Abstract
Fibrosis of parenchymal organs is caused by prolonged injury, deregulation of the normal processes of wound healing, and extensive deposition of extracellular matrix (ECM) proteins. The current review will focus on common features of fibrogenesis in parenchymal organs, and will briefly discuss common features and differences in the pathophysiology of fibrosis. Comparison of hepatic, renal, and pulmonary fibrosis has identified several common mechanisms. Common themes include a critical role for the cytokine transforming growth factor β and the generation of reactive oxygen species. Activated myofibroblasts are the common cell type that produce the excessive fibrous scar and may originate from endogenous cells such as hepatic stellate cells or fibroblasts, from the bone marrow such as fibrocytes, or from the transition of epithelial cells to mesenchymal cells. These concepts open new prospects for multidisciplinary research and the development of new therapies for fibrosis.
Keywords: fibrosis, collagen type I, inflammation, EMT
Fibrosis of parenchymal organs remains one of the leading causes of many chronic diseases in the lungs, kidneys, and liver. Chronic infections, toxic and metabolic injuries, and idiopathic inflammatory diseases can promote the development of fibrosis (1). In many cases, patients with progressive fibrosis have poor prognosis and often require organ transplantation (2). Although fibrosis is a part of the normal pathophysiologic response to injury in many tissues, extended exposure to chronic injury results in tissue fibrosis, massive deposition of extracellular matrix, scar formation, and organ failure (3). The current review will discuss common features and differences of fibrosis in parenchymal organs and will emphasize at the end that pulmonary, renal, and hepatic fibrosis share similar mechanisms.
DAMAGE OF PARENCHYMAL CELLS
Although parenchymal cells perform specialized functions in different organs, they are composed mostly of epithelial cells and develop from common sheet during embryogenesis (4). Lung epithelial cells (pneumocytes) are responsible for the gas exchange in lungs; tubular and glomerular epithelial cells participate in the process of filtration, while hepatocytes function in detoxification. Chronic injury induces damage and subsequent loss of parenchymal cells (5, 6). This cell death is a triggering event of fibrosis. Cells undergoing apoptosis actively secrete cytokines and chemokines, which activate a signal transduction pathway in the neighboring cells and release pro-fibrogenic and inflammatory cytokines, such as transforming growth factor (TGF)-β, connecrtive tissue growth factor (CTGF), and tumor necrosis factor (TNF)-α (3, 7). Necrotic cells induce an even more severe response since they do not form apoptotic bodies and release genetic material directly into the intercellular space.
LOSS OF ENDOTHELIAL CELLS
Damage of parenchymal cells is often associated with damage of endothelial cells (3, 7). Endothelial cells are not only responsible for barrier functions, nutrient transport to adjacent tissue, and regulation of lymphocytes recruitment from the blood stream; they also secrete a number of cytokines and growth factors to mediate local responses (8). All parenchymal organs are highly vascularized and damage of endothelial cells is associated with the loss of the barrier function of endothelium, release of pro-fibrogenic cytokines and flux of inflammatory cells (9).
RELEASE OF TGF-β1
TGF-β1 is the most important cytokine, promoting the development of fibrosis in all parenchymal organs (3). TGF-β1 has multiple functions during organogenesis, development, wound healing, and repair. The level of TGF-β1 is markedly elevated in the course of fibrosis (3). Not only is the production of TGF-β1 increased, but the activation from latent into biologically active form is increased as well (10). Almost every cell expresses TGF-β1 receptors; therefore, it is not surprising that TGF-β1 affects all steps of fibrosis (11). TGF-β1 is critical for activation of fibrogenic cells (12). Three sources of fibrogenic cells have been identified: activation of local fibroblasts into collagen producing myofibroblasts, BM-derived fibroblasts/fibrocytes, and fibroblasts resulting from epithelial-to-mesenchymal transition (13). TGF-β1 is one of the most potent chemoattractants for macrophages and myeloid cells. In turn, once activated, tissue macrophages and recruited macrophages became the major source of TGF-β1 in damaged tissues (3, 14). Kupffer cells, the resident macrophages in the liver, significantly contribute to TGF-β1 production in fibrotic liver (3). In addition, several other cells secrete TGF-β1. Apoptotic parenchymal cells release TGF-β1 (15). Activated myofibroblasts can themselves secrete TGF-β1. Thus, fibrotic liver hepatic stellate cells (HSCs) release a substantial amount of TGF-β1 that can maintain self activation (3).
CTGF is another important fibrogenic factor. CTGF is a downstream mediator of TGF-β1 responses that is synthesized in response to TGF-β1 signaling (16). It directly binds TGF-β1 and increases its affinity to TGF-β1 receptors, causing a sustain enhanced response (17).
COLLAGEN-PRODUCING CELLS
Despite intensive debate, a consensus is emerging that the majority of collagen-producing cells differentiate from the resident precursor cell of mesenchymal origin in response to the injury of that organ. In fibrotic lungs, resident fibroblasts proliferate and give rise to a population of collagen-secreting myofibroblasts (18). In the liver, injury induces activation of HSCs (3). These cells undergo functional and phenotypical changes that have been termed “activation.” Quiescent HSCs are the major depot of vitamin A in the mammalian body. In response to injury, quiescent HSCs lose vitamin A content, reduce expression of GFAP (glial fibril protein) and start expressing α-smooth muscle actin (α-SMA), collagen α1(I), and synamin (3). In kidneys and lungs, resident fibroblasts activate into α-SMA+, collagen α1(I)+ myofibroblasts. Of note, liver myofibroblasts retain their neural-specific markers (3), thus differentiating them from myofibroblasts from other tissues.
Bone marrow–derived cells contribute to the population of collagen-expressing cells. However, their contribution differs in different organs. Thus, BM cells contribute to almost 25 to 34% of collagen-producing cells in lungs (14). It is difficult to differentiate if these cells are represented mostly by fibrocytes or circulating mesenchymal cells (14). Overall, it is believed that a significant portion of pulmonary myofibroblasts originate in the BM. In contrast, data obtained from fibrosis research in kidneys and liver provide convincing evidence that the majority of myofibroblasts originate from the resident cells (19, 20). Thus, it is believed that BM cells contribute to 10 to 15% of collagen-producing cells in fibrotic liver cells (fibrocytes [CD45+] and mesenchymal cell precursors [CD45−]) (21, 22). This number comprises 4 to 5% of BM-derived fibrocytes and 5 to 10% of circulating mesenchymal cells (19). Similar to that, 14 to 15% of BM-derived cells (fibrocytes and mesenchymal cell precursors) contribute to renal fibrosis (21).
Epithelial-to-mesenchymal transition (EMT) is a phenomenon of cellular transdifferentiation into a different phenotype. It was first reported in kidney fibrosis, suggesting that epithelial cells such as tubular epithelial cells can undergo reprogramming in response to chronic injury and change their phenotype to mesenchymal phenotype (21). Similar events were identified in fibrotic liver. Thus, cholangiocytes, cells lining the inner surface of the bile ducts, proliferate in response to injury and were recently reported to contribute to EMT in hepatic fibrosis (23). Cholangiocytes lose cytokeratin expression and obtain fibroblastic markers Fsp1+, α-SMA+ (smooth-muscle actin), and collagen. Hepatocytes, which are also liver epithelial cells, were also reported to transdifferentiate into collagen-expressing activated myofibroblasts by changing their cell fate (24). Lung fibrosis is also accompanied by transdifferentiation of pulmonary pneumocytes into myofibroblasts (25, 26). Although there is strong evidence for multiple origins of fibroblasts, there is no information on whether these cells contribute in different ways to fibrotic disease.
INFLAMMATION AND FIBROSIS
Apoptotic or necrotic death of epithelial cells activates tissue macrophages. Macrophages, in turn, phagocytose apoptotic particles and cell debris to induce a local inflammatory response (3). Release of TGF-β triggers recruitment of bone marrow–derived inflammatory cells: neutrophils, monocytes and macrophages, and T and B lymphocytes, which in turn secrete TNF-α, IL-6, IL-1, and MCP-1 and accelerate inflammation (3). In addition, tissue- and bone marrow–derived macrophages are the major source of TGF-β1. Therefore, pro-inflammatory cells play an important role in activation of myofibroblasts. Consistent with this, mice devoid of B cells or Toll-like receptor 4 (TLR4) mutant mice are more resistant to fibrosis than are wild-type mice (27).
However, inflammation has a dual effect on fibrosis. Initial inflammatory responses that accompany fibrogenesis at the onset exacerbate liver injury (28). In contrast, recruitment of myelo-monocytic cells and natural killer (NK) cells to fibrotic organ promotes resolution of fibrosis (28, 29). In addition, the role of inflammation for progression of fibrosis varies in different organs and depends on pathological condition (30).
MICROBIAL PRODUCTS
Microbial products have a significant impact on fibrosis. Leakage of bacterial products from the intestine into the portal circulation synergistically facilitates other fibrogenic factors such as TGF-β1, oxidative stress, and mechanical injury. Thus, chronic injury increases intestinal permeability and causes release of pathogen-associated molecular patterns (PAMPS), such as bacterial cell wall components, lipopolysaccharide (LPS), peptideglycan, bacterial-derived unmethylated CpG-DNA, and endogenous ligands (HMGB-1, hyaluronan, and products of dying cells) (31). These products bind to the TLRs inducing signal transduction pathways that result in activation of innate immunity. Of the nine members of the TLRs family, TLR4 plays a key role in acceleration of fibrosis. Intestinal microflora should be considered as an important pro-fibrogenic factor (27).
OXIDATIVE STRESS
Oxidative stress is caused by increased reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radicals (3). ROS is generated endogenously or by phagocytic NADPH oxidase or by nonphagocytic NADPH. Angiotensin II–induced fibrotic actions are largely mediated by ROS generated by a nonphagocytic form of NADPH oxidase in hepatic stellate cells. Generation of endogenous ROS is associated with direct activation of the pro-fibrogenic genes in collagen-producing cells. It also decreases endogenous antioxidants that have a protective effect in models of fibrosis. However, antioxidants have not been effective in clinical trials in patients with fibrosis. In contrast, the use of the antioxidant acetylcysteine in patients with idiopathic pulmonary fibrosis (IPF) provided satisfactory results. It is proposed that an oxidant/antioxidant imbalance is involved in alveolar epithelial cell injury and thereby contributes to progressive fibrosis in IPF (32). Acetylcysteine, precursor of the major antioxidant glutathione, works as a powerful cellular detoxifying agent. Acetylcysteine, a precursor of the major antioxidant glutathione, was shown to restore depleted pulmonary glutathione levels and to improve lung function in patients with fibrotic lung disease (32, 33).
REVERSIBILITY OF FIBROSIS
Regression of fibrosis has been studied mostly in the liver (3, 34). It is associated with termination of the chronic injury, loss of TGF-β1 signaling, and decrease of pro-inflammatory cytokines (15). Recruitment of myelo-monocytic cells to injured organ is critical for the resolution of fibrosis (28). Inflammatory cells not only participate in clearance of collagen-producing cells, but also release matrix metalloproteinases (MMPs), which play an important role in ECM remodeling and regression of fibrosis. However, advanced fibrosis (cirrhosis in the liver) is resistant to collagenolysis due to formation of irreversible nonreducible cross-linked collagen and an ECM rich with elastin fibers preventing its degradation. This pathophysiologic state is considered the “point of no return” (35, 36). Loss of the integrity of the basement membrane and collapse of primary alveolar structure produces more severe damage of the lung tissue and may also prevent resolution of fibrosis (37). Even in normal wound healing there is a persistent increase in angiogenesis and number of fibroblasts (38).
SPECIFIC FEATURES OF FIBROGENEIS IN DIFFERENT PARENCHYMAL ORGANS
The mechanism of fibrosis seems to be very similar in parenchymal organs. However, there are important differences in pathogenesis of fibrosis, characteristic to the specific organ.
Liver
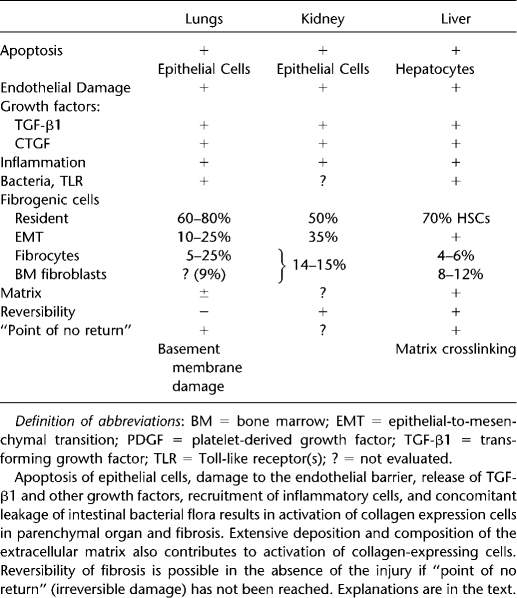
Hepatocytes represent the major epithelial cells in the liver. They are very sensitive to chronic injury, and therefore death of hepatocytes is the triggering event in liver fibrosis. Apoptotic hepatocytes secrete TGF-β1, causing activation of BM macrophages and liver resident macrophages (Kupffer cells), which produce most of TGF-β1 in fibrotic liver (Table 1) (3, 11, 28, 39, 40). TGF-β1 is the primary activator of collagen producing cells in the liver. We estimate that hepatic stellate cells (HSCs) contribute more than 80% of all collagen-producing cells. In response to injury, quiescent HSCs lose vitamin A content and undergo morphologic and functional changes to become myofibroblasts. Portal fibroblasts also undergo activation and contribute to ECM deposition. BM-derived cells, circulating mesenchymal precursor cells, and fibrocytes migrate to the damaged liver and correspond to 5 to 7% and 4 to 6% of all collagen-producing cells. EMT in the liver has been reported in hepatocytes and cholangiocytes, which reprogram their gene expression and transdifferentiate into myofibroblasts. However, this phenomenon requires further investigation. Specific ECM composition accelerates HSC activation toward collagen-producing myofibroblasts. Liver fibrosis can regress in the absence of injury. However, formation of irreversible crosslinking prevents resolution of fibrosis and is considered a point of no return.
TABLE 1.
FACTORS PLAYING AN IMPORTANT ROLE IN FIBROGENESIS OF PARENCHYMAL ORGANS
Lungs
Damage to lung epithelial cells is associated with interstitial edema, leukocyte invasion, and decreased gas exchange. Since epithelial and endothelial cells form an epithelial/endothelial barrier, damage of pneumocytes is associated with apoptosis of endothelial cells (Table 1) (7, 14, 41, 42). Impaired blood vessels lose their integrity and endorse flux of peripheral blood cells into the lung tissue. Apoptotic and recruited inflammatory cells increase local concentration of TGF-β1, which causes re-organization and activation of pulmonary fibroblasts. However, inflammation plays an important part in pulmonary fibrogenesis. The role of inflammation in IPF is controversial. The typical IPF lung pathology does not demonstrate a flux of inflammatory cells, and immunosuppressive therapy is not effective in patients with IPF. Recent data suggested that fibroblast dysfunction is a critical step in pathogenesis of IPF, rather than of inflammation. Bringardner and coworkers suggested that inflammation is indeed important for IPF. They proposed that inflammation affects the structure of the extracellular matrix, growth factor–receptor secretion, and cellular plasticity (30). An increase in cellular plasticity was documented in fibrogenesis of many tissues. Similar to the liver, pulmonary fibroblasts differentiate into ECM-producing myofibroblasts and contribute to the major population (60–80%) of all collagen-producing cells in fibrotic lung (14). In addition to local fibroblasts, pneumocytes can also transdifferentiate into myofibroblasts via EMT. Based on different models of lung injury, reported in the literature, the number of cells undergoing EMT varies from 10 to 25%. BM cells also contribute to lung fibrosis. The number of BM-derived fibroblasts and fibrocytes recruited to the injured lung can reach up to 10% and 25% of collagen-producing cells, respectively. While pro-fibrogenic and inflammatory signals play a critical role in activation of myofibroblasts in fibrotic lungs, the effect of changing composition of ECM on collagen-producing cells has not been fully evaluated. Similar to that, only a few studies have evaluated reversibility of lung fibrosis. Loss of the integrity of basement membrane often results in the collapse of primary alveoli, causing an irreversible damage associated with the point of no return (37).
Kidney
Chronic injury causes dysfunction of the tubular epithelial cells, which triggers release of fibrogenic cytokines and recruitment of inflammatory cells to injured kidneys (Table 1) (20, 21, 44, 45). Although local activation of the renin-angiotensin system (RAS) and specifically angiotensin II (Ang II) affects all parenchymal organs, its effect is more pronounced in renal fibrosis. RAS stimulates inflammation, including the expression of cytokines, chemokines, growth factors, and reactive oxygen species (46). Ang II induces vascular inflammation, endothelial dysfunction, up-regulation of adhesion molecules, and recruitment of infiltrating cells into the kidney (45). Similar to the liver, myelo-monocytic cells recruited from the bone marrow produce most of the TGF-β1 in injured kidneys. In turn, TGF-β1 induces activation of collagen-producing cells, which mostly arise from kidney resident cells. Thus, fibroblasts contribute to 50% of all collagen expressing cells in the course of renal fibrosis. Renal cortical fibroblasts maintain a quiescent state in normal kidneys, but in response to injury they proliferate and activate into myofibroblast. In contrast to other parenchymal organs, EMT significantly contributes (35%) to the collagen-expressing cells. Renal epithelial have unique characteristics. Unlike epithelium in other parenchymal organs, which develops from primary epithelial sheets, renal epithelium has two distinct embryonic origins; the mesenchymal blastema and epithelial Wolffian duct (47, 48). Most of the renal epithelium undergoing EMT during injury is of mesenchymal origin (47, 49). High plasticity of renal epithelium may cause acceleration of EMT in fibrosing kidneys. BM cells also contribute to kidney fibrosis and make up 14 to 15% of the myofibroblast population. Reversal of renal fibrosis is possible. For example, discontinuation of chronic cyclosporine eliminates nephrotoxicity and improves fibrosis. However, whether fibrotic kidneys can reverse to normal renal architecture remains unresolved, and the point of no return in the development of irreversible renal fibrosis still remains to be determined.
CONCLUSIONS
Fibrosis in all tissues is a complex mechanism, initiated to contain an injury and isolate the damaged tissue. Several mechanisms have emerged as common in fibrosis. Oxidant stress, fibrotic cytokines such as TGF-β, and fibroblast growth factors such as PDGF are important pathophysiologic factors in fibrotic diseases. The activated myofibroblasts that synthesize the extracellular matrix that forms the fibrous scar can originate from local mesenchymal cells such as fibroblasts or stellate cells. Alternatively, endothelial cells or epithelial cells can undergo a mesenchymal transition. Finally, bloodborne bone marrow cells such as fibrocytes may contribute to the myofibroblast population. The relative contribution of these cell populations appear to vary with the injured organ and the type of injury. If the etiological agent causing the injury is removed, the ability to reverse the fibrosis varies between different organs and the extent of the injury. Due to the efficiency of regeneration in the liver, this organ appears to have a higher capacity for reversal of fibrosis than the kidney or lung. The collaborative efforts of modern interdisciplinary sciences, including multiple organs biology, pathology, computational biology, immunology, and transgenic technologies, will significantly advance our understanding of fibrosis.
Supported by the National Institutes of Health (NIH) GM R01 GM041804 (D.A.B.).
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.du Bois RM. Genetic factors in pulmonary fibrotic disorders. Semin Respir Crit Care Med 2006;27:581–588. [DOI] [PubMed] [Google Scholar]
- 2.Noth I, Martinez FJ. Recent advances in idiopathic pulmonary fibrosis. Chest 2007;132:637–650. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science 1989;245:718–725. [DOI] [PubMed] [Google Scholar]
- 5.Yano T, Deterding RR, Nielsen LD, Jacoby C, Shannon JM, Mason RJ. Surfactant protein and CC-10 expression in acute lung injury and in response to keratinocyte growth factor. Chest 1997;111:137S–138S. [DOI] [PubMed] [Google Scholar]
- 6.Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect 1998;106:511–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano K, Hagimoto N, Nakanishi Y. The role of apoptosis in pulmonary fibrosis. Histol Histopathol 2004;19:867–881. [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999;18:3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suematsu M, Suzuki H, Delano FA, Schmid-Schonbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation 2002;9:259–276. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 2006;3:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol 2006;44:57–66. [DOI] [PubMed] [Google Scholar]
- 12.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci 2004;29:265–273. [DOI] [PubMed] [Google Scholar]
- 13.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 2007;22:S73–S78. [DOI] [PubMed] [Google Scholar]
- 14.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest 2007;117:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol 2006;21:S84–S87. [DOI] [PubMed] [Google Scholar]
- 16.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet 2004;363:62–64. [DOI] [PubMed] [Google Scholar]
- 17.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827. [DOI] [PubMed] [Google Scholar]
- 18.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006;45:429–438. [DOI] [PubMed] [Google Scholar]
- 20.Neilson EG. Mechanisms of disease: Fibroblasts–a new look at an old problem. Nat Clin Pract Nephrol 2006;2:101–108. [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–122. [DOI] [PubMed] [Google Scholar]
- 23.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol 2006;168:1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 2007;282:23337–23347. [DOI] [PubMed] [Google Scholar]
- 25.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 2007;293:L525–L534. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, Xu J. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332. [DOI] [PubMed] [Google Scholar]
- 28.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006;130:435–452. [DOI] [PubMed] [Google Scholar]
- 30.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 2008;10:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D, Oppenheim JJ. Antimicrobial proteins act as “alarmins” in joint immune defense. Arthritis Rheum 2004;50:3401–3403. [DOI] [PubMed] [Google Scholar]
- 32.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 33.Walter N, Collard HR, King TE Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:330–338. [DOI] [PubMed] [Google Scholar]
- 34.Friedman SL, Bansal MB. Reversal of hepatic fibrosis–fact or fantasy? Hepatology 2006;43:S82–S88. [DOI] [PubMed] [Google Scholar]
- 35.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 2007;117:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga J, Brenner D, Phan SE. Fibrosis research. methods and protocols. Totowa, NJ: Humana Press; 2005.
- 37.Takiya C, Peyrol S, Cordier JF, Grimaud JA. Connective matrix organization in human pulmonary fibrosis: collagen polymorphism analysis in fibrotic deposits by immunohistological methods. Virchows Arch B Cell Pathol Incl Mol Pathol 1983;44:223–240. [DOI] [PubMed] [Google Scholar]
- 38.Brown NJ, Smyth EA, Cross SS, Reed MW. Angiogenesis induction and regression in human surgical wounds. Wound Repair Regen 2002;10:245–251. [DOI] [PubMed] [Google Scholar]
- 39.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol 2004;40:646–652. [DOI] [PubMed] [Google Scholar]
- 40.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis 2005;10:927–939. [DOI] [PubMed] [Google Scholar]
- 41.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999;104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997;17:272–278. [DOI] [PubMed] [Google Scholar]
- 43.Kinnula VL. Redox imbalance and lung fibrosis. Antioxid Redox Signal 2008;10:249–252. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 2007;74:196–206. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 2006;21:16–20. [DOI] [PubMed] [Google Scholar]
- 46.Sironi L, Nobili E, Gianella A, Gelosa P, Tremoli E. Anti-inflammatory properties of drugs acting on the renin-angiotensin system. Drugs Today (Barc) 2005;41:609–622. [DOI] [PubMed] [Google Scholar]
- 47.Herzlinger D. Renal interstitial fibrosis: remembrance of things past? J Clin Invest 2002;110:305–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber SM, Braun GS, Segerer S, Veh RW, Horster MF. Metanephrogenic mesenchyme-to-epithelium transition induces profound expression changes of ion channels. Am J Physiol Renal Physiol 2000;279:F65–F76. [DOI] [PubMed] [Google Scholar]
- 49.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 2004;82:175–181. [DOI] [PubMed] [Google Scholar]