SUMMARY
The first step in steroidogenesis is cholesterol mobilization from cytosolic lipid droplets to the initiating rate-limiting enzyme complex located on the inner mitochondrial membrane. Angiotensin II (AngII), the primary agonist of aldosterone secretion from adrenal glomerulosa cells, is known to induce cholesterol mobilization to mitochondria. However, the role of the protein kinase C (PKC) pathway in mediating cholesterol mobilization is unknown. To determine PKC’s involvement, human adrenocortical carcinoma cells were incubated with or without PKC-activating phorbol 12-myristate 13-acetate (PMA) and mitochondrial cholesterol content assayed. Like AngII, PMA significantly elevated mitochondrial cholesterol content as well as aldosterone secretion. Thus, PKC may play a role in cholesterol mobilization to mitochondria and hence steroid production. Atrial natriuretic peptide (ANP) inhibited both AngII- and PMA-stimulated mitochondrial cholesterol content. These findings suggest that the ability of ANP to inhibit steroidogenesis induced by multiple agents may be related to its capacity to reduce cholesterol mobilization.
Keywords: Human adrenocortical carcinoma cells, NCI H295R cells, phorbol 12-myristate 13-acetate, 12-O-tetradecanoylphorbol 13-acetate, steroidogenesis, aldosterone secretion
INTRODUCTION
Steroid hormones are synthesized from cholesterol stored as cholesterol esters in cytoplasmic lipid droplets. Steroidogenesis is initiated in response to agonists of secretion via activation of cholesterol esterase (or cholesterol ester hydrolase, CEH) and mobilization of cholesterol from these lipid droplets to the outer membrane of the mitochondria. This mobilization of cholesterol is thought to involve cytoskeletal rearrangements [reviewed in (Feuilloley et al., 1996)], but the exact mechanism by which this mobilization occurs is unclear. From the outer membrane cholesterol is transferred to the inner mitochondrial membrane by a process involving the steroidogenic acute regulatory (StAR) protein [reviewed in (Miller, 2007; Stocco, 2001)]. There, the cholesterol side-chain cleavage complex (CYP11A1), the rate-limiting enzyme of steroid hormone synthesis [reviewed in (Miller, 2005)], hydrolyzes cholesterol to produce pregnenolone and initiate steroidogenesis.
In adrenal glomerulosa cells aldosterone biosynthesis is stimulated both by agents that activate adenylate cyclase and those that induce phosphoinositide hydrolysis. Thus, angiotensin II (AngII) induces aldosterone synthesis and secretion by activating the hydrolysis of phosphatidylinositol 4,5-bisphosphate and protein kinase C (PKC)/calcium-calmodulin-dependent protein kinase signaling; whereas adrenocorticotropic hormone (ACTH) stimulates cAMP production and cAMP-dependent protein kinase activity [reviewed in (Rainey et al., In press)]. Atrial natriuretic peptide (ANP) inhibits aldosterone secretion triggered by both these physiological agonists as well as a variety of pharmacologic agents [e.g., (Isales et al., 1989) and reviewed in (Barrett et al., 1989)]. This panoramic inhibitory capacity suggests that ANP must inhibit at a common step in steroidogenesis, and a likely possibility is the cholesterol mobilization phase. Indeed, Cherradi et al. (Cherradi et al., 1998) have shown that ANP decreases the amount of cholesterol found in mitochondrial contact sites (sites at which the outer and inner mitochondrial membranes are closely apposed) in AngII-stimulated bovine adrenal glomerulosa cells, although these authors suggest that this effect is the result of ANP-induced inhibition of StAR expression and cholesterol transport to contact sites.
In this report we confirm that AngII induces cholesterol mobilization to mitochondria in the human adrenocortical carcinoma cell line, NCI H295R. We also show, for the first time, that PKC-activating phorbol 12-myristate 13-acetate (PMA) increases the mitochondrial cholesterol content in and aldosterone secretion from these cells. Thus, our results suggest a possible role of PKC in the cholesterol mobilization step of steroidogenesis. Finally, ANP inhibits these effects, again highlighting the ability of this hormone to block various aspects of steroid hormone biosynthesis.
MATERIALS AND METHODS
Materials
DMEM/Ham’s F12 and antibiotic/antimycotic were obtained from Invitrogen (Carlsbad, CA). UltroSer G was purchased from Biosepra (France) under a permit from the United States Department of Agriculture. ITS+ [6.25 µg insulin, 6.25 µg transferrin, selenous acid, linoleic acid and 1.25% bovine serum albumin (BSA)] was from BD Biosciences (Franklin Lakes, NJ). BSA, aminoglutethimide, sucrose, cholesterol (as standard), horseradish peroxidase, cholesterol oxidase, p-hydroxyphenylacetic acid, sodium cholate and Tris buffer were purchased from Sigma (St. Louis, MO). Other reagents for the enzymatic cholesterol and citrate synthase assays (acetyl-Coenzyme A, citrate synthase and oxaloacetic acid) were from Boehringer (Ridgefield, CT), Roche Diagnostics (Indianapolis, IN) and Calbiochem (San Diego, CA), respectively. Solid-phase radioimmunoassay kits were purchased from Diagnostic Products (Los Angeles, CA). The Amplex Red cholesterol assay kit was obtained from Molecular Probes (Eugene, OR). The NCI H295R human adrenocrotical carcinoma cell line was generously provided by Dr. William Rainey (University of Texas Southwestern Medical Center, Dallas, TX; current address: Medical College of Georgia, Augusta, GA).
Culture of NCI H295R Human Adrenocortical Carcinoma Cells
NCI H295R cells were cultured as described in (Bird et al., 1993). Briefly, cells were grown in DMEM/Ham's F12 (1:1 vol:vol) containing 1% ITS+, 2% UltroSer G, 100 U/mL penicillin,100 µg/mL streptomycin and 0.25 µg/mL fungizone, to approximately 70–75% confluence. The cells were then incubated for 20–24 hours in serum-free DMEM/Ham's F12 (containing 0.01% BSA and antibiotic/antimycotic) prior to experimentation.
Measurement of Aldosterone Secretion
Aldosterone release into the medium was assayed using a radioimmunoassay kit from Diagnostic Products Corporation as described previously (Bollag et al., 1990).
Measurement of Mitochondrial Cholesterol Content
For determination of mitochondrial cholesterol content, cells were stimulated with the appropriate agents in serum-free DMEM/Ham’s F12 (containing BSA and antibiotic/antimycotic) in the presence of 500 µM aminoglutethimide (to inhibit cholesterol metabolism) and harvested in lysis buffer (500 µM aminoglutethimide and 250 mM sucrose in 5 mM Tris, pH 7.4). Cells were homogenized in a Potter Elvehjem homogenizer, aliquots removed for determination of protein content and citrate synthase activity and the homogenates centrifuged to remove nuclei. Mitochondria were then collected by centrifugation at 10,000 × g for 10 minutes, lysed by sonication and aliquots again taken for protein content and citrate synthase determination. Citrate synthase specific activity in the mitochondrial fraction versus the cell homogenate was measured using the spectrophotometric assay described in (Srene et al., 1963). Protein content was determined using the Biorad assay with BSA as standard. Cholesterol content was then measured in the mitochondrial fraction using the cholesterol oxidase assay described in (Cherradi et al., 1996; Gamble et al., 1978) or an Amplex Red Cholesterol kit as per the manufacturer’s instructions.
Data Analysis
Experiments were performed a minimum of three times. Experiments for which an AngII positive control [(Cherradi et al., 1996) and see below] stimulated no increase in mitochondrial cholesterol content were discarded. In addition, an enrichment of the activity of citrate synthase was required for inclusion of the experiment. For the experiments presented, the relative mean enrichment was 6 ± 1-fold. Values were analyzed for statistical significance by a one-sample t-test compared to a control of 1.0 or by analysis of variance (ANOVA) with a Student-Newmann-Keuls post-hoc test using Instat (GraphPad Software, San Diego, CA) as indicated.
RESULTS
Cherradi et al. (Cherradi et al., 1996) demonstrated previously that AngII increased mitochondrial cholesterol content in bovine adrenal glomerulosa cells by 28 ± 5%. Initially, we confirmed that in our hands AngII stimulated cholesterol mobilization to the mitochondria in the human adrenocortical carcinoma cell line, the NCI H295R cells. Thus, H295R cells were incubated for 24 hours with 10 nM AngII in the presence of 500 µM aminoglutethimide to inhibit cholesterol metabolism. We verified that AngII elicited a significant increase in mitochondrial cholesterol content of approximately 40% (to a value of 1.4 ± 0.1-fold over a control of 1.0; n=9, p<0.01 using a one-sample t test), with a mean relative enrichment of citrate synthase activity of 6.3 ± 1.4-fold. In the majority of subsequent experiments, AngII was used as a positive control to ensure responsiveness of the cells in terms of changes in mitochondrial cholesterol content. On the other hand, an elevated extracellular potassium concentration (15 mM KCl) had no effect on mitochondrial cholesterol content (1.0- ± 0.1-fold over a control of 1.0; n=6, not significant), despite inducing a 3.5- ± 0.3-fold increase in aldosterone secretion (p<0.001 versus the control of 1.0 using a one-sample t test). As a control for possible osmotic effects of KCl, an additional control in which 15 mM NaCl was added to the medium also did not exhibit significantly altered mitochondrial cholesterol content or aldosterone secretion (1.1- ± 0.2- and 1.2- ± 0.2-fold over the control of 1.0, respectively; n=3, not significant).
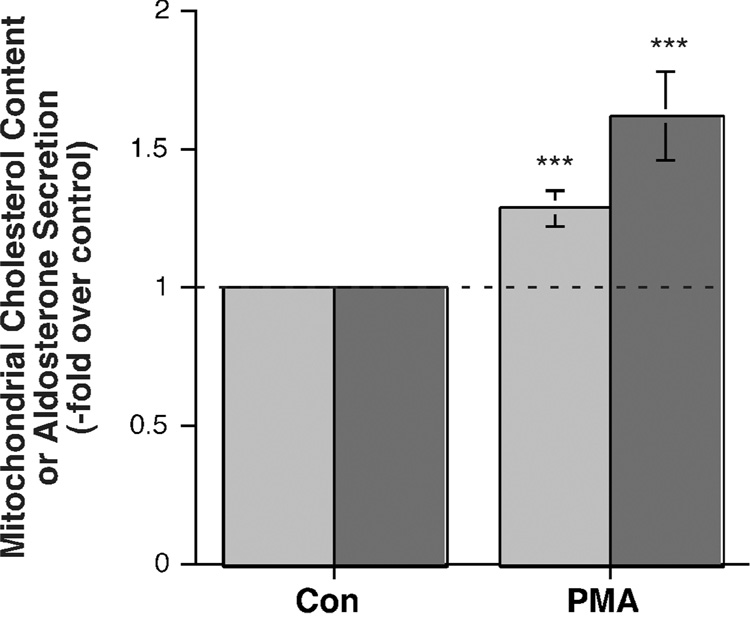
In previous experiments we and others have demonstrated that the PKC-activating phorbol ester, phorbol 12-myristate 13-acetate (PMA), can increase aldosterone secretion from bovine adrenal glomerulosa cells (Betancourt-Calle et al., 1999; Kojima et al., 1983), although other investigators have failed to observe such an effect in rat or bovine adrenal glomerulosa cells (Ganguly et al., 1992; Hajnoczky et al., 1992; Kojima et al., 1986) or the H295 cell line (Bird et al., 1995). In our hands, 10 nM PMA significantly stimulated aldosterone secretion from the H295R cells by about 1.6-fold over control (Figure 1). We also investigated the effect of PMA on mitochondrial cholesterol content and found that PMA also induced a significant increase in mitochondrial cholesterol content (Figure 1).
Figure 1. PMA stimulated aldosterone secretion from and mitochondrial cholesterol content in the human adrenocortical carcinoma cell line, NCI H295R.
H295R cells were incubated for 24 hours with serum-free medium containing 500 µM aminoglutethimide in the presence and absence of 10 nM PMA. Mitochondria were isolated and assayed for cholesterol content (light bars) as described in Methods. Values represent the means ± S.E.M. of 12 separate experiments; ***p<0.002 versus a control of 1.0 by a one-sample t-test. The mean relative enrichment of citrate synthase activity was 6.7 ± 1.7-fold. (Note that one value of relative enrichment in this set was estimated, because the actual citrate synthase activity in the homogenate could not be exactly calculated due to the loss of the homogenate protein aliquot prior to assay.) In additional, parallel experiments, H295R cells were incubated for 24 hours with medium containing no additions or 10 nM PMA. Supernatants were collected and assayed for aldosterone secretion (dark bars) by radioimmunoassay. Values represent the means ± S.E.M. of 14 separate experiments; ***p<0.002 versus a control of 1.0 by a one-sample t-test.
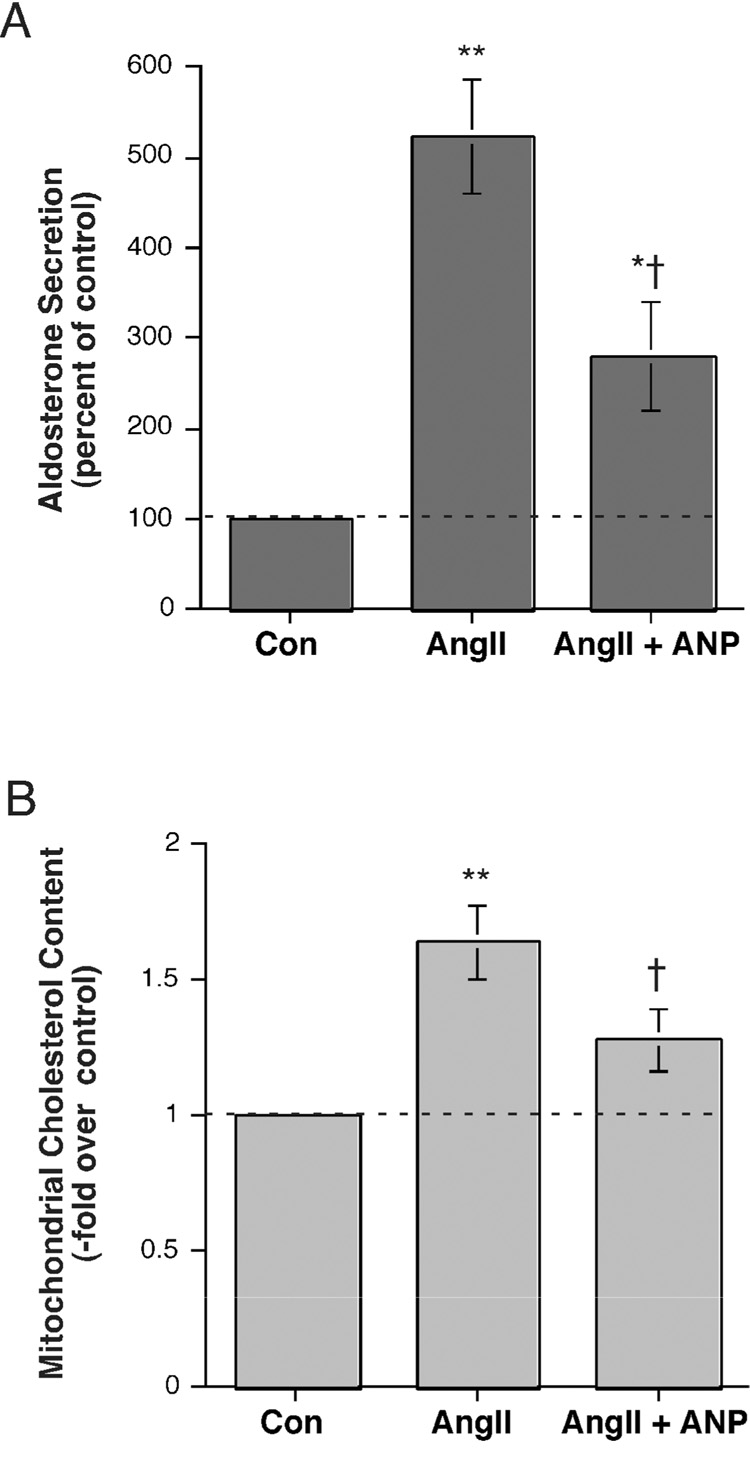
Atrial natriuretic peptide (ANP) has been reported to inhibit aldosterone secretion stimulated by a variety of physiological and pharmacological agents [reviewed in (Barrett et al., 1989)]. Therefore, we investigated the effect of ANP on AngII-induced aldosterone secretion from and mitochondrial cholesterol content in H295R cells. As shown in Figure 2A and reported previously in bovine adrenal glomerulosa cells (Calle et al., 2001) and H295R cells (Bodart et al., 1996), AngII stimulated aldosterone secretion and ANP significantly reduced this increase. A similar result was seen in terms of mitochondrial cholesterol content. Thus, AngII increased mitochondrial cholesterol content and ANP significantly decreased this rise (Figure 2B).
Figure 2. Atrial natriuretic peptide (ANP) inhibited AngII-stimulated aldosterone secretion and mitochondrial cholesterol content.
(A) H295R cells were incubated for 24 hours with serum-free medium containing no additions, 10 nM AngII or 10 nM AngII plus 100 nM ANP. Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M. of 5 separate experiments. (B) H295R cells were incubated for 24 hours with serum-free medium containing no additions, 10 nM AngII, or 10 nM AngII plus 100 nM ANP. Mitochondria were isolated and cholesterol measured as described in Methods. Values represent the means ± S.E.M. of 4 separate experiments; *p<0.05, **p<0.01 versus control and †p<0.01 versus Ang II by ANOVA and a Student-Neuman-Keul's post-hoc test. The mean relative enrichment of citrate synthase activity was 7.8 ± 2.9-fold.
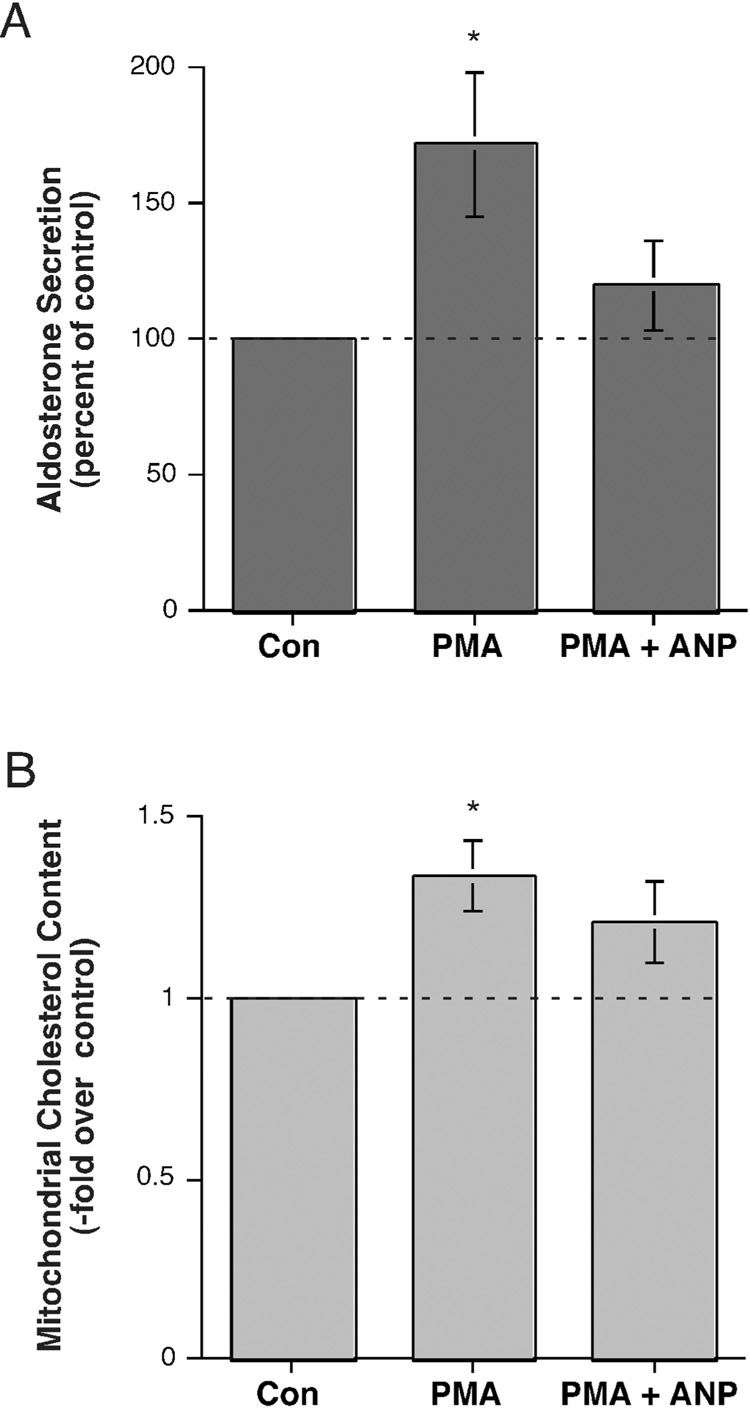
We also investigated the effect of ANP on PMA-stimulated aldosterone secretion and mitochondrial cholesterol content. PMA induced a significant elevation in the aldosterone secretory rate and this increase was returned to a value not significantly different from the control value with simultaneous exposure to ANP (Figure 3A). Similarly, the PMA-induced stimulation of mitochondrial cholesterol content was reduced to levels not significantly different from the control by ANP (Figure 3B). Experiments demonstrated that ANP alone also decreased basal aldosterone secretion by approximately 30% (to a value of 0.7 ± 0.1-fold over the control of 1.0; n=4, p<0.02 using a one-sample t test) and mitochondrial cholesterol content by roughly 40% (to a value of 0.6 ± 0.1-fold over the control of 1.0; n=4, p=0.054 versus the control of 1.0 using a one-sample t test), with a mean relative enrichment of citrate synthase activity of 9.1 ± 2.6-fold. Together these results suggest that one of the mechanisms by which ANP may function to inhibit steroidogenesis elicited by multiple agonists may be through an ability to reduce cholesterol transport to the mitochondria.
Figure 3. Atrial natriuretic peptide (ANP) inhibited PMA-stimulated aldosterone secretion and mitochondrial cholesterol content.
(A) H295R cells were incubated for 24 hours with serum-free medium containing no addition, 10 nM PMA or 10 nM PMA plus 100 nM ANP. Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M. of 8 separate experiments. (B) H295R cells were incubated for 24 hours with serum-free medium containing 500 µM aminoglutethimide and no other additions, 10 nM PMA or 10 PMA plus 100 nM ANP. Mitochondria were isolated and cholesterol measured as described in Methods. Values represent the means ± S.E.M. of 8 separate experiments; *p<0.05 versus control by ANOVA and a Student-Neuman-Keul's post-hoc test. The mean relative enrichment of citrate synthase activity was 6.7 ± 1.7-fold. (Note that one value of relative enrichment in this set was estimated, because the actual citrate synthase activity in the homogenate could not be exactly calculated due to the loss of the homogenate protein aliquot prior to assay.)
DISCUSSION
The ability of AngII to induce cholesterol mobilization to the mitochondria of bovine adrenal glomerulosa cells has been previously shown by Cherradi et al. (Cherradi et al., 1996). These investigators demonstrated an approximate 28% increase in total mitochondrial cholesterol, as well as an increase in mitochondrial contact sites (locations at which the inner and outer mitochondrial membranes are apposed). ANP was shown to decrease cholesterol in these contact sites, perhaps as a result of the capacity of ANP to reduce StAR expression (Cherradi et al., 1998). In this report, we show for the first time that ANP also inhibited cholesterol mobilization to the mitochondria of a human adrenocorticocarcinoma cell line, the NCI H295R cells, in response to AngII (Figure 2) and the phorbol ester PMA (Figure 3). This effect correlated with an ability of ANP to also reduce aldosterone secretion from these cells (Figure 2 and Figure 3), such that in both cases ANP decreased the PMA-stimulated parameter to a value not significantly different from the control. ANP-induced inhibition of steroidogenesis was previously described in the NCI H295R cells by Bodart et al. (Bodart et al., 1996), who demonstrated functional ANP receptors, the activation of which by ANP resulted in inhibition of aldosterone secretion in these cells in response to AngII. Thus, our results, together with data in the literature, suggest that ANP works by multiple mechanisms to inhibit aldosterone secretion and is able to exert its inhibitory effects towards both physiologic and pharmacologic agents, such as PMA. Indeed, these results argue that ANP exerts its action on aldosterone secretion and cholesterol transport via a pathway independent from that induced by PMA.
In this report we additionally demonstrate for the first time that the phorbol ester PMA also induced cholesterol movement to the mitochondria (Figure 1). This mobilization was accompanied by a PMA-elicited increase in aldosterone secretion (Figure 1). Our demonstration of an aldosterone secretory response to PMA in H295R cells is in contrast to a previous report indicating that PMA had no effect on aldosterone secretion (Bird et al., 1995). The reason for the discrepancy is unclear but likely relates to the fact that the magnitude of the increase in aldosterone secretion in response to PMA is much less than that to AngII, thus requiring a larger number of experiments to demonstrate statistical significance. This relatively low secretion to PMA alone is not unexpected, as Rasmussen and colleagues have previously proposed that maximal aldosterone secretion requires both a PKC signal and a calcium influx signal [reviewed in (Barrett et al., 1989; Rasmussen et al., 1995)]. Furthermore, the ability of PKC-activating PMA to trigger cholesterol mobilization suggests the possible involvement of PKC in this process, although other phorbol ester targets, such as Ras-GRP (a Ras guanine nucleotide exchange factor) or chimaerins (Rac GTPase-activating proteins) [reviewed in (Brose et al., 2002)], could also potentially mediate these effects. Also activated by PMA are two enzymes downstream of PKC, phospholipase D and protein kinase D [reviewed in (Shapiro et al., In press)], both of which are known to mediate aldosterone secretion (Bollag et al., 2002; Romero et al., 2006; Zheng et al., 2003). In addition, these enzymes are involved in intracellular trafficking [reviewed in (Jenkins et al., 2005; Wang, 2006)], suggesting their potential participation in cholesterol mobilization.
It is not clear why we observed no increase in mitochondrial cholesterol content upon stimulation with an elevated extracellular potassium concentration, despite the fact that the elevated potassium level can increase steroidogenesis. This result suggests that elevated potassium concentrations induce aldosterone secretion by increasing cholesterol transport within the mitochondria (e.g., from the outer to the inner mitochondrial membrane) rather than to the mitochondria from lipid droplets. This idea is consistent with the fact that elevated potassium is thought to function by depolarizing the plasma membrane, activating voltage-dependent calcium channel and increasing calcium influx [reviewed in (Rainey et al., In press)], as well as the finding that calcium can elicit transfer of cholesterol from the outer to inner mitochondrial membranes (Cherradi et al., 1996).
Capponi and colleagues (Cherradi et al., 2003) have shown that the activity of the cholesterol ester hydrolase, the enzyme that hydrolyzes cholesterol esters to release free cholesterol and initiate its mobilization to mitochondria, can be regulated by extracellular signal-regulated kinase (ERK)-mediated phosphorylation. Our results suggest that PKC (or another PMA-sensitive effector enzyme) also is involved in cholesterol mobilization from cytosolic lipid droplets to the mitochondrial outer membrane, although whether this is a direct effect or via activation of downstream kinases, such as ERK [reviewed in (Basu et al., 2007)], is not known. This cholesterol-mobilizing effect may then contribute to the ability of PKC-activating agonists like AngII to stimulate steroidogenesis from adrenal glomerulosa cells.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr. Rainey’s kind gift of the NCI H295R cells. We also express our profound appreciation to Dr. Robert Podolsky (Medical College of Georgia, Augusta, GA) for his consultation concerning statistical analyses. This work was supported by an American Heart Association/Southeast Affiliate grant-in-aid award #0051573B (to RAC and CMI) and an American Heart Association grant-in-aid award #0350166N and a National Institutes of Health award #HL070046 (to WBB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barrett PQ, Bollag WB, Isales CM, McCarthy RT, Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989;10(4):1–22. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Basu A, Sivaprasad U. Protein kinase Ce makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt-Calle S, Bollag WB, Jung EM, Calle RA, Rasmussen H. Effects of angiotensin II and ACTH on MARCKS phosphorylation in glomerulosa cells. Mol. Cell. Endocrinol. 1999;154:1–9. doi: 10.1016/s0303-7207(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: A model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mathis JM, Mason JI, Rainey WE. Ca2+-regulated expression of steroid hydroxylases in H295R human adrenocortical cells. Endocrinology. 1995;136:5677–5684. doi: 10.1210/endo.136.12.7588323. [DOI] [PubMed] [Google Scholar]
- Bodart V, Rainey WE, Fournier A, Ong H, De Lean A. The H295R human adrenocortical cell line contains functional atrial natriuretic peptide receptors that inhibit aldosterone biosynthesis. Mol. Cell. Endocrinol. 1996;118:137–144. doi: 10.1016/0303-7207(96)03776-8. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Barrett PQ, Isales CM, Liscovitch M, Rasmussen H. A potential role for phospholipase-D in the angiotensin-II-induced stimulation of aldosterone secretion from bovine adrenal glomerulosa cells. Endocrinology. 1990;127(3):1436–1443. doi: 10.1210/endo-127-3-1436. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Jung EM, Calle RA. Mechanism of angiotensin II-induced phospholipase D activation in adrenal glomerulosa cells. Mol. Cell. Endocrinol. 2002;192:7–16. doi: 10.1016/s0303-7207(02)00134-x. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- Calle RA, Bollag WB, White S, Betancourt-Calle S, Kent P. ANPs effect on MARCKS and StAR phosphorylation in agonist-stimulated glomerulosa cells. Mol. Cell. Endocrinol. 2001;177:71–79. doi: 10.1016/s0303-7207(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Brandenburger Y, Rossier MF, Vallotton MB, Stocco DM, Capponi AM. Atrial naitruretic peptide inhibits calcium-induced steroidogenic acute regulatory protein gene transcription in adrenal glomerulosa cells. Mol. Endocrinol. 1998;12:962–972. doi: 10.1210/mend.12.7.0132. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Pardo B, Greenberg AS, Kraemer FB, Capponi AM. Angiotensin II activates cholesterol ester hydrolase in bovine adrenal glomerulosa cells through phosphorylation mediated by p42/p44 mitogen-activated protein kinase. Endocrinology. 2003;144:4905–4915. doi: 10.1210/en.2003-0325. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Rossier MF, Vallotton MB, Capponi AM. Calcium stimulates intramitochondrial cholesterol transfer in bovine adrenal glomerulosa cells. J. Biol. Chem. 1996;271:25971–25975. doi: 10.1074/jbc.271.42.25971. [DOI] [PubMed] [Google Scholar]
- Feuilloley M, Vaudy H. Role of the cytoskeleton in adrenocortical cells. Endocr. Rev. 1996;17:269–288. doi: 10.1210/edrv-17-3-269. [DOI] [PubMed] [Google Scholar]
- Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J. Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- Ganguly A, Chiou S, Fineberg NS, Davis JS. Greater importance of Ca(2+)-calmodulin in maintenance of ang II- and K(+)-mediated aldosterone secretion: lesser role of protein kinase C. Biochem. Biophys. Res. Commun. 1992;182:254–261. doi: 10.1016/s0006-291x(05)80138-x. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Varnai P, Buday L, Farago A, Spat A. The role of protein kinase-C in control of aldosterone production by rat adrenal glomerulosa cells: activation of protein kinase-C by stimulation with potassium. Endocrinology. 1992;130(4):2230–2236. doi: 10.1210/endo.130.4.1547736. [DOI] [PubMed] [Google Scholar]
- Isales CM, Bollag WB, Kiernan LC, Barrett PQ. Effect of ANP on sustained aldosterone secretion stimulated by angiotensin II. Am. J. Physiol. 1989;256:C89–C95. doi: 10.1152/ajpcell.1989.256.1.C89. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62(19–20):2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I, Lippes H, Kojima K, Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983;116:555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Kojima I, Shibata H, Ogata E. Phorbol ester inhibits angiotensin-induced activation of phospholipase C in adrenal glomerulosa cells. Its implication in the sustained action of angiotensin. Biochem. J. 1986;237:253–258. doi: 10.1042/bj2370253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology. 2005;146:2544–2550. doi: 10.1210/en.2005-0096. [DOI] [PubMed] [Google Scholar]
- Miller WL. StAR search--What we know about how the steroidogenic acute regulatory protein mediates mtiochondrial cholesterol import. Mol. Endocrinol. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bollag WB, Isales CM. Aldosterone regulation. In: Singh AJ, Williams GH, editors. Textbook of Nephro-Endocrinology. Atlanta, Ga: Elsevier, Inc.; (In press) [Google Scholar]
- Rasmussen H, Isales CM, Calle R, Throckmorton D, Anderson M, Gasalla-Herraiz J, McCarthy R. Diacylglycerol production, Ca2+ influx, and protein kinase C activation in sustained cellular responses. Endocr. Rev. 1995;16:649–681. doi: 10.1210/edrv-16-5-649. [DOI] [PubMed] [Google Scholar]
- Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006;147(12):6046–6055. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- Shapiro BA, Bollag WB. Angiotensin II signaling in the adrenal cortex: a role for protein kinases C and D in acute aldosterone secretion? Hauppauge, New York: NovaScience Publishers; (In press) [Google Scholar]
- Srene PA, Brazil H, Gonen L. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta CHem. Scand. 1963;17 Suppl. 1 [Google Scholar]
- Stocco DM. Tracking the role of a star in the sky of the new millenium. Mol. Endocrinol. 2001;15:1245–1254. doi: 10.1210/mend.15.8.0697. [DOI] [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Zheng X, Bollag WB. AngII induces transient phospholipase D activity in the H295R glomerulosa cell model. Mol. Cell. Endocrinol. 2003;206:113–122. doi: 10.1016/s0303-7207(03)00211-9. [DOI] [PubMed] [Google Scholar]