Abstract
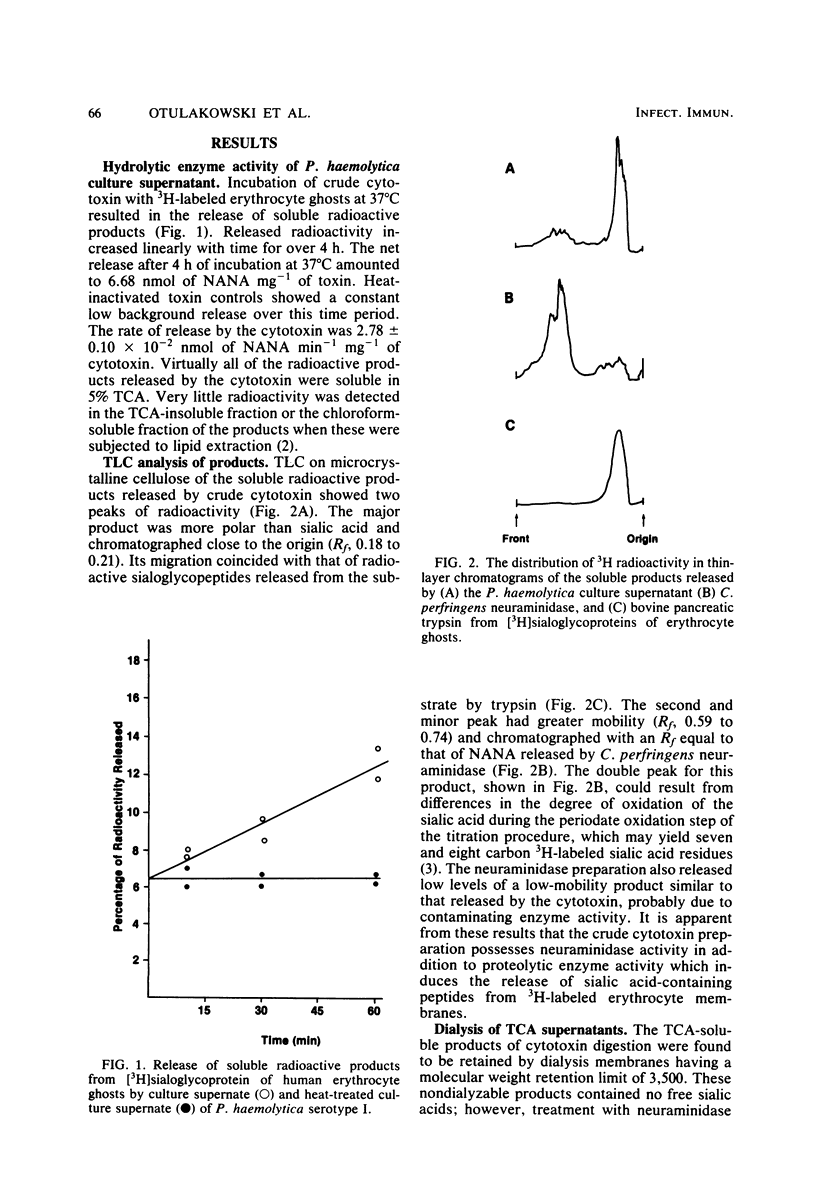
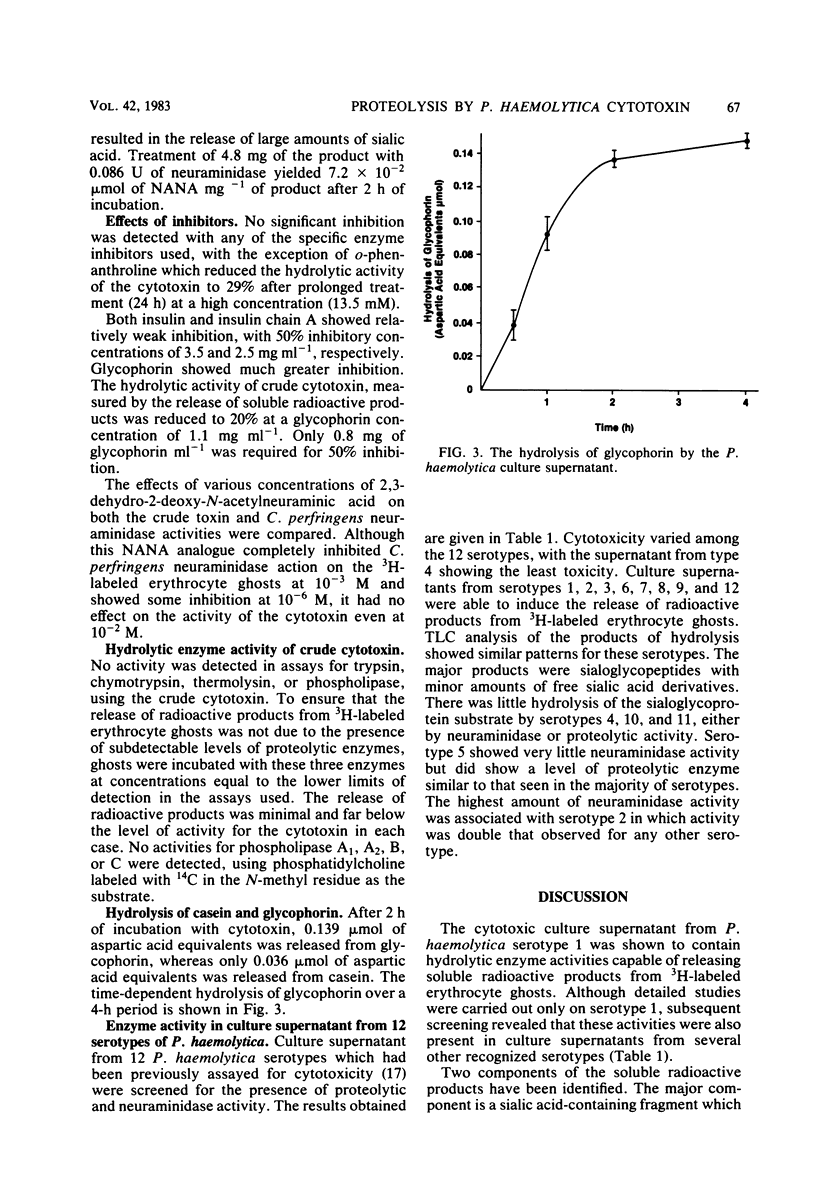
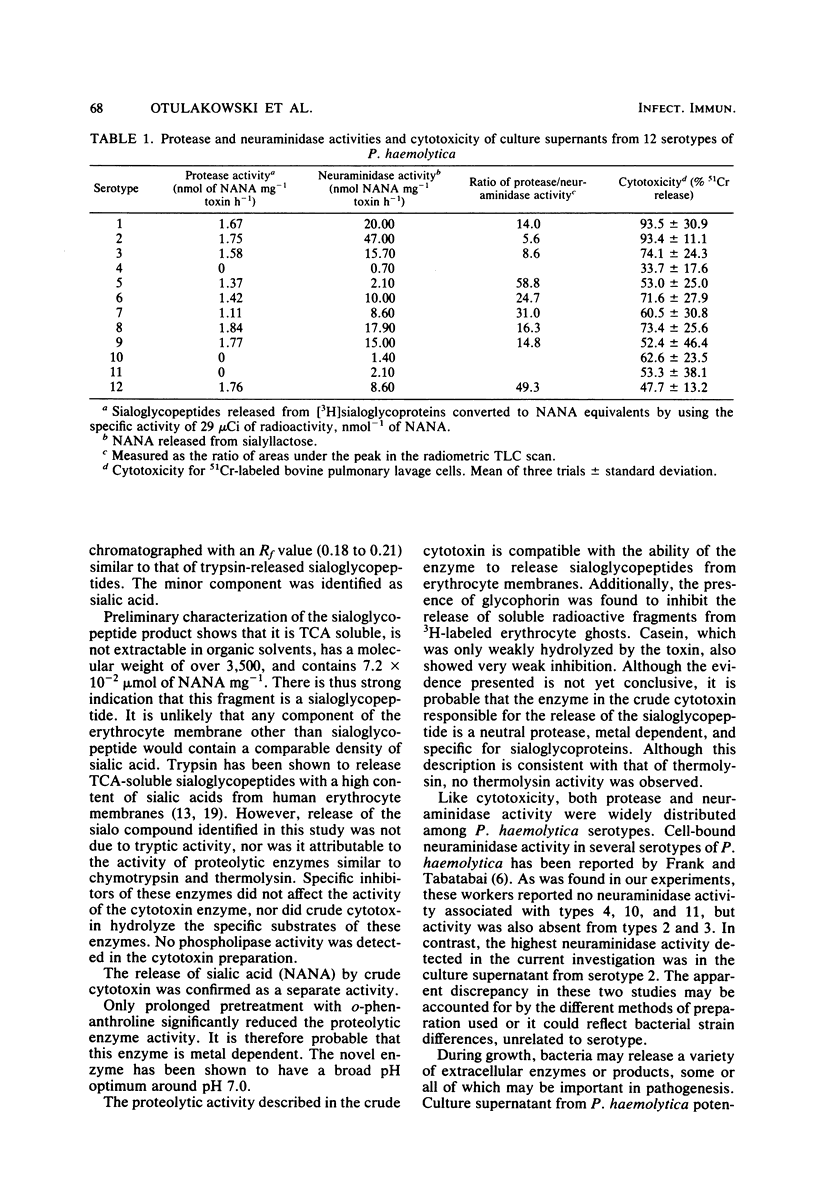
Proteolytic enzyme activity releasing sialo glycopeptides from 3H-labeled human erythrocyte ghosts was detected in cytotoxic (leukotoxic) culture supernatants from 9 of 12 Pasteurella haemolytica serotypes. Microcrystalline cellulose thin-layer chromatograms of radioactive water-soluble products showed the following two radioactive peaks: a high-mobility minor peak (Rf, 0.54 to 0.74), identified as sialic acid, and a low-mobility major peak (Rf, 0.18 to 0.21), partially characterized as a trichloroacetic acid-soluble, sialic acid-rich fragment with a molecular weight of greater than 3,500, not extractable by chloroform. The sialic acid content of this fragment after treatment with Clostridium perfringens neuraminidase was estimated to be 7.2 X 10(-2) mumol mg-1. The presence of neuraminidase as a separate activity in some culture supernatants was confirmed. It is considered to be responsible for the observed release of free sialic acid. Preliminary studies with the crude enzyme showed that it has a broad pH optimum around pH 7.0 and that activity is not affected by inhibitors of trypsin, chymotrypsin, thermolysin, thio and serine enzymes, nor by an inhibitor of neuraminidase, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid. Activity was, however, inhibited by o-phenanthroline at a high concentration after prolonged treatment. The enzyme hydrolyzed glycophorin at a rate four times higher than the rate for casein. Free glycophorin inhibited the enzyme-induced release of radioactive products from 3H-labeled ghosts. It is speculated that the novel enzyme is a neutral protease, probably metal-dependent, with specificity for sialoglycopeptides. The possible relationship of this protease to the previously reported host species-specific leukotoxicity of P. haemolytica and its potential role in virulence is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Benson M. L., Thomson R. G., Valli V. E. The bovine alveolar macrophage. II. In vitro studies with Pasteurella haemolytica. Can J Comp Med. 1978 Jul;42(3):368–369. [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld O. O., Gallop P. M., Liao T. H. Modification and introduction of a specific radioactive label into the erythrocyte membrane sialoglycoproteins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):242–251. doi: 10.1016/0006-291x(72)90369-5. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Feder J. A spectrophotometric assay for neutral protease. Biochem Biophys Res Commun. 1968 Jul 26;32(2):326–332. doi: 10.1016/0006-291x(68)90389-6. [DOI] [PubMed] [Google Scholar]
- Frank G. H., Tabatabai L. B. Neuraminidase activity of Pasteurella haemolytica isolates. Infect Immun. 1981 Jun;32(3):1119–1122. doi: 10.1128/iai.32.3.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hambrey P. N., Mellors A., Tizard I. R. The phospholipases of pathogenic and non-pathogenic Trypanosoma species. Mol Biochem Parasitol. 1981 Feb;2(3-4):177–186. doi: 10.1016/0166-6851(81)90098-0. [DOI] [PubMed] [Google Scholar]
- Leake E. S., Wright M. J., Kreger A. S. In vitro effect of purified proteases of Pseudomonas aeruginosa on rabbit lung macrophages. Exp Mol Pathol. 1978 Oct;29(2):241–252. doi: 10.1016/0014-4800(78)90042-4. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Ikemoto S. A sialoglycopeptide liberated from human red cells by treatment with trypsin. Nature. 1966 Oct 8;212(5058):198–199. doi: 10.1038/212198a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reimerdes E. H., Klostermeyer H. Determination of proteolytic activities on casein substrates. Methods Enzymol. 1976;45:26–28. doi: 10.1016/s0076-6879(76)45005-x. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Pasteurella haemolytica cytotoxin: production by recognized serotypes and neutralization by type-specific rabbit antisera. Am J Vet Res. 1983 Apr;44(4):715–719. [PubMed] [Google Scholar]
- Taha B. H., Carubelli R. Mammalian neuraminidase: intracellular distribution and changes of enzyme acitivity during lactation. Arch Biochem Biophys. 1967 Mar;119(1):55–61. doi: 10.1016/0003-9861(67)90428-6. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Thorne G. M., Gorbach S. L. General characteristics: nomenclature of microbial toxins. Pharmacol Ther. 1981;13(1):193–203. doi: 10.1016/0163-7258(81)90071-1. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


