dividing the percentage decline in platelet counts and ANC into four groups (< 25%, 25% to 50%, 50% to 75%, and 75%) and analyzing this using the Cochran-Armitage test for trend. P values and odds ratios (ORs) for the variables selected for the predictive model are provided, with a two-sided P value of .05 considered significant. For univariate analyses, P values are presented as unadjusted, and adjusted (using a Bonferroni correction) where appropriate.
RESULTS
Patients
Baseline patient demographic data have been reported previously and are listed in Table 1. In the MDS-003 study, 59 patients (40%) had baseline neutropenia, 42 patients (28%) had baseline thrombocytopenia, and 84 patients (57%) had neutropenia, thrombocytopenia, or both. In the MDS-002 study, 83 patients (39%) had baseline neutropenia, 71 patients (33%) had baseline thrombocytopenia, and 118 patients (55%) had either cytopenia or both.
Table 1.
Patient Baseline Demographics
| Characteristic | MDS-003 (n = 148) | MDS-002 (n = 214) |
|---|---|---|
| Age, years | ||
| Median | 71 | 72 |
| Range | 37-95 | 27-94 |
| Duration of MDS, years | ||
| Median | 2.5 | 2.2 |
| Range | 0.1-20.7 | 0.0-12.9 |
| RBC transfusion rate/8 weeks | ||
| Median | 6 | 4 |
| Range | 0-18 | 1-24 |
| Female sex | ||
| No. | 97 | 76 |
| % | 66 | 36 |
| IPSS score | ||
| Low/intermediate-1 | ||
| No. | 120 | 168 |
| % | 81 | 79 |
| Intermediate-2/high | ||
| No. | 8 | 8 |
| % | 5 | 4 |
| Unclassified | ||
| No. | 20 | 38 |
| % | 14 | 18 |
Abbreviations: MDS, myelodysplastic syndromes; IPSS, International Prognostic Scoring System.
Treatment Outcomes
Overall response rates.
Overall response rates and information on patient discontinuations can be found in the online-only Appendix.
The relationship between early cytopenias and TI: Univariate analyses.
MDS patients with del(5q) were more likely to develop treatment-related thrombocytopenia than non–del(5q) patients (P < .001), although the overall rates of treatment-related neutropenia were similar (P = .67; Table 2). In the MDS-003 study, 124 patients (84%), 33 of whom had baseline thrombocytopenia and 91 of whom had normal platelet counts at baseline, developed treatment-related thrombocytopenia, and 61 patients (41%) developed neutropenia (12 of whom had baseline neutropenia and 49 of whom had normal baseline neutrophil counts). Within the first 8 weeks of therapy, the mean platelet count decline was 75.6%, and the ANC decline was 55.2%. In the MDS-002 study, 87 patients (43%) developed thrombocytopenia (33 patients with baseline thrombocytopenia and 54 without), whereas 92 patients (43%) developed neutropenia (30 with baseline neutropenia and 62 without). Within the first 8 weeks, the mean platelet count decline was 39.1%, and the mean ANC decline was 39.8%.
Table 2.
Lenalidomide Therapy Treatment–Related* Thrombocytopenia or Neutropenia
| Cytopenia on Therapy | MDS-003
|
MDS-002
|
P‡ | ||||
|---|---|---|---|---|---|---|---|
| n | N† | % | n | N† | % | ||
| Thrombocytopenia | 124 | 148 | 84 | 87 | 204 | 43 | < .001 |
| Baseline thrombocytopenia§ | 33 | 42 | 79 | 33 | 68 | 49 | .003 |
| No baseline thrombocytopenia | 91 | 106 | 86 | 54 | 136 | 40 | < .001 |
| Neutropenia | 61 | 148 | 41 | 92 | 210 | 43 | .67 |
| Baseline neutropenia‖ | 12 | 60 | 20 | 30 | 83 | 36 | .042 |
| No baseline neutropenia | 49 | 88 | 56 | 62 | 127 | 49 | .34 |
Treatment-related thrombocytopenia is defined as a ≥ 50% decrease in platelet count from baseline for MDS-002 and MDS-003. Treatment-related neutropenia is defined as a ≥ 50% decrease in absolute neutrophil count (ANC) from baseline for MDS-002 and a ≥ 75% decrease in ANC for MDS-003.
No. of patients with at least one nonmissing baseline and postbaseline neutrophil or platelet count measurement.
Fisher's exact test, two-sided.
Baseline platelet count of < 150,000/μL.
Baseline ANC of < 2000/μL.
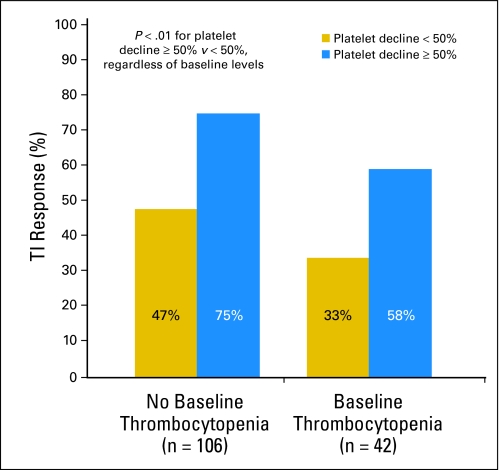
The development of thrombocytopenia was correlated with attainment of TI, regardless of baseline platelet count, for patients with del(5q). Overall, 70% of del(5q) patients whose platelet count decreased by ≥ 50% achieved TI, as compared with 42% whose platelet count remained stable or declined by less than 50% (unadjusted P = .01, adjusted P = .03). For patients without baseline thrombocytopenia, 75% of patients who experienced a ≥ 50% decrease in platelet count achieved TI, as compared with 47% of those whose platelet count remained stable or declined by less than 50%; for patients with baseline thrombocytopenia, the numbers were 58% and 33%, respectively (Fig 1).
Fig 1.
RBC transfusion independence (TI) response for del(5q) patients with and without thrombocytopenia at baseline, comparing patients who develop significant treatment-related thrombocytopenia (platelet count decline ≥ 50%) with those who do not (platelet count decline < 50%). P < .01 for platelet count decline ≥ 50% v platelet count decline less than 50%, regardless of baseline platelet level.
For patients without del(5q) on the MDS-002 study, treatment-related thrombocytopenia had no relationship to TI; 28% of patients without baseline thrombocytopenia whose platelet count decreased by ≥ 50% achieved TI, as compared with 38% of those whose platelet count remained stable or decreased by less than 50% (unadjusted P = .3); for those with baseline thrombocytopenia, the numbers were 9% and 20%, respectively (unadjusted P = .3).
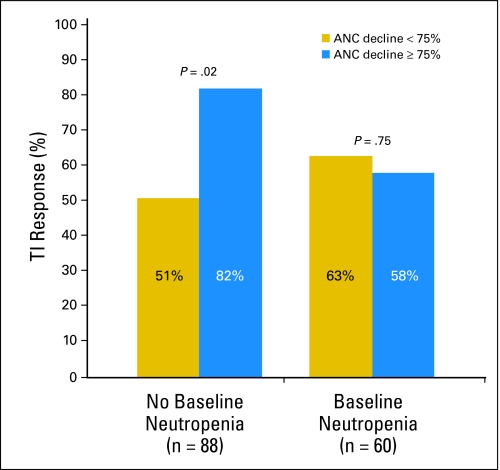
Analysis of treatment-associated neutropenia showed that the TI outcome for patients with del(5q) was dependent on the interaction between baseline neutropenia and the degree of early treatment-related neutropenia. Among patients without baseline neutropenia, 82% of those whose ANC decreased by ≥ 75% achieved TI, as compared with 51% of those whose ANC remained stable or decreased by less than 75% (unadjusted P = .02, adjusted P = .06). For patients with baseline neutropenia, treatment-related declines in ANC were not correlated with TI response (P = .75, Fig 2). There was no relationship between treatment-related neutropenia and TI among patients in the MDS-002 study; 23% of those whose ANC decreased by ≥ 50% achieved TI, as compared with 31% of those whose ANC remained stable or decreased by less than 50% (unadjusted P = .3 for those with baseline normal ANC); 21% of those whose ANC decreased by ≥ 50% achieved TI, as compared with 30% of those whose ANC remained stable or decreased by less than 50% (unadjusted P = .4 for those with baseline neutropenia). No change in the results occurred within the MDS-002 study for ANC or platelet count declines of 25%, 50%, or 75% within 4, 8, or 16 weeks of therapy. Within MDS-003, a further evaluation of TI response rate by dividing the percentage decline of platelet into four groups revealed a highly significant association using the Cochran-Armitage test for a decline in platelet count (P = .005) and in ANC (P = .02). In addition, 80% of patients with del(5q) who experienced declines in both platelet and neutrophil counts achieved TI, as compared with 64% of patients who experienced a decline in platelets or neutrophils and 42% of patients who had neither treatment-related declines (P = .008).
Fig 2.
RBC transfusion independence (TI) response for del(5q) patients with and without neutropenia at baseline, comparing patients who develop significant treatment-related neutropenia (absolute neutrophil count [ANC] decline ≥ 75%) with those who do not (ANC decline < 75%).
Relationship of cytopenias to TI: Multivariate analyses.
Analysis of continuous variables on the MDS-003 study for percent decline in ANC and platelet count showed that a platelet decrease in the first 8 weeks (P < .001), baseline platelet count (P = .04), age (P = .03), lactate dehydrogenase (normal, high; P = .09), duration of MDS before study entry (P = .02), and baseline transfusion burden (U/8 weeks; P = .01) had independent predictive powers for attainment of TI (Table 3). Platelet count decline also correlated significantly with cytogenetic response, proving to have a direct effect on the del(5q) clone (P = .02).
Table 3.
Factors Associated With Transfusion Independence Response in Patients With Del(5q) Based on Multivariate Analysis
| Analyses | MDS-003
|
|
|---|---|---|
| OR | P | |
| Continuous variables analysis | ||
| Decline in platelet count* | 1.04 | < .001 |
| Baseline platelet count | 1.00 | .04 |
| Age | 0.95 | .03 |
| Lactate dehydrogenase | 0.29 | .09 |
| Duration of MDS before study entry | 0.85 | .02 |
| Baseline transfusion burden | 0.81 | .01 |
| Categorized variables analysis | ||
| Decline in platelets (≥ 50%, < 50%) | 4.68 | .008 |
| Decline in ANC (≥ 75%, < 75%)† | 4.56 | .056 |
| RBC transfusion burden (≤ 4 U/8 weeks, > 4 U/8 weeks) | 0.85 | .04 |
| Duration of MDS before study entry (≤ 2 years, > 2 years) | 0.88 | .06 |
Abbreviations: OR, odds ratio; MDS, myelodysplastic syndromes; ANC, absolute neutrophil count.
Maximum percent decline.
In patients with normal baseline ANC.
On the basis of modeling using categorized variables, a treatment-related decrease in platelet count of ≥ 50% (P = .008, OR = 4.67) was predictive of TI among patients with del(5q) (Table 3). A decrease in ANC of ≥ 75% (P = .056; OR = 4.56) was significant for patients who entered the trial with normal ANC counts according to NCI-CTC standards. Baseline transfusion burden (P = .04; OR = 0.85) and duration of MDS before study entry (P = .06; OR = 0.88) were also selected as contributing factors to response in this model.
In the MDS-002 study, no relationship could be established in multivariate analyses, using continuous or categoric variable modeling, between declines in ANC (P = .22) or platelet counts and achievement of TI (P = .85). Factors predictive of TI response in non-del(5q) patients are listed in Table 4. In addition to these factors selected in the categoric variable modeling, association with TI response was demonstrated for French-American-British subtype (P = .02; OR = 4.06) and blast count (P = .07; OR = 1.25).
Table 4.
Factors Associated With Transfusion Independence Response in Non-del(5q) Patients Based on Multivariate Analysis
| Categorized Variables Analysis | MDS-002
|
|
|---|---|---|
| OR | P | |
| RBC transfusion burden, < 4 U/8 weeks, ≥ 4 U/8 weeks | 3.95 | < .001 |
| Baseline platelet count, < 150,000/μL, ≥ 150,000/μL | 2.67 | .03 |
| Duration of MDS before study entry, < 2 years, ≥ 2 years | 2.99 | .005 |
| LDH, ≤ ULN, > ULN | 3.29 | .05 |
| FAB subtype, RA/RARS, other | 4.06 | .02 |
| Blast count, ≤ 5%, > 5% | 1.25 | .07 |
Abbreviations: OR, odds ratio; MDS, myelodysplastic syndromes; LDH, lactate dehydrogenase; ULN, upper limit of normal; FAB, French-American-British; RA, refractory anemia; RARS, RA with ringed sideroblasts.
DISCUSSION
A variety of mechanisms of action are believed to contribute to therapeutic response in MDS. These range from abrogation of hematopoietic suppressive effects of pro-inflammatory apoptotic cytokines to direct cytotoxicity, akin to chemotherapeutic agents. Drugs effective in eliminating the dysplastic clone, and thus potentially in yielding a complete remission, would also be expected to cause profound cytopenias to restore polyclonal, effective hematopoiesis.8-10,22-25
This is the first study, to our knowledge, to analyze the relationship between the development of treatment-induced cytopenias and response to therapy in MDS. Specifically, we found that among lower-risk MDS patients with a del(5q) cytogenetic abnormality whose platelet counts decreased by ≥ 50%, and for those with no baseline neutropenia whose neutrophils declined by ≥ 75%, packed RBC TI was more likely to be achieved, as compared with patients not experiencing the same magnitude of cytopenias. These findings were maintained in the multivariate analyses when controlling for other known predictors of response.
Consistent with the notion that TI response is dictated by effective suppression of the MDS clone, lower-risk MDS patients without the del(5q) cytogenetic abnormality, in whom severe cytopenias occurred at half the rate as in their del(5q) counterparts, achieved TI 2.5 times less frequently, and no relationship could be established between the development of neutropenia or thrombocytopenia and hematologic response. TI is a meaningful clinical outcome, as it was maintained in patients with the del(5q) abnormality for a median of more than 2 years.20 This theory is bolstered by the association between treatment-related cytopenias and cytogenetic response. In fact, cytogenetic response in the MDS-003 study is more correlative to TI response, as 61 of 62 patients with partial or complete cytogenetic responses no longer required transfusions. Clinically, however, treating physicians are less likely to assess cytogenetic status during the first 8 weeks of treatment and are more likely to focus on treatment-related cytopenias, the occurrence of which may be predictive of clonal suppression. Although using cytogenetic response as a primary end point would have been intriguing, serial bone marrow draws for cytogenetic assessments were not required. Thus not all patients had follow-up assessments and were, therefore, not assessable for cytogenetic response.
There are two possible explanations for the discrepant findings in the relationship of cytopenias to TI response. First, the number of cytopenic events and rate of TI was insufficient in the MDS-002 study to reliably establish a link to TI response. Although this may be true for the development of thrombocytopenias, which occurred at approximately half the rate in MDS-002 compared with that in MDS-003, rates of neutropenia were similar between the studies. The second consideration is that the cytotoxic effects of lenalidomide in the MDS-003 study were specific for the del(5q) clone, as were cytopenias (particularly thrombocytopenia, the negative sequelae of the thrombocytosis associated with the 5q− syndrome), whereas in the MDS-002 study, cytopenias were not specific to any one MDS phenotype. Recent preliminary work has identified haploinsufficiency of the ribosomal protein encoding the RSP14 gene as being causative of the characteristic phenotype that defines the 5q− syndrome (by RNA interference screen).26 Another group has shown that cell-cycle regulatory phosphatases Cdc25C and PP2A determine the sensitivity of del(5q) MDS cells to lenalidomide.27 It is possible that these are the mechanisms underlying the phenotypic picture of thrombocytosis in some patients with the del(5q) abnormality, and thus the preferential suppression of platelet production is the first indicator of efficacy.
The observation that cytopenias are related to response in patients with the del(5q) abnormality raises the question of whether the lenalidomide dose should be titrated to the development of cytopenias in an effort to maximize response. Most patients in the MDS-003 study (84%) required a dose reduction from 10 mg/d to 5 mg/d or even 5 mg every other day. Because the reason for dose reduction was protocol-directed (for the development of cytopenias in almost all cases), this factor was collinear with a decline in ANC or platelet count and could not be included in the multivariate analyses. This study establishes a direct link between the development of cytopenias and response to lenalidomide but was not designed to demonstrate causality and thus is hypothesis-driving. Prospective clinical trials in development, and those currently accruing patients, are exploring lenalidomide dose titration in MDS, the use of GFs to support patients through treatment-related cytopenias, and high-dose lenalidomide in del(5q) acute myeloid leukemia (Southwest Oncology Group study S0605).
This study is limited by the number of patients enrolled onto the MDS-002 and MDS-003 studies. It is possible that, for example, if more patients with baseline neutropenia were enrolled in MDS-003, a relationship could have been established between decline in neutrophil count and response, as was established for those without baseline neutropenia. In MDS-002, as is true with many MDS studies,9,22,28,29 inclusion criteria are based on IPSS criteria,7 which are still relevant with respect to prognosis but predate more contemporary classification schemes30,31 and allow for considerable patient heterogeneity, which may prevent identification of a relationship between cytopenia and response in a select disease subset. However, the data from MDS-003 represent the largest collection of patients with del(5q) treated with active therapy to date, whereas patient numbers from MDS-002 eclipse those of the two phase III registration trials for other non-GF therapies.8,9 Specific questions regarding MDS subsets more likely to respond to particular therapies, and predictors of those responses, will be answered as future techniques incorporate more refined histopathologic and immunologic determinants, along with updated cytogenetic criteria and methods of detecting cytogenetic abnormalities (such as fluorescent in situ hybridization and high-resolution genomic or single-nucleotide polymorphism array technology).32-34
In conclusion, in lower-risk MDS patients with the del(5q) cytogenetic abnormality, treatment-related cytopenias, particularly those involving a significant decrease in platelet count, correspond to achievement of TI. In lower-risk MDS patients without this cytogenetic abnormality, no such relationship could be established. This finding supports a direct cytotoxic effect of lenalidomide specific to the del(5q) clone that may be obligate for TI response.
Analyses were performed to determine the appropriate cutoff point in establishing a relationship between a platelet or absolute neutrophil count (ANC) decline of 25%, 50%, or 75% and treatment outcome. The final determination of the cutoff point was based on having adequate patient numbers to perform informative univariate analyses. Cytopenia cutoff points for the MDS-003 study were assessed at 25%, 50%, and 75% declines for both ANC and platelet counts to find a useful descriptive univariate summary of the data for ease of clinical interpretation. TI response rate was also assessed by dividing the percentage decline in platelet counts and ANC into four groups (< 25%, 25% to 50%, 50% to 75%, and 75%) and analyzing this using the Cochran-Armitage test for trend.
In the MDS-003 study, 97 patients (66%) achieved TI, and 73% of patients achieved a partial or complete cytogenetic response. In the MDS-002 study, 56 patients (26%) achieved TI, whereas only nine patients (19%) with informative cytogenetic abnormalities had a cytogenetic response (four patients [9%] had a complete response).
In the MDS-003 study, during the first 8 weeks of therapy, 10 patients dropped out because of toxicities; nine patients had an ANC decline of less than 75% and one patient had no follow-up data. Three of the 10 patients had a platelet decline of less than 50%, six patients had platelet declines of ≥ 50%, and one patient had no follow-up data. In the MDS-002 study, 18 patients dropped out because of toxicities; 15 patients had an ANC decline of less than 75%, two patients had an ANC decline of ≥ 75%, and one patient had no follow-up data. Eleven of the 18 patients had a platelet count decline of less than 50%, and seven patients had platelet declines of ≥ 50%.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Kenton Wride, Celgene (C); Robert Knight, Celgene (C) Consultant or Advisory Role: Mikkael A. Sekeres, Celgene (C), Pharmion (C); Jaroslaw P. Maciejewski, Celgene (C), MGI Pharma (C), Amgen (C), Alexion (C), Genzyme (C); Aristotle A.N. Giagounidis, Celgene (C); Azra Raza, Celgene (C), Pharmion (C), Novartis (C); Alan F. List, Celgene (C), Pharmion (C), MGI Pharma (C), Kanisa (C), S*BIO (C) Stock Ownership: Kenton Wride, Celgene Honoraria: Alan F. List, Celgene, Pharmion, MGI Pharma Research Funding: Mikkael A. Sekeres, Celgene; Jaroslaw P. Maciejewski, Genzyme; Azra Raza, Telik, Seattle Genetics, Merck, Pharmion; Alan F. List, Celgene, MGI Pharma Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mikkael A. Sekeres, Alan F. List
Collection and assembly of data: Robert Knight
Data analysis and interpretation: Mikkael A. Sekeres, Kenton Wride, Alan F. List
Manuscript writing: Mikkael A. Sekeres
Final approval of manuscript: Mikkael A. Sekeres, Jaroslaw P. Maciejewski, Aristotle A.N. Giagounidis, Robert Knight, Azra Raza, Alan F. List
Acknowledgments
We thank the many patients across the world who gave their time to participate in these multi-institutional trials, along with the study personnel and investigators involved.
Appendix: Study Eligibility and Definition of Transfusion Independence as the Primary End Point
Study eligibility required a confirmed diagnosis of primary myelodysplastic syndrome (MDS) according to the French-American-British criteria (Bennett JM, Catovsky D, Daniel MT, et al. Br J Haematol 51:189-199, 1982), transfusion-dependent anemia (defined as requiring ≥ 2 U of packed RBCs [pRBCs] within the 8 weeks before study enrollment), a minimum platelet count of 50,000/μL, and a neutrophil count of 500/μL. Patients were aged ≥ 18 years, had an adequate Eastern Cooperative Oncology Group performance status, and good renal and hepatic function. Patients with clinically significant coexisting medical conditions, known allergy or hypersensitivity to thalidomide, and women of childbearing potential with a positive pregnancy test were excluded. All patients provided written, informed consent before enrollment into both trials, and both studies were approved by local institutional review boards at participating centers.
Lenalidomide was administered at a dose of 10 mg/d for 21 or 28 days of each 28-day cycle20 (Raza A, Reeves JE, Feldman EJ, et al. Am Soc Hematol Annual Meeting Abstracts 108:78a, 2006; abstr 250). Treatment was interrupted for adverse events of grade more than 3 and resumed at a dose of 5 mg daily, or every other day, after resolution of the adverse effects. Dose reductions for cytopenias were based on absolute neutrophil and platelet counts, and not percentage declines, as specified by the individual protocols. CBC counts were assessed weekly during the first 8 weeks and every other week thereafter. Bone marrow and cytogenetics examinations were performed after 24 weeks of therapy. The primary end point for both studies was the frequency of transfusion independence (TI). The primary end point for this study was TI, defined as no requirement for pRBC transfusions over a minimum 8-week period, at any time during the study. TI is considered to be a major hematologic improvement along erythroid cell lines by the International Working Group MDS response criteria and a hematologic improvement along erythroid cell lines using the modified International Working Group response criteria.11,12 Transfusion frequency and pretransfusion hemoglobin values within the 8 weeks preceding study treatment served as reference values for assessment of response. As both studies were conducted at multiple institutions, and individual patient requirements and provider thresholds for blood transfusions vary, these could not be mandated. Instead, the MDS-003 and MDS-002 studies provided general guidelines of transfusing 2 U of pRBC for a decline in hematocrit to less than 25%. For both studies, hemoglobin values triggering RBC transfusions were assessed and found to be similar before and during study treatment, indicating that transfusion thresholds did not differ from baseline. For both the MDS-002 and MDS-003 studies, TI required a period of 56 consecutive days during which no transfusions were given and during which the hemoglobin concentration increased by at least 1 g/dL to account for the potential of varying transfusion thresholds.
published online ahead of print at www.jco.org on November 17, 2008.
Supported in part by National Institutes of Health Grant No. U54RR19397-03 (M.A.S., J.P.M., and A.F.L.) and by research funding from Celgene Corporation to M.A.S.
Presented in part at the Ninth International Symposium on Myelodysplastic Syndromes, May 16-19, 2007, Florence, Italy, and at the American Society of Hematology Annual Meeting, December 11, 2007, Atlanta, GA, and published in abstract form at the meetings.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.List AF, Vardiman J, Issa JP, et al: Myelodysplastic syndromes. Hematol Am Soc Hematol Educ Program 297-317, 2004 [DOI] [PubMed]
- 2.Harris NL, Jaffe ES, Diebold J, et al: The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November, 1997. Ann Oncol 10:1419-1432, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ma X, Does M, Raza A, et al: Myelodysplastic syndromes: Incidence and survival in the United States. Cancer 109:1536-1542, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Rollison DE, Hayat M, Smith M, et al: First report of national estimates of the incidence of myelodysplastic syndromes and chronic myeloproliferative disorders from the U.S. SEER Program. Blood 108:77a, 2006. (suppl; abstr 247) [Google Scholar]
- 5.Sekeres MA, Kantarjian H, List A, et al: P127 Prospective cross-sectional analysis of cytopenias and transfusion needs of MDS patients in the USA. Leuk Res 31:S108, 2007. (suppl 1) [Google Scholar]
- 6.Golshayan AR, Jin T, Maciejewski J, et al: Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol 137:125-132, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Greenberg P, Cox C, LeBeau MM, et al: International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079-2088, 1997 [PubMed] [Google Scholar]
- 8.Silverman LR, Demakos EP, Peterson BL, et al: Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol 20:2429-2440, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Issa JP, Rosenfeld CS, et al: Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 106:1794-1803, 2006 [DOI] [PubMed] [Google Scholar]
- 10.List A, Dewald G, Bennett J, et al: Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 355:1456-1465, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Kantarjian H, et al: Myelodysplastic syndromes standardized response criteria: Further definition. Blood 98:1985, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Greenberg PL, Bennett JM, et al: Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108:419-425, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Silverman LR, McKenzie DR, Peterson BL, et al: Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol 24:3895-3903, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Corral LG, Kaplan G: Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis 58:I107-I113, 1999. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies FE, Raje N, Hideshima T, et al: Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98:210-216, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Geitz H, Handt S, Zwingenberger K: Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology 31:213-221, 1996 [DOI] [PubMed] [Google Scholar]
- 17.List A, Tate W, Glinsmann-Gibson B: The immunomodulatory thalidomide analog, CC-5013, inhibits tropic response in VEGF in AML cells by abolishing cytokine-induced PI3/Akt activation. Blood 100:139a, 2002. (suppl; abstr 521) [Google Scholar]
- 18.Parman T, Wiley MJ, Wells PG: Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med 5:582-585, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Buesche G, Dieck S, Giagounidis A, et al: Anti-angiogenic in vivo effect of lenalidomide (CC-5013) in myelodysplastic syndrome with del(5q) chromosome abnormality and its relation to the course of disease. Blood 106:113a, 2005. (suppl; abstr 372) [Google Scholar]
- 20.List AF, Dewald GW, Bennett JM, et al: Long-term clinical benefit of lenalidomide (Revlimid) treatment in patients with myelodysplastic syndrome and chromosome deletion 5q. Blood 108:78a, 2006. (suppl; abstr 251) [Google Scholar]
- 21.Raza A, Reeves JE, Feldman EJ, et al: Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 111:86-93, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian H, Oki Y, Garcia-Manero G, et al: Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 109:52-57, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Raza A, Meyer P, Dutt D, et al: Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes. Blood 98:958-965, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Schiller GJ, Slack J, Hainsworth JD, et al: Phase II multicenter study of arsenic trioxide in patients with myelodysplastic syndromes. J Clin Oncol 24:2456-2464, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Vey N, Bosly A, Guerci A, et al: Arsenic trioxide in patients with myelodysplastic syndromes: A phase II multicenter study. J Clin Oncol 24:2465-2471, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ebert BL, Pretz J, Bosco J, et al: Identification of RPS14 as the 5q-syndrome gene by RNA interference screen. Blood 110:8a, 2007. (suppl; abstr 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei S, Rocha K, Williams A, et al: Gene dosage of the cell cycle regulatory phosphatases Cdc25C and PP2A determines sensitivity to lenalidomide in del(5q) MDS. Blood 110:43a, 2007. (suppl; abstr 118) [Google Scholar]
- 28.Roboz GJ, Ritchie EK, Allen-Bard S, et al: Arsenic trioxide (ATO) is safe and effective in combination with low-dose Ara-C (LDAC) for the treatment of IPSS-2 myelodysplastic syndrome (MDS) and poor-prognosis acute myeloid leukemia (AML) in elderly patients. Blood 106:786a, 2005. (suppl; abstr 2805) [Google Scholar]
- 29.Sekeres MA, Kalaycio ME, Erba HP, et al: P155 Arsenic trioxide (ATO) and gemtuzumab ozogamicin (GO) in high-risk MDS or AML arising from MDS. Leuk Res 31:S125, 2007. (suppl 1) [Google Scholar]
- 30.Malcovati L, Germing U, Kuendgen A, et al: A WHO classification-based prognostic scoring system (WPSS) for predicting survival in myelodysplastic syndromes. Blood 106:316a, 2005. (suppl; abstr 788) [Google Scholar]
- 31.Vardiman JW, Harris NL, Brunning RD: The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292-2302, 2002 [DOI] [PubMed] [Google Scholar]
- 32.O'Keefe CL, Tiu R, Gondek LP, et al: High-resolution genomic arrays facilitate detection of novel cryptic chromosomal lesions in myelodysplastic syndromes. Exp Hematol 35:240-251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babicka L, Ransdorfova S, Brezinova J, et al: Analysis of complex chromosomal rearrangements in adult patients with MDS and AML by multicolor FISH. Leuk Res 31:39-47, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Gondek LP, Haddad A, O'Keefe C, et al: SNP karyotyping in myelodysplastic syndromes (MDS) reveals the presence of cryptic karyotypic abnormalities, including uniparental disomy, and has important prognostic implications. Blood 108:265a, 2006. (suppl; abstr 853) [Google Scholar]