Abstract
Human studies and animal models suggest that mechanical as well as biological processes contribute to acute lung injury. While mechanical stresses and bacterial products can directly alter the endothelial and epithelial barriers in the lungs, a growing body of evidence suggests that synergistic interactions between low levels of mechanical stress and bacterial products in the lungs can cause or exacerbate acute lung injury. New approaches to disrupting these synergistic interactions between mechanical stress and innate immunity have the potential to reduce the incidence or improve the outcome of acute lung injury in humans.
Keywords: lung injury, epithelium, VILI, apoptosis
Acute lung injury (ALI) is an important problem for the U.S. population and the health care system. ALI affects more than 200,000 people in the United States each year, with more than 75,000 deaths, and annual health care costs exceeding $2 billion (1). The mortality rate rises with age, and the annual mortality from ALI exceeds the combined mortality from breast cancer and AIDS. More than 75% of the cases of ALI are associated with infections, either in the lungs or elsewhere in the body, and mechanical ventilation is a lifesaving mode of treatment (1). Although advances have been made in understanding the pathophysiology of lung injury, our understanding of the causal mechanisms is incomplete. Accumulating evidence suggests that mechanical stresses and innate immunity pathways alone and in combination can cause or compound lung injury, but the mechanisms responsible are not completely understood.
MECHANICAL STRESS AND LUNG INJURY
The observation that mechanical stress alters lung physiology was made more than 40 years ago (2, 3), and several excellent reviews have appeared summarizing the links between mechanical stresses and lung injury (4–6). In normal humans, transpulmonary pressures are low during tidal breathing, and tidal volumes of up to 15 ml/kg are well tolerated in patients without lung injury, such as those with neuromuscular diseases. The work of Tschumperlin and Margulies showed that basement membrane surface area in isolated rat lungs does not increase until the lungs are inflated to approximately 45 to 50% of total lung capacity (7). Thus, in normal lungs, the alveolar walls fold and unfold during tidal breathing when changes in distending forces are minimal, and significant stretching of the alveolar walls does not occur.
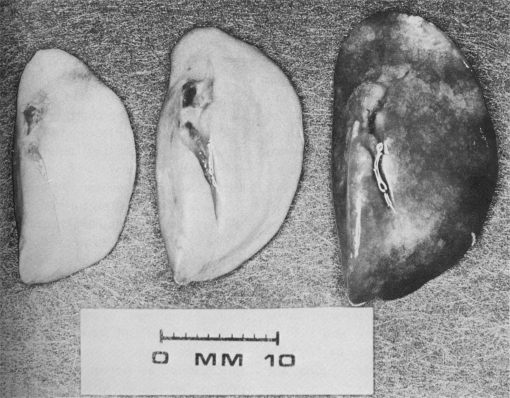
When inspiratory pressures rise significantly during mechanical ventilation, the situation changes. The classic work of Webb and Tierney showed that very high inspiratory pressures could injure rodent lungs, and that positive end-expiratory pressure (PEEP) had a protective effect (Figure 1) (8). Static inflation pressures exceeding 30 cm H2O increased albumin permeability in isolated sheep lungs (9), and peak airway pressures above 35 cm H2O increased fluid filtration from the vasculature of isolated perfused dog lungs (10). The adverse effects of high inflation pressures are rapid, as Dreyfuss and coworkers showed that ventilating rat lungs with a peak airway pressure of 45 cm H2O produced lung edema and endothelial injury in as little as 5 minutes, and ultrastructural evidence of endothelial and epithelial injury by 20 minutes (11, 12). Bachofen and Weibel observed that humans dying of ALI had ultrastructural evidence of severe epithelial injury (13), suggesting parallels between events in human lungs and the ventilated animal models. However, the pressures required to cause endothelial and epithelial damage in the animal preparations are much higher than the mean airway pressures produced in the lungs of normal humans, even when ventilated with tidal volumes of 10 to 15 ml/kg, as well as in many patients with lung injury.
Figure 1.
Rodent lungs ventilated with varying peak inspiratory pressures (Pinsp) and positive end expiratory pressure (PEEP). Left: Pinsp = 14 cm H2O, PEEP = 0 cm H2O. Middle: Pinsp = 45 cm H2O, PEEP = 10 cm H2O. Right: Pinsp = 45 cm H2O, PEEP = 0 cm H2O. Reprinted by permission from Reference 8.
The advent of computerized tomography showed that ALI was much more heterogeneous than originally thought, and that the effective alveolar volume in many patients with ALI was significantly reduced because of extensive areas of alveolar filling and collapse (14, 15). This observation led to the concept that distending pressures caused by a specific tidal volume could vary markedly in different regions of injured lungs, with much higher local pressures in areas of relatively uninjured alveolar units. Studies with rat alveolar epithelial cells in vitro supported the concept that cyclic stretch is more injurious than tonic stretch and that small amplitude cyclic stretch superimposed on a tonic stretch (the in vitro equivalent of PEEP) is protective (16). This is consistent with the concept that repeated opening and closing of alveolar units leads to local injury.
Mechanical stress on alveolar walls can produce several different consequences in injured lungs, including the physical disruption of alveolar epithelial and endothelial cells, disruption of the alveolar wall basement membrane, and activation of stretch-responsive signaling pathways in the alveolar walls and airspace leukocytes, which produce inflammation and an increase in endothelial and epithelial permeability. Neutrophils have been implicated in mediating the inflammatory aspects of ventilator-associated lung injury, and mice lacking the major neutrophil chemokine receptor, CXCR2, are protected from ventilator-associated lung injury (17–19). In addition to local effects in the lungs, emerging evidence suggests that injurious mechanical ventilation can contribute to distant organ injury (20). In the NIH ARDSnet trial of two different mechanical ventilation strategies in patients with acute lung injury, the patients treated with the lower tidal volume had significantly more days alive and free of circulatory failure, renal dysfunction, and serious coagulation abnormalities (21). The patients treated with the lower tidal volume also had lower concentrations of IL-8 and IL-6 in plasma, consistent with a prior randomized trial showing that a lung protective ventilatory strategy reduced inflammation in the lungs and systemic circulation (22, 23). In rabbits treated with intratracheal HCl to produce lung injury, an injurious ventilatory strategy with relatively high tidal volumes and low PEEP was associated with significant renal injury (24).
While a number of studies suggest that mechanical stress, reflected by high tidal volume, high airway pressures, or cyclic airway opening and closing can cause lung injury, ventilation of normal animals with lower tidal volumes and airway pressures approaching the values used in patients does not cause significant lung injury, so it is likely that other factors act with or in addition to mechanical stress in causing or exacerbating lung injury (25, 26).
BIOLOGICAL STRESS AND LUNG INJURY
Activation of innate immunity in the lungs is critical to host defense against infection. A range of microbial products activate a series of Toll like receptors (TLR) on the surface of leukocytes and lung mesenchymal cells, which activate NF-κB and other intracellular pathways, leading to acute neutrophilic inflammation and an increase in microvascular permeability in the lungs (27, 28). Mice with targeted deletion of MyD88, a key protein required for most TLR signaling, have major defects in antimicrobial host defenses (29). Over 75% of the cases of ALI in a major community survey were associated with infections, either in the lungs or elsewhere (1), and bacterial products such as lipopolysaccharide are detectable in the lungs of many patients with ALI, whether or not overt bacterial infection is detectable (30). Thus, innate immune mechanisms are likely to be activated in the lungs of most patients before and after the onset of ALI.
One of the key advances in understanding ALI was the discovery that bacterial products have additive or synergistic effects with mechanical stress in inducing or worsening experimental ALI (31). Treatment of experimental animals with either intravenous or intrapulmonary gram-negative endotoxin enhances inflammation and permeability responses to mechanical ventilation, even when the tidal volumes are in the range of 10 to 15 ml/kg (25, 26). Alveolar macrophages produce a range of proinflammatory cytokines when exposed to bacterial products, and exposure of alveolar macrophages to cyclic stretch markedly enhances the cytokine responses to endotoxin (32). Other Toll-like receptor pathways are also likely to produce synergistic interactions with mechanical stress in the lungs, increasing the likelihood of lung injury when diverse microbes or viruses enter the lungs. In addition to bacterial cell wall products like gram-negative endotoxin, whole bacteria such as Staphylococcus aureus and Escherichia coli also synergize with mechanical ventilation to worsen lung inflammation, enhance systemic inflammation, and worsen liver and renal function (33). TLR receptors also recognize endogenous molecules that are released at sites of tissue injury, called “alarmins” or “danger-associated molecular patterns” (DAMPs) (34, 35). Several of these, such as HMGB1, matrix fragments, and oxidized phospholipids, appear to activate cells via TLR4, providing endogenous stimuli that could amplify the response to mechanical ventilation in inflamed lungs in the absence of bacterial products (Table 1) (36).
TABLE 1.
ALARMINS THAT COULD BE PRESENT IN THE LUNGS
| Putative Receptor | |
|---|---|
| Heat Shock Proteins (HSP) | |
| HSP 22 | TLR2,4 |
| HSP 60 | TLR2,4 |
| HSP 70 | TLR2,4 |
| Extracellular matrix products | |
| Biglycan | TLR2, 4 |
| Hyaluronan | TLR2, 4 |
| Fibronectin domains | TLR4 |
| HMGB1 | TLR2,4 |
| Oxidized phospholipids | TLR4 |
| Oxidized LDL | TLR4 |
| β-defensins | TLR4 |
| Surfactant protein A | TLR4 |
Modified from Reference 36.
While microbial products enhance the response of the lungs to mechanical stress, several lines of evidence suggest that mechanical ventilation also enhances the responsiveness to microbial products in the lungs, providing reciprocal interactions between innate immunity and mechanical stress. For example, ventilation of normal rabbits with high tidal volumes (Vt = 20 ml/kg) increases the expression of CD14 on alveolar macrophages and alveolar epithelial cells, which is a key accessory molecule in TLR-dependent microbial recognition (37). Alveolar macrophages recovered from rabbits ventilated with high tidal volume expressed more cell surface CD14, and produced more TNF-α in response to lipopolysaccharide (LPS) in vitro (37). Consistent with these findings, mechanical ventilation rapidly increases CD14 gene expression in the lungs of adult mice (38).
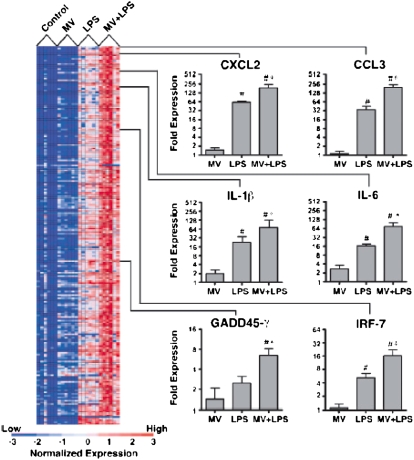
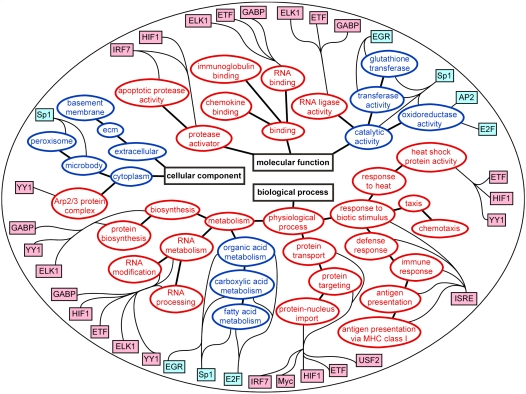
Gene microarray studies show the complexity of the interactions between mechanical ventilation and innate immunity in the lungs (39). In normal mice, the combination of a modest tidal volume (10 ml/kg) and a low dose of intratracheal LPS (5 ng/kg) has a major synergistic effect on gene expression in the lungs as compared with mechanical ventilation or intratracheal LPS alone (Figure 2) (40). Enhanced genes include chemokines, cytokines, intracellular signaling proteins, and transcriptional activators such as interferon response factor-7 (IRF-7). In addition, gene expression analysis suggests that a network of transcription factors is activated by the combination of endotoxin and mechanical ventilation in the lungs (41). Figure 3 shows a functional map of differentially expressed genes and the transcription factors that contribute to their regulation. This type of approach highlights the complexity in biological systems, which complicates the evaluation of interactions between mechanical stress and innate immunity in the lungs. This complexity must be taken into account in designing strategies to protect the lungs from the adverse effects of mechanical ventilation and microbial products. The computational approach to identifying clusters and pathways of gene activation should be valuable in developing new hypotheses about the mechanisms involved.
Figure 2.
Gene expression in the lungs of mice treated with mechanical ventilation (Vt = 10 ml/kg) or LPS (5 ng/gm intratracheally), or the combination of mechanical ventilation + LPS. Blue = unchanged or down-regulated; red = up-regulated. Reprinted by permission from Reference 40.
Figure 3.
Modular gene regulatory map of biological modules and related transcription factors expressed in the lungs of mice treated with the combination of mechanical ventilation (Vt = 10 ml/kg) and gram-negative bacterial endotoxin. Red = up-regulated; blue = down-regulated. Reprinted by permission from Reference 41.
Studies in normal humans show a great deal of variation in the intensity of TLR activation by microbial products, and subpopulations of people who are high or low responders to LPS and other bacterial products have been identified in the normal population (42). It follows that there is likely to be a significant degree of individual variation in the synergism between mechanical stress and activation of innate immunity in the lungs, consistent with the clinical observation that people vary widely in their responses to serious lung infections. The development of rapid genetic or biological markers of susceptibility could help to identify patients who are at greatest risk for severe lung injury when they require mechanical ventilation.
CELL DEATH AND ACUTE LUNG INJURY
Studies by Bachofen and Weibel showed that epithelial injury is a hallmark of acute lung injury (13). Ware and Matthay used a physiologic approach to show that epithelial function is impaired in most patients with acute lung injury, and that patients with the worst epithelial function have a poor prognosis (43). The death of epithelial cells and other cells in the lungs can occur by regulated (apoptosis) or nonregulated mechanisms (necrosis), and both are likely to be important at the intersection between mechanical and biological stresses in the lungs. Local regions of high mechanical stress are likely to cause direct necrosis of alveolar epithelial cells and injury to the alveolar basement membrane, and studies using intravital dyes to identify dying cells in the lungs support this concept (5, 44).
Regulated cell death is mediated by a family of death receptors, which activate a series of intracellular caspases, leading to cleavage of nuclear DNA and cellular involution (45). A separate mitochondrial pathway causes cell death when oxidants or other stresses damage mitochondrial membrane proteins, releasing cytochrome c into the cytoplasm. The lung fluids of patients with acute lung injury contain biologically active soluble Fas ligand (sFasL), which triggers apoptosis via the Fas receptor on distal lung epithelial cells (46, 47). The source of the alveolar sFasL is not clear, but sFasL is shed from cell surfaces by the action of matrix metalloproteinase 7 (MMP-7), which is found on the surface of lung epithelial cells (48). The Fas receptor is expressed on human and murine alveolar epithelial cells, suggesting that the alveolar surface is a target for Fas dependent apoptosis when biologically active sFasL accumulates in the airspaces.
Lung tissue specimens from patients with acute lung injury show caspase activation in alveolar wall cells, supporting an apoptotic mechanism of cell death in injured human lungs (47). Apoptosis pathways are activated in the lungs of dogs with lung injury and treated with mechanical ventilation (49). The combination of mechanical ventilation and intratracheal endotoxin increases the concentration of sFasL in the lungs of mice, and induces caspase-dependent epithelial apoptosis in the lungs. Direct activation of epithelial Fas in the lungs of rabbits using human sFasL, or in mice using a Fas-activating antibody, causes alveolar wall apoptosis and lung inflammation (50, 51). Repeated activation of Fas causes lung fibrosis within 3 weeks, mediated in part by matrix metalloproteinase-12 (MMP-12), which is a prominent product of Fas activated murine and human alveolar macrophages (52).
Apoptosis also can be initiated or amplified by TLR signaling pathways in response to microbial products or endogenous ligands. Stimulation of TLR2 by bacterial lipoproteins and activation of TLR4 signaling through the TRIF adaptor protein leads to cellular apoptosis (53). It therefore seems likely that some of the endogenous alarmins released from dying cells or disrupted extracellular matrix might trigger or accentuate apoptosis responses via TLR2 and TLR4, particularly when combined with mechanical stress. Consistent with this concept, treatment of rabbits with intratracheal HCl and a lung protective ventilatory strategy was associated with apoptosis in airway and alveolar epithelial cells (24). Interestingly, ventilation of the rabbits with an injurious ventilatory strategy (higher Vt and lower PEEP) was associated with necrosis in the lungs, rather than apoptosis, consistent with the idea that more severe mechanical stresses cause direct necrosis of lung cells (5). The additional role of microbial products was not studied, but the intratracheal instillation of acid is likely to have caused the release of a variety of endogenous “alarmins” from dying cells in the lungs, providing an opportunity for TLR pathways to compound the lung injury due to mechanical ventilation.
Thus, activation of the Fas pathway by sFasL in the airspaces, or activation of TLR receptors by microbial or endogenous ligands can cause or amplify cell death in the lungs. Because some evidence suggests that mechanical stress enhances the activity of the Fas/FasL system in the lungs, it is likely that mechanical ventilation and TLR signaling also have the potential to cause a synergistic increase in regulated cell death in injured lungs. More information is needed about the interactions between mechanical stress and cell death pathways in the lungs.
SUMMARY AND UNANSWERED QUESTIONS
Mechanical stress and innate immunity pathways interact to amplify lung inflammation and lung injury. Innate immunity evolved to provide a warning about the presence of infectious agents in tissue, and to activate defenses to eliminate them. In contrast, mechanical ventilation is a recent development designed to support the gas exchange function of the lungs, so the pathways activated by mechanical ventilation are not necessarily either adaptive or beneficial. A number of important questions remain about the mechanisms by which mechanical and biological events interact to initiate or compound acute lung injury. The specific intersection points in the signaling pathways activated by mechanical stretch and TLR receptors remain to be identified. The relative importance of apoptosis and necrosis in causing endothelial and epithelial death in injured ventilated lungs is not completely clear. Reagents have been developed to block TLR-dependent pathways in experimental animals and humans, and these could be used to dissociate the synergistic interactions between mechanical stress and innate immunity. Reducing set tidal volume and using “lung protective” ventilation in patients with acute lung injury has become the recommended practice, yet mortality from ALI remains unacceptably high. Whether transient inhibition of innate immunity pathways will further reduce the onset or severity of acute lung injury during mechanical ventilation without increasing the chances of infectious complications in the lungs is an important question that remains to be answered.
Supported in part by NIH Grants HL081764 and HL073996–01 (T.R.M.), and the Medical Research Service of the Department of Veterans Affairs.
Conflict of Interest Statement: T.R.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield LJ, Ebert PA, Benson DW. Effect of positive pressure ventilation on surface tension properties of lung extracts. Anesthesiology 1964;25:312–316. [DOI] [PubMed] [Google Scholar]
- 3.Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs' lungs. J Appl Physiol 1966;21:1453–1462. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294–323. [DOI] [PubMed] [Google Scholar]
- 5.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med 2005;171:1328–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos CC, Slutsky AS. The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 2006;68:585–618. [DOI] [PubMed] [Google Scholar]
- 7.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 1999;86:2026–2033. [DOI] [PubMed] [Google Scholar]
- 8.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis 1974;110:556–565. [DOI] [PubMed] [Google Scholar]
- 9.Egan EA, Nelson RM, Olver RE. Lung inflation and alveolar permeability to non-electrolytes in the adult sheep in vivo. J Physiol 1976;260:409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J Appl Physiol 1984;57:1809–1816. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 1985;132:880–884. [DOI] [PubMed] [Google Scholar]
- 12.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 1988;137:1159–1164. [DOI] [PubMed] [Google Scholar]
- 13.Bachofen A, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56. [PubMed] [Google Scholar]
- 14.Gattinoni L, Presenti A, Torresin A, Baglioni S, Rivolta M, Rossi F, Scarani F, Marcolin R, Cappelletti G. Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1986;1:25–30. [DOI] [PubMed] [Google Scholar]
- 15.Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J. Preservation of normal lung regions in the adult respiratory distress syndrome: analysis by computed tomography. JAMA 1986;255:2463–2465. [PubMed] [Google Scholar]
- 16.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells: effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 2000;162:357–362. [DOI] [PubMed] [Google Scholar]
- 17.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2/CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest (In press) [DOI] [PMC free article] [PubMed]
- 18.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest 2002;110:1603–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care 2005;11:82–86. [DOI] [PubMed] [Google Scholar]
- 20.Slutsky AS, Tremblay LN. Multiple system organ failure. is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721–1725. [DOI] [PubMed] [Google Scholar]
- 21.NIH ARDSNet Group. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 22.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1–6. [DOI] [PubMed] [Google Scholar]
- 23.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54–61. [DOI] [PubMed] [Google Scholar]
- 24.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003;289:2104–2112. [DOI] [PubMed] [Google Scholar]
- 25.Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L533–L542. [DOI] [PubMed] [Google Scholar]
- 26.Bregeon F, Delpierre S, Chetaille B, Kajikawa O, Martin TR, Autillo-Touati A, Jammes Y, Pugin J. Mechanical ventilation affects lung function and cytokine production in an experimental model of endotoxemia. Anesthesiology 2005;102:331–339. [DOI] [PubMed] [Google Scholar]
- 27.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc 2005;2:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev 2000;173:27–38. [DOI] [PubMed] [Google Scholar]
- 29.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 2004;172:3377–3381. [DOI] [PubMed] [Google Scholar]
- 30.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;155:937–944. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and C-Fos M-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat J-L, Nicod LP, Chevrolet J-C. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol Lung Cell Mol Physiol 1999;275:L1040–L1050. [DOI] [PubMed] [Google Scholar]
- 33.Dhanireddy S, Altemeier WA, Matute-Bello G, O'Mahony DS, Glenny RW, Martin TR, Liles WC. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest 2006;86:790–799. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheim JJ, Tewary P. de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol 2007;601:185–194. [DOI] [PubMed] [Google Scholar]
- 36.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface toll-like receptors. Semin Immunol 2007;19:3–10. [DOI] [PubMed] [Google Scholar]
- 37.Moriyama K, Ishizaka A, Nakamura M, Kubo H, Kotani T, Yamamoto S, Ogawa EN, Kajikawa O, Frevert CW, Kotake Y, et al. Enhancement of the endotoxin recognition pathway by ventilation with a large tidal volume in rabbits. Am J Physiol Lung Cell Mol Physiol 2004;286:L1114–L1121. [DOI] [PubMed] [Google Scholar]
- 38.Smith LS, Gharib SA, Wurfel MM, Martin TR. Age-dependent differences in CD14 expression in the lungs in response to mechanical ventilation. Am J Respir Crit Care Med 2007;175:A95. (abstract).
- 39.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 2007;4:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 2005;175:3369–3376. [DOI] [PubMed] [Google Scholar]
- 41.Gharib SA, Liles WC, Matute-Bello G, Glenny RW, Martin TR, Altemeier WA. Computational identification of key biologic modules and transcription factors in acute lung injury. Am J Respir Crit Care Med 2006;173:653–658. [DOI] [PubMed] [Google Scholar]
- 42.Wurfel MM, Park WY, Radella F, Ruzinski J, Sandstrom A, Strout J, Bumgarner RE, Martin TR. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J Immunol 2005;175:2570–2578. [DOI] [PubMed] [Google Scholar]
- 43.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 44.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med 2003;167:1057–1063. [DOI] [PubMed] [Google Scholar]
- 45.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol [Epub ahead of print] 2008 Jan 4. [DOI] [PubMed]
- 46.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas-ligand induces epithelial cell apoptosis in humans with acute lung Injury (ARDS). J Immunol 1999;163:2217–2225. [PubMed] [Google Scholar]
- 47.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and Fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 2002;161:1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ethell DW, Kinloch R, Green DR. Metalloproteinase shedding of Fas ligand regulates beta-amyloid neurotoxicity. Curr Biol 2002;12:1595–1600. [DOI] [PubMed] [Google Scholar]
- 49.Simon BA, Easley RB, Grigoryev DN, Ma SF, Ye SQ, Lavoie T, Tuder RM, Garcia JG. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L851–L861. [DOI] [PubMed] [Google Scholar]
- 50.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 2001;158:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol 2001;281:L328–L335. [DOI] [PubMed] [Google Scholar]
- 52.Matute-Bello G, Wurfel MM, Lee JS, Park DR, Frevert CW, Madtes DK, Shapiro SD, Martin TR. Essential role of MMP-12 in Fas-induced lung fibrosis. Am J Respir Cell Mol Biol 2007;37:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser WJ, Offermann MK. Apoptosis induced by the Toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 2005;174:4942–4952. [DOI] [PubMed] [Google Scholar]