Abstract
Background:
To manage public expenditures in the mid-1990s, British Columbia implemented evidence-based drug coverage policies, including “reference pricing.” Industry lobbied against the province's policy, arguing that reference pricing harms patients and that it is inconsistent with federal and provincial legislation. Researchers and the courts have studied and rejected industry's claims. However, industry also threatened to halt R&D investment in British Columbia and continues to so threaten other provinces contemplating evidence-based drug coverage policies. The purpose of this study is to review evidence regarding these threats.
Methods:
Provincial-level R&D data for 1988–2006 were used to analyze the impact of BC PharmaCare's policies on pharmaceutical R&D in British Columbia. We used statistical analyses to determine whether the province's policies affected BC-based R&D as expressed in two ways: (1) as inflation-adjusted expenditure per capita in British Columbia and (2) as the ratio of expenditure per capita in the province to expenditure per capita in the rest of Canada.
Results:
Evidence-based drug coverage policies had no statistically significant negative effects on BC-based pharmaceutical R&D. BC R&D was slightly above expected trends in 1997 and slightly below expected trends in 1998 and 1999 (though not statistically significantly in either case). From 2001 to 2003, BC R&D was (statistically significantly) above expected trends.
Conclusions:
While they are part of the politics of the pharmaceutical sector, claims and threats regarding connections between coverage policy and location of R&D investment are not borne out in British Columbia's experience. This is likely because, as suggested by business and economic literature, firms locate R&D based on the expected cost-to-firm and productivity of the R&D investment itself. Prudent policy would therefore manage pharmaceutical expenditures using evidence-based policies and pursue scientific and economic development goals through direct and strategic government investment in local scientific capacity.
Abstract
Contexte :
Afin de gérer les dépenses publiques au milieu des années 1990, la Colombie-Britannique a mis en œuvre des politiques d'assurance-médicaments fondées sur des preuves – y compris l'établissement du coût en fonction du produit de référence. L'industrie s'est élevée contre la politique de la province, soutenant qu'elle était nuisible pour les patients et qu'elle contrevenait aux lois fédérales et provinciales. Des chercheurs et des tribunaux ont examiné puis rejeté les revendications de l'industrie. Toutefois, cette dernière a également menacé de mettre fin aux investissements en R&D en Colombie-Britannique et continue de menacer d'autres provinces qui envisagent d'adopter des politiques d'assurance-médicaments fondées sur des preuves. La présente étude vise à examiner les preuves relatives à ces menaces.
Méthodes :
Nous avons utilisé des données provinciales en R&D de 1988 à 2006 pour analyser l'incidence des politiques d'assurance-médicaments de la Colombie-Britannique sur la R&D pharmaceutique dans la province. Nous nous sommes servis d'analyses statistiques pour déterminer si les politiques de la province influençaient la R&D en C.-B. – la R&D étant exprimée de deux manières : (1) les dépenses par habitant ajustées en fonction de l'inflation en Colombie-Britannique et (2) le rapport des dépenses par habitant dans la province et des dépenses par habitant dans le reste du Canada.
Résultats :
Les politiques d'assurance-médicaments fondées sur des preuves n'ont pas eu d'incidence négative statistiquement importante sur la R&D pharmaceutique en C.-B. La R&D dans cette province dépassait légèrement les attentes en 1997 et était juste en deçà de celles-ci en 1998 et en 1999 (bien que ces différences soient statistiquement négligeables dans les deux cas). De 2001 à 2003, la R&D en C.-B. a dépassé les attentes, et ce, d'une manière statistiquement significative.
Conclusions :
Bien qu'elles fassent partie de la politique du secteur pharmaceutique, les revendications et les menaces concernant les liens entre les politiques d'assurance-médicaments et l'emplacement des investissements en R&D ne se sont pas corroborées par l'expérience de la Colombie-Britannique. C'est probablement parce que, comme le suggère la documentation économique et industrielle, les sociétés choisissent l'emplacement des projets de R&D en fonction des coûts prévus et de la productivité des investissements en R&D proprement dits. Une politique prudente permettrait donc de gérer les dépenses pharmaceutiques avec des politiques fondées sur des preuves, et de poursuivre des objectifs scientifiques et de développement économique grâce à des investissements gouvernementaux stratégiques dans les capacités scientifiques locales.
Governments around the world struggle with the need to manage pharmaceutical expenditures in ways that provide equitable and sustainable access to necessary medicines. They are also mindful that the pharmaceutical industry is a major sector for economic and scientific activities (Jacobzone 2000; Morgan et al. 2008). In the 1990s, BC PharmaCare – the public drug plan in British Columbia – began to manage public expenditure on pharmaceuticals using a series of coverage policies focused on paying only for scientifically established health outcomes (Morgan et al. 2004). These policies are best represented by BC PharmaCare's reference pricing policy, which was implemented for three drug classes in 1995 (nitrate drugs, histamine-2 blockers and nonsteroidal anti-inflammatory drugs [NSAIDs]) and two additional drug classes in 1997 (angiotensin-converting enzyme [ACE] inhibitors and calcium-channel blockers). In effect, reference pricing limits public subsidies for drugs in select classes based on the price of lowest-cost alternatives within those classes. Any product would be exempted from British Columbia's reference pricing policy if the manufacturer could provide scientific evidence to substantiate claims of superiority in terms of clinically relevant patient health outcomes (Morgan et al. 2004).
Industry strongly opposed BC PharmaCare's approach to coverage policy and, in particular, the use of reference pricing. Manufacturers launched advertising campaigns suggesting that reference pricing would have negative effects on patient health and the healthcare system and initiated a lawsuit challenging the legality of the policy (Coutts 1995; Mullens 1997; Brunt et al. 1998). Several independent research studies and the BC courts have vindicated government on these counts (Grootendorst and Holbrook 1999; Hazlet and Blough 2002; Morfitt et al. 2002; Schneeweiss, Soumerai et al. 2002; Schneeweiss, Walker et al. 2002; Schneeweiss et al. 2003, 2004). Industry also argued that British Columbia would lose on investment because BC PharmaCare's policies were “unfriendly” towards patented pharmaceutical manufacturers. This contention has been less thoroughly investigated and is the subject of this paper.
Impact of BC PharmaCare Policies on R&D in BC
Data
Patented Medicine Prices Review Board (PMPRB) data on pharmaceutical company R&D expenditures provide information necessary to determine whether British Columbia's evidence-based drug coverage policies, and in particular its reference pricing policy, had a significant effect on local R&D investment. The PMPRB collects industry self-reported data on amounts that pharmaceutical companies spend on R&D activities in each province. While firms may have incentives to overstate R&D amounts – in order to appear to have lived up to promised levels of R&D (Kalant and Shrier 2006) – such incentives should not affect this analysis of BC PharmaCare's policy impacts. For example, in their analysis of the national impact of changes in drug patent policy, Grootendorst and Di Matteo (2007) found comparable results using the PMPRB data versus data from Statistics Canada. We used PMPRB data because publicly available Statistics Canada data on pharmaceutical R&D are not available at a regional level. PMPRB reports R&D expenditures by companies marketing patented drugs that belong to the brand-name industry association (Canada's Research-Based Pharmaceutical Companies, or Rx&D) and by all pharmaceutical companies marketing patented drugs. For this analysis we used the latter set of data.
Methods
We searched for evidence of an impact of PharmaCare policy in BC-based R&D by pharmaceutical companies in two ways. First, we searched for changes in inflation-adjusted pharmaceutical R&D expenditure per capita in British Columbia, controlling for pre-policy time trends. Second, just as researchers use other economic sectors to control for general trends in R&D when studying total pharmaceutical sector R&D (Grootendorst and Di Matteo 2007), we used trends in pharmaceutical R&D in the rest of Canada to control for factors that might be affecting BC-based R&D in ways other than the specific PharmaCare policies studied here. To do this, we looked for changes in the ratio of expenditure per capita in British Columbia to expenditure per capita in the rest of Canada, controlling for pre-policy time trends in that ratio.
We performed time series analyses (using SAS for Windows v.9) to test for changes in trends or levels of BC-based pharmaceutical R&D. The models computed were linear ordinary least squares regressions with co-variance matrices adjusted for autocorrelation. In separate regressions (owing to lack of statistical degrees of freedom), we tested for policy impacts following 1995 (the year reference pricing was initiated for three drug classes) and following 1997 (the year the program was expanded to two further classes). Finally, after visual inspection of the data, we tested for temporary changes in BC-based R&D during the periods of 1998 to 2000 and 2001 to 2003 because BC-based R&D in those periods appeared to be below and above trends, respectively. There was an as-yet-unexplained 36% decrease in pharmaceutical company spending on BC-based R&D in 2006. Findings of our statistical analysis were not affected by the exclusion of that data point.
Results
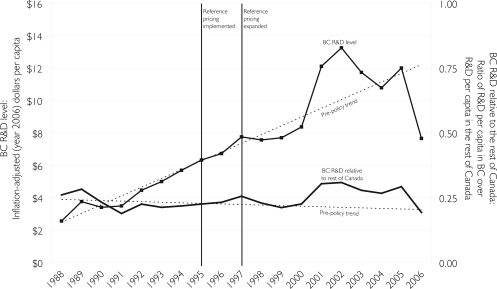
Figure 1 illustrates pharmaceutical R&D expenditure for British Columbia in inflation-adjusted (year 2006) dollars per capita and as a ratio relative to R&D expenditure per capita in the rest of Canada. The figure also illustrates forecast data based on a best-fitting time series regression model using the pre-policy data spanning 1988 to 1997. Forecasts from 1988 to 1995 are similar, but suggest a more modest policy impact because the increase in BC-based R&D from 1995 to 1997 was more rapid than pre-policy trends. We chose to illustrate the 1988 to 1997 model in order to increase the chance of detecting a negative impact of BC PharmaCare's policies.
FIGURE 1.
Per capita R&D expenditure in British Columbia by patent-holding pharmaceutical companies in inflation-adjusted (year 2006) dollars and as a ratio of per capita expenditure in the rest of Canada, 1988 to 2006
There were no statistically significant changes in either the level or the trend of BC-based pharmaceutical R&D in absolute terms or relative to the rest of Canada following the implementation (1995) or expansion (1997) of reference pricing. However, per capita investment in British Columbia plateaued from 1998 to 2000. While the decline is not statistically significant (p=.51 for per capita levels, p=.17 for ratios relative to the rest of Canada), if the fall in BC-based R&D from 1998 to 2000 were attributable to BC PharmaCare's policies, the potential R&D lost (in comparison to trend) would be valued at $6.5 million (year 2006 dollars), or roughly $2 million per year for three years. From 2001 to 2003, BC-based R&D increased to statistically significant levels above trends (p<.01 for per capita levels and for ratios relative to the rest of Canada). The increase in BC-based R&D investment by patent-holding drug companies from 2001 to 2003 would be valued as a windfall (in comparison to trend) of $28.5 million (year 2006 dollars), or about $9 million per year for three years.
Relative to the rest of Canada, pharmaceutical company R&D in British Columbia was low and on a slightly – though not statistically significant – downward trend through the pre-policy era (1988 to either 1995 or 1997). During the pre-policy period, per capita spending on R&D in the province was approximately 75% to 80% lower than per capita spending on R&D in the rest of Canada. The relative size of BC-based R&D investment trended slightly – though not statistically significantly – upward through the post-policy period. From 2001 to 2005, per capita pharmaceutical R&D was approximately 70% lower in British Columbia than in the rest of Canada.
Discussion
Policy analysis is often challenging because of the difficulty of finding valid counter-factuals against which to compare policy experience. In this case, it is hard to know with certainty what R&D investment would have been without reference pricing policy. Evidence suggests that reference pricing in British Columbia did not cause any significant changes in R&D expenditures in the province by the pharmaceutical industry, either in absolute terms or compared with the rest of Canada. BC-based pharmaceutical R&D continued to grow following the implementation of evidence-based drug coverage policies – indeed, it did so slightly more quickly following these policies than preceding them. Industry will, however, continue to claim the policy created a hostile environment that decreased investment potential. Pharmaceutical companies have long cited local market conditions as influences on R&D investment decisions (Taggart 1991; OECD 2006). Taggart (1991) describes this as surprising “because there seems to be no prima facie reasoning that would immediately lead to this conclusion”; in other words, it defies basic economic logic.
How so? Pharmaceutical companies are businesses before anything else. As such, they make investment decisions based on expected costs and benefits. For example, literature on location of R&D from this sector and others states that, on the cost side of R&D investments, firms will consider such factors as the effect of tax breaks on the cost-to-firm of local R&D spending (Taggart 1991; Cornet and Rensman 2001; Davis and Meyer 2004; OECD 2006; Pazderka 2007). It is notable that Canada's R&D tax breaks are among the most generous in the world (OECD 2005). However, as evidenced by Canada's relatively poor R&D performance (Guellec and de la Potterie 2001; Harris 2005; Conference Board of Canada 2007; Howitt 2007), tax breaks are not sufficient to make significant local R&D investment of value to firms.
An increasing amount of research suggests that the most important consideration in R&D investment decisions – even more than tax breaks – is the availability, accessibility and quality of local technical infrastructure and scientific capacity (Jaffe 1989; Cockburn and Henderson 1996; Mansfield and Lee 1996; Porter 1998, 2000; Kuemmerle 1999; Davis and Meyer 2004). These factors are critical insofar as they relate to the research productivity and therefore expected return from a firm's R&D investments. Across many studies, the availability and cost of high-quality labour ranks as a crucial determinant of R&D location (Taggart 1991; Cornet and Rensman 2001; OECD 2006); also important is the location of productive universities and related laboratories (Jaffe 1989; Cockburn and Henderson 1996; Mansfield and Lee 1996; Kuemmerle 1999; Davis and Meyer 2004).
Thus, firms may never have intended to cut R&D in British Columbia or to increase R&D investment in the province more quickly than they actually did over the past decade. However, they may find that the rhetoric of punishment serves to build opposition to evidence-based drug coverage policies in other jurisdictions. For threats of punishment to be credible, pharmaceutical companies must be united in their local “boycott” and must sustain their support for it for sufficiently long to make it clear to local and foreign decision-makers that firms will punish themselves (by forgoing otherwise profitable local scientific endeavours) in order to punish governments that employ certain drug coverage policies. Such coordination among competing firms may be unsustainable if the area in question is otherwise attractive for R&D investment.
Conclusion
Despite industry claims, we found no evidence to suggest that pharmaceutical manufacturers pulled R&D investment from British Columbia following BC PharmaCare's implementation of evidence-based policies, and reference pricing in particular, in the mid-1990s. The reason: threats of punishment do not stand up against business fundamentals. Industry will invest in local R&D based on the costs and benefits incurred from that scientific investment. Such factors are totally independent of local coverage policy except to the extent that firms try to associate them through the rhetoric of rewards and punishment. Even in the case of a policy as harshly opposed as reference pricing in British Columbia, the threats are not credible because firms will maintain their R&D investments as long as R&D fundamentals are unchanged.
Government policies most likely to affect R&D investment are those concerning the availability and cost of specialized researchers and facilities and proximity of academic research facilities. Prudent public policy would therefore manage pharmaceutical expenditures using evidence-based policies – which evidence from British Columbia shows can achieve cost-control and patient health goals – and pursue scientific and economic development goals through direct and strategic government investment in local scientific capacity. Provinces like British Columbia would be well advised to consider strategic support of scientific research in other areas, such as biotechnology, rather than competing (at significant cost to taxpayers) to overcome the pull of historical concentration of pharmaceutical investments in other, distant locations.
Acknowledgements
This project was supported by the Centre for Health Services and Policy Research (CHSPR) at the University of British Columbia. CHSPR is supported in part by the BC Ministry of Health and a Research Unit Award from the Michael Smith Foundation for Health Research (MSFHR). Steve Morgan is supported by a New Investigator award from the Canadian Institutes of Health Research (CIHR) and Scholar Award from MSFHR. The views presented here are solely those of the authors and not necessarily those of CHSPR, the BC Ministry of Health, CIHR or MSFHR.
Footnotes
This is a abridged version of a longer paper available online at http://www.longwoods.com/product.php?productid=19524
Contributor Information
Steve Morgan, Assistant Professor, Department of Health Care and Epidemiology, University of British Columbia; Research Lead, Program in Pharmaceutical Policy, UBC Centre for Health Services and Policy Research, Vancouver, BC.
Colleen Cunningham, Former Co-op Student, UBC Centre for Health Services and Policy Research, Graduate Program in Economics, Simon Fraser University, Vancouver, BC.
References
- Brunt H.J., Campbell N.L., Maclure M., Cassels A. Assessing the Effectiveness of Government and Industry Media Campaigns on Seniors' Perceptions of Reference-Based Pricing Policy. Journal of Applied Gerontology. 1998;17(3):19. [Google Scholar]
- Cockburn I., Henderson R. Public–Private Interaction in Pharmaceutical Research. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(23):12725–30. doi: 10.1073/pnas.93.23.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conference Board of Canada. How Canada Performs: A Report Card on Canada. Ottawa: Author; 2007. p. 154. [Google Scholar]
- Cornet M., Rensman M. The Location of R&D in the Netherlands: Trends, Determinants and Policy. The Hague: CPB Netherlands Bureau for Economic Policy Analysis; 2001. CPB Document. [Google Scholar]
- Coutts J. Drug Makers Taking BC's War on Prices to Court: Provincial Policy Instructs Doctors to Prescribe Cheapest Remedies for Three Common Problems. The Globe and Mail. 1995. Dec 19, p. A.10.
- Davis L.N., Meyer K.E. Subsidiary Research and Development, and the Local Environment. International Business Review. 2004;13(3):359–82. [Google Scholar]
- Grootendorst P., Di Matteo L. The Effect of Pharmaceutical Patent Term Length on Research and Development and Drug Expenditures in Canada. Healthcare Policy. 2007;2(3):63–84. [PMC free article] [PubMed] [Google Scholar]
- Grootendorst P., Holbrook A. Evaluating the Impact of Reference-Based Pricing. Canadian Medical Association Journal. 1999;161(3):273–74. [PMC free article] [PubMed] [Google Scholar]
- Harris R. Commentary. Vol. 211. Toronto: C.D. Howe Institute; 2005. May, Canada's R&D Deficit – And How to Fix It. pp. 1–24. [Google Scholar]
- Hazlet T.K., Blough D.K. Health Services Utilization with Reference Drug Pricing of Histamine (2) Receptor Antagonists in British Columbia Elderly. Medical Care. 2002;40(8):640–49. doi: 10.1097/00005650-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Howitt P. Commentary. Vol. 246. Toronto: C.D. Howe Institute; 2007. Apr, Innovation, Competition and Growth: A Schumpeterian Perspective on Canada's Economy. pp. 1–15. [Google Scholar]
- Jaffe A.B. Real Effects of Academic Research. American Economic Review. 1989;79(5):957. [Google Scholar]
- Kalant N., Shrier I. Research Output of the Canadian Pharmaceutical Industry: Where Has All the R&D Gone? Healthcare Policy. 2006;1(4):21–34. [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle W. Foreign Direct Investment in Industrial Research in the Pharmaceutical and Electronics Industries – Results from a Survey of Multinational Firms. Research Policy. 1999;28(2–3):179–93. [Google Scholar]
- Mansfield E., Lee J.-Y. The Modern University: Contributor to Industrial Innovation and Recipient of Industrial R&D Support. Research Policy. 1996;25(7):1047–58. [Google Scholar]
- Morfitt G.L., Esdaile J., Gladstone A., Moleschi M., Saxton A. Report of the Reference Drug Program Consultation Panel. Victoria, BC: Ministry of Health; 2002. p. 6. [Google Scholar]
- Morgan S., Bassett K., Mintzes B. Outcomes-Based Drug Coverage in British Columbia. Health Affairs (Millwood) 2004;23(3):269–76. doi: 10.1377/hlthaff.23.3.269. [DOI] [PubMed] [Google Scholar]
- Morgan S., McMahon M., Greyson D. Balancing Health and Industrial Policy Objectives in the Pharmaceutical Sector: Lessons from Australia. Health Policy. 2008 doi: 10.1016/j.healthpol.2008.01.003. (in press) [DOI] [PubMed] [Google Scholar]
- Mullens A. Report on Health and Pharmaceuticals: BC's Contentious Drug Policy in Spotlight. Reference-Based Pricing Draws Praise, Criticism. The Globe and Mail. 1997. Oct 28, p. C1.
- Organisation for Economic Co-operation and Development (OECD) R&D and Innovation: Creating and Diffusing Knowledge. Paris: Author; 2005. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) Trends and Recent Developments in Foreign Direct Investment. Paris: Author; 2006. [Google Scholar]
- Pazderka B. Commentary: The Effect of Pharmaceutical Patent Term Length on R&D and Drug Expenditures in Canada. Healthcare Policy. 2007;2(3):85–89. [PMC free article] [PubMed] [Google Scholar]
- Porter M.E. Clusters and the New Economics of Competition. Harvard Business Review. 1998;76(6):77–90. [PubMed] [Google Scholar]
- Porter M.E. Location, Competition and Economic Development: Local Clusters in a Global Economy. Economic Development Quarterly. 2000;14(1):15–34. [Google Scholar]
- Schneeweiss S., Dormuth C., Grootendorst P., Soumerai S.B., Maclure M. Net Health Plan Savings from Reference Pricing for Angiotensin-Converting Enzyme Inhibitors in Elderly British Columbia Residents. Medical Care. 2004;42(7):653–60. doi: 10.1097/01.mlr.0000129497.10930.a2. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S., Soumerai S.B., Glynn R.J., Maclure M., Dormuth C., Walker A.M. Impact of Reference-Based Pricing for Angiotensin-Converting Enzyme Inhibitors on Drug Utilization. Canadian Medical Association Journal. 2002;166(6):737–45. [PMC free article] [PubMed] [Google Scholar]
- Schneeweiss S., Soumerai S.B., Maclure M., Dormuth C., Glynn R.J. Clinical and Economic Consequences of Reference Pricing for Dihydropyridine Calcium Channel Blockers. Clinical Pharmacology & Therapeutics. 2003;74(4):388–400. doi: 10.1016/S0009-9236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S., Walker A.M., Glynn F.J., Maclure M., Dormuth C., Soumerai S.B. Outcomes of Reference Pricing for Angiotensin-Converting Enzyme Inhibitors. New England Journal of Medicine. 2002;346(11):822–29. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- Taggart J.H. Determinants of the Foreign R&D Locational Decision in the Pharmaceutical Industry. R&D Management. 1991;21(3):229–40. [Google Scholar]