Abstract
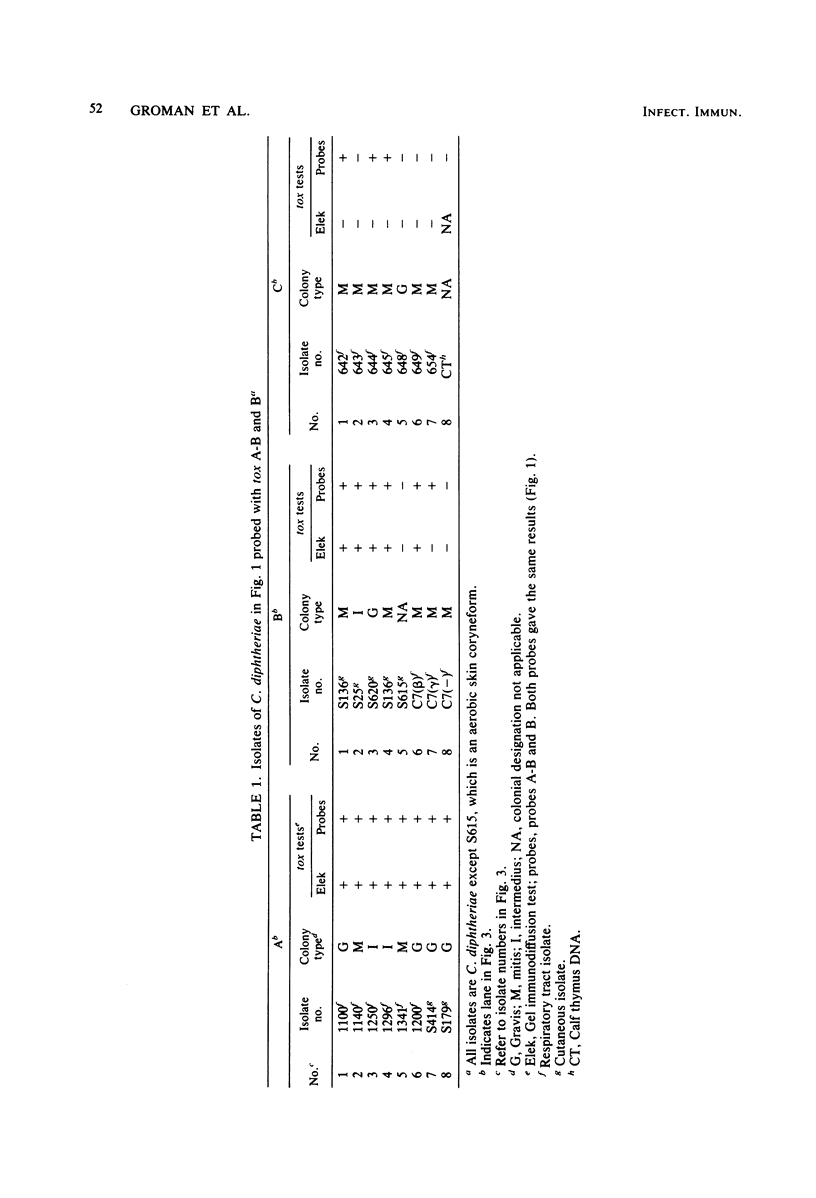
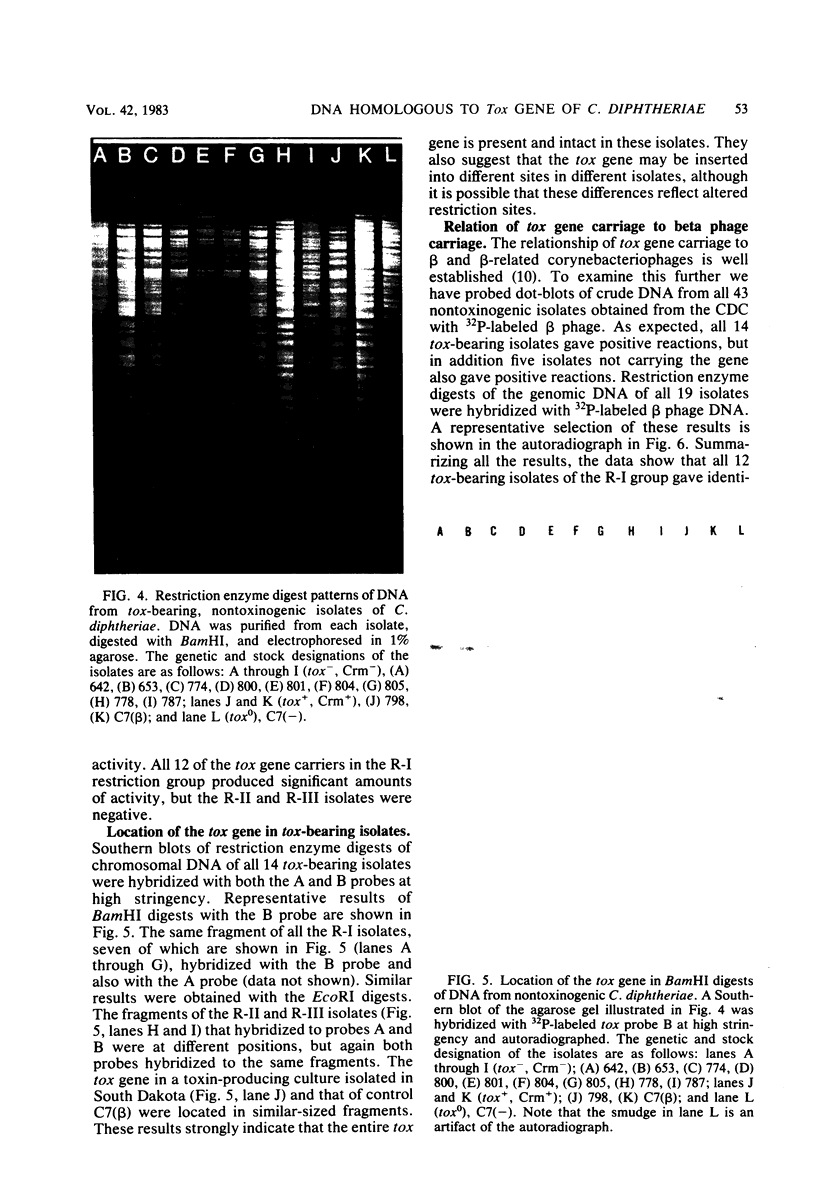
Three probes have been described which can be used to detect the presence of DNA sequences homologous to the tox gene of Corynebacterium diphtheriae. Probes "A" and "B" detected sequences coding for A and B fragments of diphtheria toxin, respectively. The third "A-B" probe contained both the A and B coding sequences. The B probe was completely unambiguous in detecting only toxin-related sequences, and the A probe was only slightly less so. The efficacy of the probes was tested on a series of toxinogenic and nontoxinogenic isolates of C. diphtheriae. All isolates which were toxinogenic as characterized by the gel immunodiffusion technique gave positive reactions with the probes. Of particular interest was the finding that 14 of 43 nontoxinogenic isolates also carried DNA homologous to both the A and B probes. All 14 isolates were nontoxinogenic by the rabbit intracutaneous test as well as by the gel immunodiffusion test; however, 12 of them produced ADP-ribosylating activity, whereas two were negative. The isolates producing ADP-ribosylating activity belonged to a cohort of cultures, of which 11 were isolated in South Dakota and 1 was isolated in Montana. Genomic DNAs of all 12 appeared to be identical when restriction enzyme digest patterns were compared, and the same fragment carried the tox gene in all of them. The tox-bearing nontoxinogenic isolates from Alaska and Florida each had unique restriction patterns and did not produce ADP-ribosylating activity. A number of genomic fragments of all the tox-bearing nontoxinogenic isolates hybridized with beta converting phage DNA. The significance of these observations to the natural history of diphtheria was discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck G. A., Groman N. B. Identification of deoxyribonucleic acid restriction fragments of beta-converting corynebacteriophages that carry the gene for diphtheria toxin. J Bacteriol. 1981 Oct;148(1):153–162. doi: 10.1128/jb.148.1.153-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Physical mapping of beta-converting and gamma-nonconverting corynebacteriophage genomes. J Bacteriol. 1981 Oct;148(1):131–142. doi: 10.1128/jb.148.1.131-142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. J., Michel J. L., Rappuoli R., Murphy J. R. Restriction map of corynebacteriophages beta c and beta vir and physical localization of the diphtheria tox operon. J Bacteriol. 1981 Oct;148(1):124–130. doi: 10.1128/jb.148.1.124-130.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Conversion in Corynebacterium diphtheriae with phages originating from nontoxigenic strains. Virology. 1956 Dec;2(6):843–844. doi: 10.1016/0042-6822(56)90066-6. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M. Genetic factors in Corynebacterium diphtheriae conversion. J Bacteriol. 1955 Dec;70(6):637–640. doi: 10.1128/jb.70.6.637-640.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Comparative studies with tox plus and tox minus corynebacteriophages. J Virol. 1970 Jun;5(6):783–784. doi: 10.1128/jvi.5.6.783-794.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K. Characterization and genetic mapping of nontoxinogenic (tox) mutants of corynebacteriophage beta. J Virol. 1976 Jul;19(1):195–207. doi: 10.1128/jvi.19.1.195-207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Isolation and characterization of tox mutants of corynebacteriophage beta. J Virol. 1976 Jul;19(1):220–227. doi: 10.1128/jvi.19.1.220-227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS E. I. Induction of toxigenicity in non-toxigenic strains of C. diphtheriae with bacteriophages derived from non-toxigenic strains. Proc Soc Exp Biol Med. 1955 Oct;90(1):91–93. doi: 10.3181/00379727-90-21948. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Schiller J., Groman N., Coyle M. Plasmids in Corynebacterium diphtheriae and diphtheroids mediating erythromycin resistance. Antimicrob Agents Chemother. 1980 Nov;18(5):814–821. doi: 10.1128/aac.18.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Murphy J. R., Davis B. D. Precursor in cotranslational secretion of diphtheria toxin. J Bacteriol. 1980 Jan;141(1):184–189. doi: 10.1128/jb.141.1.184-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]