Abstract
The sleep medicine community has increasingly recognized the necessity that clinical care be based on high-quality levels of evidence. Although research supports a favorable influence of positive airway pressure (PAP) therapy on risk for significant adverse outcomes in patients with severe obstructive sleep apnea–hypopnea (OSAH), well-designed trials are still required to elucidate the effect of PAP on health, quality of life, and economic risks in patients with milder OSAH. Similarly, although there is strong evidence supporting various PAP titration strategies and PAP modalities in patients with severe OSAH without significant medical and psychiatric comorbidities, there is insufficient high-level evidence assessing and comparing the clinical efficacy and health care cost implications of various titration paradigms and various PAP modalities in individuals with milder OSAH and those with comorbid conditions. For ethical and other reasons, it may not be possible to apply a randomized controlled design to address all questions. However, whichever design is employed, it must be rigorously developed with attention to all potential confounders with adequate power to provide compelling, high-quality evidence.
Keywords: obstructive sleep apnea, positive airway pressure, therapy of sleep apnea
Wright and colleagues generated considerable self-examination within the field of sleep medicine with the publication of their literature review of the health consequences of obstructive sleep apnea–hypopnea (OSAH) and the effectiveness of continuous positive airway pressure (CPAP) therapy for this disorder (1). The authors concluded that perhaps with the exception of reducing sleepiness and motor vehicle crashes, evidence of benefit from treatment with CPAP was, at best, weak. Wright and coworkers supported their contention by citing numerous flaws in the studies that were published to that time. Inadequacies were identified in research design and analyses, including lack of randomized controlled trials, as well as failure to account for carryover effects and potential confounders.
It is evident that well-designed and conducted randomized controlled trials (RCTs) yield high-quality evidence, but they carry costs as well as benefits. Several authors (2–4) have asserted that the hierarchy of research design quality, as reflected by the evidence-grading pyramid, is flawed and artificial, with the results of many well-done non-RCTs withstanding the test of time. It has been further argued that RCT design may rigidly restrict the intervention to a single paradigm, whereas in clinical practice, physicians adjust therapies according to the specific clinical features of individual patients (2, 4). Thus, the generalizability of the results of randomized controlled clinical trials to clinical care may be imperfect. Moreover, these authors contended that similar evidence from more than one, well-done observational study usually corroborates the results of an RCT examining the same issue. RCTs are also usually costly (5) and, if comparably reliable evidence can be obtained from observational studies, limited resources may receive wider and more efficient allocation. Finally, RCTs may not always be ethical or feasible such as when randomization to a control arm puts the subject at what is deemed to be a significant likelihood of risk.
Although RCTs may not be necessary to assess every outcome and every intervention, it must be acknowledged that the results of observational studies may not withstand the challenge of well-designed reexamination with an RCT, as has been the case with the use of estrogen replacement therapy to prevent cardiovascular disease in postmenopausal women (6–8). In observational studies of PAP intervention, the referent group is often represented by individuals who either decline or who are nonadherent to therapy. This engenders notable concern that a patient's decision to accept or decline to use therapy to treat OSAH may be influenced by a variety of coexistent unmeasured or unmeasurable patient factors that are, in fact, the primary determinant(s) of outcome; the presence or absence of the intervention reflecting an epiphenomenon of the operative unmeasured or otherwise unassessed factors.
While recognizing the intrinsic strengths and weaknesses of various study designs and questioning the absolute and exclusive primacy of RCTs (9, 10), sleep medicine investigators accepted the challenge to examine and maximize the rigor of research design and robustness of analyses. In this perspective, we present our view of the state of the evidence, such as it may be, regarding the effectiveness of PAP therapy following the publication of the above-cited article by Wright and coworkers (1). Because of the inherent limitations of this forum, we focus the discussion on representative issues in two areas. First, we will discuss clinical outcomes, including sleepiness, health-related quality of life issues, and systemic hypertension. The impact of PAP on cardiovascular disease and longevity is also selected for this representative discussion both because of its inherent importance and to allow examination of the strength of the evidence across the wider scope of patients with OSAH. Subsequently, we provide an appraisal of the state of the evidence for various PAP titration and initiation strategies as well as several currently prescribed PAP modalities to facilitate development of a blueprint for enhanced patient care in the context of the wide-spectrum clinical environments and resource availability.
OSAH, SLEEPINESS, AND HEALTH-RELATED QUALITY OF LIFE
Patients with OSAH often present with sleepiness and its attendant consequences, including complaints of impaired memory and concentration, irritability, and inadvertent episodes of sleep. These symptoms not only lower the individual patient's quality of life, but create a societal burden through the higher rates of automobile crashes among patients with OSAH compared with the general population (11–14).
Compelling evidence that PAP is effective in ameliorating sleepiness among patients with this complaint at baseline has been demonstrated in a meta-analysis including 745 patients with a range of OSAH severity enrolled in 12 randomized parallel group or cross-over trials (15). This meta-analysis assessed studies that provided the control groups with measures including counseling about conservative measures (lateral position therapy, weight loss, avoidance of alcohol and sedatives), an oral placebo pill, and sham PAP. Perceived sleepiness, as assessed by the Epworth Sleepiness Scale (ESS), improved to a substantially greater extent in patients receiving PAP compared with control subjects, with a mean improvement of 2.94 points on a 24-point scale. Objective sleepiness, measured by mean sleep latency on either a multiple sleep latency test or maintenance of wakefulness test in 482 subjects, improved by 0.93 minutes in PAP compared with control. The magnitude of changes in ESS score and sleep latency in the meta-analysis was statistically significant, and the improvement in ESS score appeared noteworthy. The change in objective sleepiness was less impressive than that of subjective sleepiness, and there were no data linking changes in mean sleep latency to clinically relevant outcomes. When the meta-analysis was restricted to the trials enrolling patients with greater subjective sleepiness (ESS ⩾ 11), the results were more striking, whereas an analysis of the remaining five trials revealed nonsignificant differences. It is plausible that the apparent lack of influence of PAP intervention among patients with less subjective sleepiness at baseline resulted from a floor effect in measurement of the ESS score. It also merits note that in this meta-analysis, the improvements in sleepiness were independent of the type of control therapy, suggesting that the mode of placebo control per se is less important than the active therapy regarding the evaluated effects.
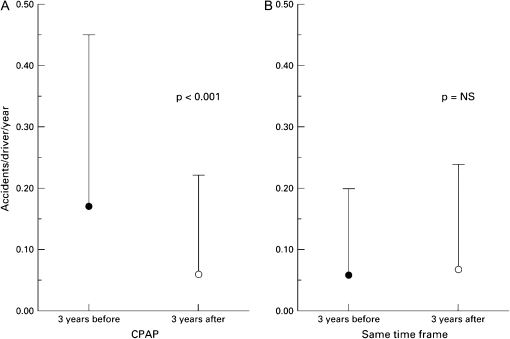
Prevention of motor vehicle crashes (MVCs) is of critical importance to patients and society. Because of the known impact of sleepiness on the risk of MVCs, the effect of PAP on this outcome has been investigated only in nonrandomized studies, comparing rates of accidents in patients before and after treatment (14, 16–19). The patients in these studies were typical of a sleep clinic population, predominantly men with symptomatic, severe OSAH (mean apnea + hypopnea index [AHI]: 35–60), and MVCs were assessed by self-report. Because patients may underreport accidents, some studies included a review of driving records from the Department of Motor Vehicles, or included a matched control group, strengthening the validity of the findings. In addition, the inclusion of a matched control group enhanced the study design (14, 17, 18). These studies all revealed reduction in the rate of MVCs after initiation of PAP, whether assessed as rate of accidents per person per year or by miles driven. Barbé and colleagues (14) observed that at baseline, patients with OSAH had higher MVC rates than did control subjects matched for age and sex. However, although the crash rate fell by 60% among patients after treatment with PAP, there was a similar percent reduction in the control group. On the other hand, George (18) observed that after initiation of PAP therapy, crash rates fell to the level seen in matched control subjects in whom the crash rate was stable over the study interval (Figure 1). This discrepancy may result from differences in the study designs. Control subjects in the study by Barbé and coworkers were aware that they were enrolled in a research project, and therefore the improvements in their driving record may have resulted from increased attentiveness to safe driving practices during the observation period. In contrast, data obtained from the control subjects in the study by George were taken from Department of Motor Vehicle records and these individuals were not personally contacted by investigators. Therefore, it is possible that the benefit from increased awareness of motor vehicle safety to MVC risk is comparable to treatment with PAP, or that heightened attention to safety confounds assessment of the specific benefits of PAP in this regard.
Figure 1.
Average (±SD) number of automobile crashes per driver per year over the 3-year interval before initiation of positive airway pressure therapy (solid circles) and during the 3-year interval after initiation of positive airway pressure therapy (open circles) in (A) patients with sleep apnea–hypopnea compared with (B) control subjects over the same time periods. Reprinted by permission from Reference 18.
We are unaware of any studies that have specifically addressed MVCs in nonsleepy patients with OSAH. Although randomized trials in which sleepy individuals might be allocated to a control group and monitored for MVC frequency are unethical, a randomized trial examining nonsleepy patients with mild OSAH may be feasible and might advance our understanding of appropriate patient groups that should be treated with PAP. There are challenges in designing and analyzing such a study. Because the baseline rate of MVC is probably low in this population a suitably long observation interval is necessary to determine whether there is a treatment effect. In addition, the potential impact of heightened awareness alone on vehicular safety, differences in driving practices (e.g., hours per day, time of day, cell phone use), and the influence of occupation on MVC are potential confounders that must be considered. The preceding issues notwithstanding, at this time we believe that it is reasonable to expect that PAP treatment of sleepy patients with OSAH will lower the MVC risk.
In controlled trials in diverse populations, patients with OSAH who were treated with PAP compared with placebo intervention showed significant improvement in general measures of quality of life, employing instruments such as the Short Form-36 (20–22), and in sleep-specific quality of life measures in most (23–25), but not all studies (26). In one parallel group trial that included only nonsleepy patients, no significant changes in quality of life were found (27). Thus in general, patients with OSAH, particularly if sleepy, experience improved quality of life from PAP. However, in view of the somewhat variable conclusions reached across these studies, it seems warranted to determine whether there are two subpopulations of patients with OSAH who are more likely to obtain quality of life benefit from PAP, with careful consideration of confounding factors as well as the interactions between confounding factors.
OSAH AND HYPERTENSION
The relation between untreated OSAH and hypertension has been convincingly established by a number of observational studies with adequate adjustment for confounding demographic and lifestyle factors (28–30) and confirmed prospectively (31). A canine model of sleep apnea convincingly demonstrated that experimentally induced upper airway occlusion that reasonably mimicks severe OSA in humans led to hypertension during both sleep and wakefulness. Moreover, the hypertension resolved when the animals were subsequently permitted to sleep undisturbed (32). The evidence for a causal role of OSAH in the development of hypertension is among the strongest for any cardiovascular condition.
The impact of PAP therapy on blood pressure in patients with OSAH has been investigated in numerous small trials with variable results. In an attempt to reconcile these inconsistencies, Haentjens and colleagues performed a meta-analysis including 572 patients in 12 randomized trials, reporting the outcome of 24-hour ambulatory blood pressure (33). These investigators identified important between-trial heterogeneity in study design and patient characteristics. Both parallel groups and cross-over trials were analyzed; placebo treatments were either sham PAP (27, 34–40) or oral tablet (24, 26, 41, 42), and study duration ranged from 1 to 12 weeks. OSAH severity and symptomatology, as well as the percentage of subjects with documented hypertension or receiving antihypertensive treatment, varied between trials. In aggregate, treatment with PAP lowered mean 24-hour blood pressure by 1.69 mm Hg, reflecting a statistically, and arguably clinically significant, reduction (43). Significant predictors of a more robust decrease in blood pressure in conjunction with PAP treatment included more severe OSAH at study entry (measured as the AHI or the number of arousal events per hour of sleep) and greater nightly of use of PAP. In this meta-analysis, subjective sleepiness, assessed by the ESS, did not predict blood pressure response to PAP, as had been hypothesized in some of the component trials. Similarly, trials that included a higher percentage of hypertensive participants were not more likely to show a benefit with PAP. Potentially relevant factors that were not assessed in the meta-analysis included potential genetic influences, lifestyle choices (e.g., exercise, alcohol consumption), duration of OSAH, and duration of the trial. This meta-analysis, although well done, shares the strengths and weaknesses of this statistical technique, namely that the unit of analysis is the trial rather than the individual participant, and therefore evaluating the effect of particular patient characteristics such as baseline blood pressure is not possible. The latter may be of particular issue in that it may take more than several months of PAP therapy to see a reduction in sympathetic nervous system activation (44). Despite the study heterogeneity, we believe the evidence is reasonably compelling that PAP modestly reduces blood pressure in at least some patients with OSAH, particularly in those with more severe disease (24, 34, 37). Careful studies to identify specific subpopulations most likely to derive antihypertensive benefit from PAP are still needed.
Epidemiologic studies and meta-analyses teach that improved blood pressure control leads to improved outcomes such as reduced development of coronary artery disease, congestive heart failure, and stroke (43, 45, 46). There remain many important, unanswered questions. In the absence of symptoms or other clinical issues such as cardiac risk factors, does the goal of lowering blood pressure provide sufficient justification for recommending PAP treatment to hypertensive patients with OSAH? Furthermore, is treatment with PAP as good as, or superior to, adopting a more health-promoting lifestyle or more intensive pharmacologic therapy in reducing adverse outcomes in hypertensive patients with OSAH, and what is the long-term effect of PAP on blood pressure control? In addition to effects on blood pressure, PAP therapy directly reduces cardiac afterload, ameliorates hypoxemia, and fosters improved sleep continuity. At least in theory, these factors argue in favor of PAP over conventional antihypertensive drug treatment per se. An RCT with “hard” cardiovascular endpoints to answer these questions would require an extremely large sample size and long duration. The study design would need to address the confounding impacts of baseline OSAH severity, variable adherence to PAP, changing clinical circumstances including changing medications and lifestyle over a long period, as well as potential genetic influences in the study groups. For these reasons, such a study is unlikely to be conducted. On the basis of the current evidence of a modest blood pressure decrement in patients with OSAH, we believe that difficult-to-control hypertension should be considered when making the decision to initiate PAP therapy, especially in those patients with established cardiovascular risk factors or disease. We recognize that this approach is, at best, largely based on extrapolated data with a critical need for accrual of high-quality evidence to provide definitive clinical guidance.
OSAH, CARDIOVASCULAR DISEASE, AND LONGEVITY
Mortality associated with OSAH, and the benefits of treatment in this regard, are issues of obvious and elemental importance to patients, their health care provider(s), and society. Implicit in this is the need to establish criteria for initiating therapeutic interventions, over and above those related to lifestyle changes.
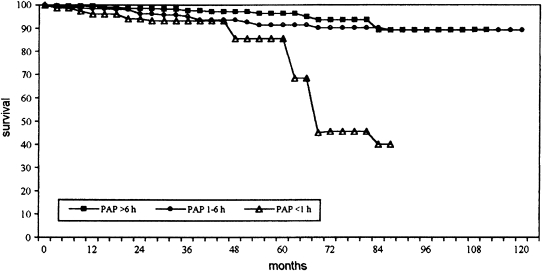
Marin and coworkers (47) reported a substantially higher incidence of fatal as well as nonfatal cardiovascular events in a cohort of individuals with severe OSAH (AHI > 30) who declined PAP therapy compared with untreated patients with mild-to-moderate OSAH (AHI = 5–30) without daytime sleepiness and snorers without OSAH (AHI < 5). Moreover, PAP treatment of a separate population of individuals including those with severe OSAH and those with mild-to-moderate OSAH plus severe daytime sleepiness was associated with substantially greater cumulative survival compared with that of individuals with comparably severe AHI but who declined therapy. In addition, survival of patients with severe OSAH and patients with mild-to-moderate OSAH plus severe daytime sleepiness who were treated with PAP was approximately equivalent to that of untreated patients with mild-to-moderate OSAH (without severe daytime sleepiness). This was an observational study and it is possible that the acceptance of treatment was a marker for a concomitant shift to healthy lifestyle choices and/or greater adherence to other medications, whereas those patients with OSAH who declined treatment may have continued to make unwise choices that enhanced exposure to cardiovascular mortality and morbidity. However, baseline measures of cardiovascular risk were comparable in the treated and untreated groups and it seems less likely that acceptance of PAP therapy marked a lifestyle epiphany to such a degree that it provides a primary explanation for the long-term survival differences. It remains possible that the decision to decline or adhere to PAP therapy is a marker for other difficult-to-assess healthy habits, such as adherence to prescribed medications, that might have impacted mortality. In addition, bias may have been introduced by unexplored medical, psychiatric, or socioeconomic factors that influenced the decision to accept and adhere to PAP and influenced outcome. Because severe daytime sleepiness was a criterion for therapy in patients with mild to moderately increased AHI, it would have been informative to know the specific predictive value of this symptom and cardiovascular outcome. Furthermore, the investigators did not report details of the patient education regarding potential health consequences associated with nontreatment in those individuals who went on to be treated with positive pressure and those who did not. Thus, while incapable of providing unique attribution of improved survival to PAP therapy per se, the data provided by Marin and coworkers represent compelling evidence that adherence to positive pressure therapy confers survival benefit in patients with a severely elevated AHI, and perhaps those with mild to moderate elevation and severe daytime sleepiness. Confidence in this conclusion is supported by other studies (48–50). Although subject to the same considerations inherent to an observational study as discussed above, a study by Campos-Rodriguez and coworkers (48) assessed the dose–response relationship between PAP adherence and survival. Individuals with average daily PAP use in excess of approximately 4 hours (range, 1–6 h) experienced a survival benefit relative to a group with an average daily use of 0.3 hour (<1 h/d) (Figure 2). It is unfortunate that these investigators did not analyze the dose–response relationship between adherence and survival in terms of shorter bins of daily PAP exposure, as this would have provided greater clinical insights. Thus, although they were not RCTs, several cohort-based observational studies provide a consistent conclusion regarding the effect of positive pressure therapy on survival of patients with more severe OSAH.
Figure 2.
Cumulative survival of patients with obstructive sleep apnea–hypopnea by categories of average daily use of positive airway pressure therapy. Patients with average daily use less than 1 hour had significantly lower cumulative survival than those with an average daily use of 1–6 hours and those with an average daily use exceeding 6 hours. There was no difference in the groups with average daily use of 1–6 hours and more than 6 hours. Reprinted by permission from Reference 48.
Biological and physiological plausibility of improved cardiovascular mortality and morbidity reinforce the conclusions of the observational cohort studies. Within-subject comparisons conducted during longitudinal studies have demonstrated a reduction of biomarkers reflecting proatherogenic inflammation after initiation of PAP therapy in patients with moderate or greater OSAH (51–57). These studies did not include a placebo-controlled group. Although conceivable that some factor(s) other than therapy per se was primarily operational in reducing systemic and in vitro evidence of inflammation, the likelihood of this is diminished by the observation that an effect was evident after as little as 4–8 weeks of therapy (53–55) and without a change in body mass index. Similarly, with some exceptions (58, 59), noncontrolled within-subject studies have demonstrated that PAP therapy is associated with a decrease in markers of oxidative stress (60–64). The influence of potentially confounding variables was generally not considered in these studies. Furthermore, the sample sizes were small and generally reflected patients with more severe OSAH, at least in terms of AHI. These limitations notwithstanding, the results are consistent with those of the cohort survival and cardiovascular morbidity studies.
Finally, in addition to the biological plausibility that PAP therapy reduces mortality and morbidity, a retrospective study examined the temporal pattern of death in patients with and without OSAH. Although the data were not adjusted for specific cardiovascular risk factors, the analysis suggested that there is a dose–response relationship between the AHI and the relative risk of sudden nocturnal cardiac death (65). There was no difference in prevalent risk factors between the patients with OSAH and individuals without OSAH. The effect of PAP therapy was not evaluated in this study. However, although excess mortality due to sudden death attributable to OSAH has not been reported, PAP therapy has been shown to reduce prevalent dysrhythmias in these patients (66, 67). Conceptually, at least, this should reduce risk. Similarly, randomized trials comparing PAP therapy with usual care have demonstrated that the former improves left ventricular ejection fraction in patients with severe OSAH and reduced pretreatment left ventricular function (68, 69). On the other hand, one randomized cross-over trial comparing autotitrating CPAP (A-CPAP) versus sham CPAP in patients with left ventricular dysfunction and moderate to severe OSAH failed to show a differential effect on left ventricular function (70). As the authors of this report noted, however, the AHI was not as severely elevated in their study population as in the other studies. Moreover, follow-up sleep studies to assess the impact of A-CPAP on OSAH were not performed. Unfortunately, there was no fixed-CPAP treatment arm, which would have provided insight into whether or not the lack of effect reflected unresponsiveness to CPAP or just the A-CPAP mode of delivery.
In light of the aggregate data, is it mandatory to conduct large, randomized controlled clinical trials to determine whether individuals with severe OSAH, at least as defined by the AHI, who use positive pressure therapy are likely to experience improved long-term survival relative to nonuse? From the perspective that the existent, relatively large observational cohort studies provide consistent results in this regard, and the fact that these results are congruent with biological and physiological plausibility, an affirmative conclusion seems reasonably well founded. It remains nonetheless prudent to maintain some concern that the improved survival might ultimately be due to unassessed factors, rather than PAP per se, and that adherence to this therapy is a marker of these beneficial factors. Although ethical considerations preclude an RCT design to explore this issue further in symptomatic patients and individuals with severe OSAH, larger observational studies that include newer techniques to address potential bias (71) seem warranted. In our view the evidence is of sufficiently high quality that clinicians should educate patients with severe OSAH that acceptance of, and adherence to, positive pressure therapy is associated with improved survival and reduced cardiovascular morbidity. Without minimizing the importance of research to uncover fundamental roots of pathogenesis, we suspect that patients are more interested in outcome than specific attribution.
The further challenge for our field is to determine the risks and benefits; or, in other words, the outcomes of treating individuals with mild and moderate OSAH with PAP or, for that matter, any intervention that alleviates sleep-disordered breathing events. The impact of various disease modifiers such as sleepiness and comorbid conditions on outcome must also be assessed. Acquisition of data in all of these regards will provide the bonus of enabling development of evidence-based guidelines for treatment of OSAH across the spectrum of disease severity.
EVIDENCE-BASED DATA ON PAP TITRATION AND TREATMENT PARADIGMS
Technician adjustment of applied PAP guided by monitoring during overnight, in-laboratory full polysomnography (PSG; multivariable monitoring that provides information about stage architecture and continuity, breathing pattern, oxyhemoglobin saturation, heart rhythm and rate, and in some circumstances abnormal body movements) has been considered by many clinicians and investigators to be the “gold standard” for determining the PAP prescription. However, the degree to which this represents the optimal approach has been called into question by the high prevalence of OSAH (72, 73), engendering increased demand for limited sleep laboratory and personnel resources, in turn giving rise to long waiting lists, increased costs, and delayed care with potentially serious health consequences (47, 48, 50, 74). Consequently, practitioners of sleep medicine have recognized the importance of developing and validating alternative strategies to establish the PAP prescription. In the context of this discussion, the quality of research and resulting evidence concerning these strategies is addressed, with a focus on methods of pressure titration as well as the various PAP treatment modes.
PAP Titration Strategies
The level of PAP that eliminates events that reflect OSAH varies from patient to patient in a way that is poorly predictable; for example, there is no single pressure that is effective for all patients. Thus, individualized pressure prescription is necessary. Further complicating the issue is the fact that the requisite pressure for a given individual may not be constant across circumstances, with pressure requirements potentially varying with changes in weight, sleep position, medication, alcohol, and age.
In the early years of PAP therapy, the main endpoint of titration was the elimination of apneas. Subsequently, elimination of hypopneas, snoring, and inspiratory flow limitation as well as optimization of sleep continuity have been suggested titration goals (75, 76). At present, there are no standard, validated evidence-based guidelines for pressure adjustment, and therefore the criteria vary from center to center. There are important, unresolved issues including (1) lack of universal agreement regarding the type of events that must be addressed during titration; (2) the relative merits of different PAP titration paradigms, for example, sleep technician adjustment of PAP over a full-night PSG (full-night PSG titration) versus initiation and titration of PAP during the second portion of the night-time PSG after a diagnosis of OSAH during the first portion of the PSG (split-night PSG titration) versus autoadjusting CPAP (A-CPAP) titration; and (3) lack of clear evidence-based criteria to facilitate matching individual patients to the specific titration paradigm that engenders the greatest likelihood of clinical success.
Which Events Need to Be Corrected
There is a general consensus that the presence of obstructive apneas and hypopneas (identified by reduced but not absent airflow accompanied by oxyhemoglobin desaturation or arousal) warrants an increase in the magnitude of applied PAP. Inspiratory flow limitation is recognized when increasing (e.g., progressively more negative) esophageal pressure swings are unaccompanied by increasing airflow (77), consistent with increased upper airway resistance. The physiological and clinical significance of prolonged periods of flow limitation (PPFL; arbitrarily defined as flow-limited periods during sleep lasting more than 2 min) (78) remains uncertain. Some studies have demonstrated or inferred the association between blood pressure elevation during sleep and intervals during which there are large negative intrathoracic pressure swings (79–81). It is conceivable, therefore, that PPFL could be harmful, particularly in patients with cardiovascular disease (82). The data were derived from studies examining small numbers of patients, with short follow-up intervals, or nonclinically representative experimental conditions (79–82). In addition, the fact that PPFL may be present in otherwise healthy, snoring individuals, for example, during delta or slow wave sleep, provides cause for some reservation concerning the need to correct PPFL. To our knowledge, there is no high-quality evidence regarding the outcome advantages and disadvantages associated with PAP titration that is directed to eliminate PPFL in patients with OSAH with and without cardiovascular and other medical comorbidities. Therefore, appropriately powered, randomized trials with patients with OSAH allocated to groups with titration strategies that do or do not target PPFL for elimination with subsequent long-term monitoring are needed. Moreover, the study populations should be sufficiently large to permit assessment in clinically relevant subgroups, with the most important representing those patients with cardiovascular comorbidities. Studies performed in animal models, in which it is possible to use invasive measurements under well-controlled experimental conditions, will be of interest (83). Although recognizing the limitations of the existing evidence, in view of the physiological data indicating that PPFL is associated with potentially important disadvantageous physiological consequences we believe that the potential benefits of eliminating PPFL outweigh possible risks. Pending the availability of evidence-based guidance it seems reasonable to direct clinical titration of PAP to eliminate PPFL.
PAP Titration Strategies
Full-night PSG titration.
Historically, patients who have been diagnosed as having OSAH that is deemed to warrant PAP treatment have titration of pressure over an entire night during a technician-attended PSG. Titration is usually done by a trial-and-error process, by the technician who is attending the PSG and who adjusts the applied pressure until those respiratory and sleep parameters that are considered to be clinically important are reduced to the degree judged by the clinician to be acceptable. One study has reported that, because of upper airway hysteresis in some patients, the optimal CPAP value during the latter portion of the titration night is slightly lower than that required earlier in the night, suggesting that some individuals are prescribed higher pressure than required as a result of the currently conventional titration paradigm (84). Such a reduction may be particularly useful in patients requiring high levels of pressure. However, given concern that the pressure-lowering benefit of hysteresis may not be sustained or extend into the following nights, as well as the lack of clinical outcome data, RCTs are needed before the clinical applicability of this strategy is considered. These data do provide some conceptual basis for prescribing A-CPAP as a treatment mode, as is discussed subsequently in this article.
Titration using A-CPAP to determine fixed-pressure CPAP prescription.
A-CPAP devices are intended to detect breathing disturbances or absence of such disturbances over specified intervals and modify the applied PAP upward or downward, respectively, in real time, according to a device-specific algorithm (the algorithm features, and use of A-CPAP as an ongoing treatment modality, are discussed below). This PAP modality was originally designed for treatment but was quickly adopted for use in CPAP titration such that a single “best” level positive pressure could be identified from the overnight profile of pressure that was applied by the A-CPAP device with prescription of that single or fixed-pressure CPAP level (85–90). Stradling and coworkers (87) randomized patients with OSAH to receive a fixed-pressure CPAP prescription for home use that was derived either by an overnight technician titration or after a night of A-CPAP with examination of the overnight A-CPAP profile from which the lowest pressure that eliminated most “obstructive events” (which were not further defined) was selected. The average level of prescribed positive pressure did not differ significantly between the titration strategies. More recently, Masa and coworkers (89) studied patients with severe OSAH (AHI > 30) and reported that after 3 months of CPAP, there were no significant differences in the level of prescribed CPAP, improvement in AHI, or subjective sleepiness when the fixed-pressure CPAP prescription was determined by technician titration of CPAP or was derived from examination of the applied pressure profiles during A-CPAP application over three nights while the patient slept at home. Mulgrew and coworkers (90) randomized sleepy patients with OSAH to receive fixed-pressure CPAP at a prescription determined either during a sleep laboratory technician titration or from the A-CPAP profiles obtained over 1 week of nightly use at home. After that 3 months of therapy, there was no significant difference between the two titration strategies regarding the change in AHI from baseline, score on the Epworth Sleepiness Scale (a validated measure of subjective sleep propensity during common activities [91]), or quality of life (measured by a validated instrument, the Sleep Apnea Quality of Life Index [92]). It is important to note that before randomization, all participants had a personal education and interface-fitting session with a coordinator as well as an opportunity to wear CPAP during wakefulness in an effort to facilitate tolerance. In addition, the protocol permitted further manual adjustment of pressure in the A-CPAP titration, but not the technician titration group, in response to a suggestion of persistent OSAH on overnight oximetry performed during the first week of fixed-pressure CPAP therapy. It is also important to recognize that trials examining the use of A-CPAP to identify a single, fixed-pressure prescription generally excluded patients with OSAH with medical and psychiatric morbidities.
Although use of A-CPAP to identify a fixed-pressure CPAP prescription is cost-effective relative to a full-night titration in selected patient populations (93), there are not enough studies to substantiate this conclusion across the broad clinical spectrum of patients with OSAH (e.g., patients with mild–moderate OSAH or with comorbidities). Randomized controlled clinical trials comparing full-night PSG and A-CPAP-titration to derive a fixed-pressure CPAP prescription are particularly needed in these groups of patients who are commonly seen in clinical practice. The primary outcomes of these studies should include clinical symptoms, cognitive and quality of life tests, and adherence to PAP therapy. We believe that there is sufficient evidence to justify determination of a fixed-pressure prescription from a trial of home A-CPAP provided the patient is typical of those evaluated in the published studies (e.g., unequivocal, symptomatic OSAH and without serious medical or psychiatric comorbidities) and is provided with in-depth education about OSAH and its treatment as well as an opportunity to select the best-fitting and most comfortable interface. This paradigm is not suitable for those individuals who have only limited comprehension of OSAH and its therapy, those with abnormal craniofacial anatomy, and those for whom a satisfactory interface has not been clearly identified beforehand.
Split-night PSG titration.
Split-night PSG titration is an attended, in-laboratory, overnight procedure during which sleep and breathing variables are recorded for diagnostic purposes during the first 2 hours of the sleep period, after which, if specific criteria are met, CPAP titration is performed during the remainder of the night. Split-night PSG titration may provide a pressure prescription that is comparable to that of full-night PSG titration in patients who demonstrate frequent obstructive events early in the sleep period (94–97). In a case-controlled study, McArdle and coworkers (94) observed no significant difference between patients receiving a split-night PSG titration and those having a full-night PSG titration regarding subjective sleepiness. Similarly, when considering the entire study population there was no difference between the two titration strategies regarding the cumulative percentage of patients who continued to use CPAP over time. There was a suggestion that it was lower in those patients with a baseline AHI < 30 who had a split-night titration compared with those who had a full-night PSG titration. However, the number of patient with AHI < 30 was quite small and this issue requires definitive examination with an RCT design. Surprisingly, there are few prospective studies of this strategy in patients with clinical comorbidities and across the entire spectrum of OSAH severity, especially milder degrees of OSAH. This issue notwithstanding, a report by the American Academy of Sleep Medicine describing the evidence base of practice parameters for the use of PAP therapy includes the split-night PSG titration protocol as an acceptable alternative to the standard approach (98, 99).
Deutsch and coworkers have provided a useful cost-effectiveness analysis that compared the full-night PSG titration strategy with the split-night PSG titration strategy as well as with a paradigm that includes diagnostic monitoring home A-CPAP titration to establish a fixed-pressure CPAP prescription (93). Results of the comparison between the full-night PSG titration and home A-CPAP titration strategies have been described (see above). The authors employed a decision tree model incorporating typical clinical algorithms for each strategy to compare their cost-effectiveness from a third-party payer perspective over a 5-year period. Probabilities and test characteristics were derived from previously published data. Cost estimates were based on the 2004 Medicare Fee Schedule and survival rates were taken from National Center for Health Statistics data and published studies. Effectiveness was measured as quality-adjusted life years. This analysis, as well as previously published studies in this area, demonstrated that split-night PSG titration is cost-effective in a clinic population with a high prevalence of symptomatic patients with moderate-to-severe disease (93, 100). However, more studies are needed in this area, especially in patients with less severe OSAH and those with comorbidities.
Use of predictive equations to establish positive pressure prescription.
Employing a predictive equation represents another potential strategy to identify a therapeutic level of PAP (50, 101–104). Most studies addressing this issue examined primarily patients with severe OSAH and evaluated relatively small sample sizes without describing power calculations. West and coworkers (102) reported comparable symptomatic improvement in patients using fixed-pressure CPAP that was established using a formulaic approach, patients for whom a fixed-pressure CPAP prescription was derived from home A-CPAP profiles, and patients who used nightly A-CPAP as a treatment modality. However, traditional full-night PSG titration was not used as a control. Hukins (105) evaluated the practice of initiating CPAP at a pressure (termed “arbitrary” pressure by the investigator) that was determined by a formula based on the patient's body mass index. Outcomes were compared with those determined after a traditional full-night PSG titration. Of note, the arbitrary pressure paradigm permitted the initial pressure to be reduced in response to patient intolerance of the initial setting. On the other hand, the initial arbitrary pressure was empirically increased in response to reports of snoring or persistent sleepiness. After 3 months of treatment, the formulaic and full-night PSG titration paradigms provided comparable improvement in subjective sleepiness and health-related quality of life assessed by the Short Form-36 quality of life questionnaire. Despite comparability of improvement in these outcomes, the investigators reported that objective PSG assessment of OSAH in patients, using the arbitrary pressure paradigm (after any adjustments for intolerance, persistent snoring, or sleepiness), resulted in an increase in the treatment pressure increase from 11.4 ± 2.0 to 13 ± 2.0 cm H2O (mean ± SD). Sleep architecture and overnight oxyhemoglobin saturation during application of the arbitrary pressure were not reported. In another randomized trial, Masa and colleagues (89) observed that the formula-derived CPAP prescription did not completely abolish all sleep-disordered breathing events. Moreover, a number of patients with comorbid conditions were excluded.
In our view, the absence of controlled, long-term outcome studies across the spectrum of OSAH severity and patients with health comorbidities, as well as the concerns raised by the Hukins study and the study by Masa and coworkers, preclude routine clinical application of formulaically derived positive pressure prescriptions. Conceivably, use of this paradigm may be appropriate in underresourced areas, but there must be particular efforts to ensure patient education and close follow-up with a knowledgeable clinician.
Treatment of OSAH with PAP Modalities Other than Fixed-Pressure CPAP
Use of A-CPAP for ongoing treatment.
Although conventional, fixed-pressure CPAP therapy is effective in most patients with OSAH, the application of a single pressure value over time has potential drawbacks because the collapsibility of the upper airway varies not only during a single night (e.g., shifting body position, alcohol ingestion with changing blood levels overnight) but also long term (changes in body mass index). A-CPAP devices are intended to modify the applied pressure in real time, according to that required to maintain upper airway patency. In theory, at any given time, these devices apply the lowest effective pressure. Because fixed-pressure CPAP is generally titrated to maintain upper airway patency under circumstances of greatest collapsibility and is maintained at this level throughout the night, treatment with A-CPAP should result in application of a lower average overnight pressure. In concept, this may reduce side effects, improve comfort, and facilitate adherence to therapy. In general, studies comparing A-CPAP and fixed-pressure CPAP applied during PSG monitoring have demonstrated comparable efficacy in reducing the AHI, at least when the A-CPAP adjustment algorithm is not perturbed by excessive air leaks through the mouth or at the mask–skin interface. In a meta-analysis of published randomized trials comparing treatment with A-CPAP and fixed-pressure CPAP devices, Ayas and coworkers (106) observed that, as expected, the average applied overnight pressure is significantly lower with A-CPAP while comparably low AHIs are achieved. However, there was no difference between the two modalities regarding subjective sleepiness or adherence to therapy. The authors concluded that, in general, A-CPAP therapy does not offer advantages in the evaluated outcomes over fixed-pressure CPAP and the latter remains the standard for PAP treatment. More recent clinical studies addressing this question (104, 107–111) have not provided evidence to warrant modification of this conclusion. RCTs are clearly needed to determine whether there are subpopulations of patients with OSAH that would derive outcome and adherence benefit from A-CPAP therapy. Conversely, these trials would identify populations in whom this modality may be disadvantageous. Careful attention to potential confounders is critically important in designing these studies. Finally, studies are needed to examine the utility of A-CPAP in “salvaging” patients with OSAH who are unresponsive to, will not accept, or are inadequately adherent to fixed-pressure CPAP such that they can be satisfactorily treated.
Bilevel PAP.
Unlike CPAP, which by definition applies the same pressure throughout the ventilatory cycle, bilevel PAP permits independent adjustment of the pressure applied during inspiration and that applied during expiration. Application of expiratory PAP prevents the static upper airway occlusion (e.g., at end expiration) whereas inspiratory PAP prevents the dynamic upper airway obstruction that is related to inspiratory physiology. Laboratory evaluations have demonstrated the capacity for bilevel PAP to achieve comparable improvement in AHI as fixed-pressure CPAP but at a lower expiratory pressure (112). However, although limited, the available evidence does not reveal added benefit from an initial PAP prescription delivering bilevel PAP compared with fixed-pressure CPAP in the general OSAH population (113, 114). There is some evidence from case series studies suggesting that bilevel PAP offers a treatment advantage in subpopulations of patients, for example, those with obesity hypoventilation syndrome and hypercapnic patients with chronic obstructive pulmonary disease (115, 116). These studies were neither large nor long-term. Given the potential advantages of bilevel PAP, blinded randomized studies with long-term follow-up are needed to elucidate the advantages and disadvantages of this modality as initial treatment of patients with medical comorbidities and as “salvage” therapy in those patients who are unresponsive to, do not accept, are intolerant of, or inadequately adhere to fixed-pressure CPAP.
Pressure-relief PAP.
Pressure-relief PAP is a variant of fixed-pressure CPAP and reflects reduction of the applied CPAP during expiration in proportion to the patient's expiratory flow with subsequent increase in pressure to the prescribed “fixed” level at end expiration (e.g., when expiratory flow has ceased). The goal is to reduce the expiratory load and improve patient adherence. Prospective, randomized studies have demonstrated that pressure-relief CPAP is as effective as conventional fixed-pressure CPAP in improving AHI and sleep efficiency, and in reducing the arousal index (the average number of arousals per hour of sleep) (117, 118). However, these studies did not demonstrate that use of this modality conferred an adherence advantage. As in the case of bilevel PAP, adequately powered, prospective, randomized studies should be directed to investigate the possibility that there are subgroups of patients who will benefit from this modality and under what, if any, circumstances benefit will be accrued.
Considerations in Assessing Performance of PAP Devices
Fixed-pressure CPAP.
Translation of PAP device–generated mask pressure to a therapeutically effective constant pressure in the upper airway may be adversely affected by factors that increase system resistance (e.g., long tubing and/or water condensation) and by dynamic variables (breathing frequency and tidal volume) (119). It is important to understand this possibility when patients express complaints regarding persistent or recurrent symptoms or comfort of therapy. Although this is less of an issue with the newer generation of CPAP devices, with their greater ability to compensate for changes in circuit resistance, swings in airway pressure associated with extremes in breathing pattern, and air leaks through the mouth or at the mask–skin interface, it nonetheless should be considered by the clinician to whom the specifications regarding the mechanical capabilities of the device should be made available by the manufacturer through comprehensive and clinically relevant bench testing.
A-CPAP.
A-CPAP devices are not easy to implement in practice. First, the device needs to incorporate sensors and software to automatically and reliably detect and classify abnormal breathing events. The system should be sufficiently robust to distinguish between sleep-disordered breathing events (e.g., snoring, apneas, hypopneas, and flow limitation) that require pressure adjustment from common events that should not result in a modification of the CPAP applied to the patient (e.g., cough, sighs, swallowing, speaking, mouth breathing, leaks, and events associated with arousal from sleep). Another implementation difficulty concerns the algorithm to modify the pressure applied in response to various detected events. As mentioned above, there remains the open question of the optimal target variables that should drive clinical PAP titration. In view of the limited consensus in the clinical community regarding which variable(s) should be used to define abnormal breathing events, the presence or absence of which should “drive” titration, various proprietary algorithms have been incorporated into A-CPAP devices across different manufacturers. The large number of A-CPAP devices, and the fact that their adjustment algorithms are usually undisclosed for commercial reasons, reinforces the need for bench testing with public access to the results as well as well-designed clinical trials (120).
CONCLUSIONS
Since Wright and coworkers (1) brought undeniable awareness to the deficiencies in OSAH research up to that time, there has been increasing attention given to employing study designs that provide the highest possible level of evidence to address important clinical questions. In view of scientific, ethical, and feasibility considerations, no single interventional trial design is optimally suited to examine all questions regarding PAP therapy for OSAH. However, regardless of clinical trial design, acquisition of high-quality evidence mandates that investigators engage proper methods to establish and evaluate a sample size that is requisite to address the study hypothesis. This becomes increasingly important as greatly needed studies are designed to examine PAP treatment outcomes in individuals with less severe OSAH in whom the treatment effect size may be smaller, as well as in trials assessing and comparing efficacy, outcomes, and cost-effectiveness of various titration strategies and different PAP modalities in specific subpopulations, such as those with medical, psychiatric, and socioeconomic comorbidities.
In conjunction with increased attentiveness to acquisition of high-quality evidence, we have gained notable insights regarding PAP therapy for OSAH. In the process, important gaps in our knowledge have been highlighted. Although some attribution to unassessed health and lifestyle factors cannot be definitively excluded, studies provide strong evidence that use of PAP therapy by patients with severe OSAH favorably influences incident cardiovascular disease and long-term survival. This conclusion is buttressed by physiological and biological plausibility. With further attention to exploring the potential contribution(s) of heretofore unassessed factors, future trials should provide a definitive answer. Well-designed and executed outcome studies of patients with milder OSAH and assessment of interactions with medical, psychiatric, and expanded lifestyle habits (e.g., exercise patterns), and socioeconomic status will be challenging, but critically important in view of the prevalence of less severe disease within the population. Moreover, with increasing appreciation of the broad biological and clinical effects of sleep-disordered breathing (e.g., endocrine, hepatic, and renal) and the potential impact of treatment, it would seem prudent to expand the spectrum of assessed outcomes to provide an expansive compendium of the high quality of evidence.
Currently available evidence reasonably supports the expectation that PAP treatment of patients with severe OSAH who also have persistent hypertension will result in reduction of blood pressure over the 24-hour interval. However, well-designed, long-term interventional trials are required to identify specific subgroups of hypertensive patients with OSAH who have greater and lesser likelihoods of obtaining a meaningful antihypertensive effect from PAP therapy. Moreover, we need to know whether reduction in blood pressure with PAP therapy of OSAH has unique advantages compared with pharmacologic antihypertensive intervention.
It is intuitively appealing to consider that PAP treatment reduces the likelihood of motor vehicle crashes and currently available data support this posit. Although further examination of this issue with RCTs is not ethically viable, well-designed long-term observational studies that consider the broad spectrum of potential confounders may provide clinically important insight as well as information of considerable societal and medicolegal importance.
Studies have examined a number of strategies to identify the optimally therapeutic and tolerated level of positive pressure. In general, these investigations provide evidence that a clinically appropriate pressure prescription can usually be derived through any one of a variety of paradigms. However, the study populations that were examined have generally been limited to patients with OSAH without health comorbidities and have not included patients with milder disease. Thus, the results of these studies cannot be extrapolated to the general OSAH population. Much the same conclusions are reached when considering the evidence regarding the clinical utility and cost-effectiveness of the various PAP modalities. Although there has been reasonable demonstration that these modalities stabilize the upper airway in patients with OSAH, the evidence base is of insufficient quality to determine whether there are unique long-term benefits conferred by these modalities in specific clinical subgroups of patients with OSAH.
It is essential to acknowledge the overlap of information provided by studies of PAP therapy, including effective titration paradigms and intervention-related outcomes, and information that defines the nature of OSAH as a disease. For example, what physiological variable(s) and to what degree must these variables be perturbed to elicit symptoms and adverse outcomes? Indeed, the argument becomes circular in that a call for efficacy and outcome studies in individuals with “milder” OSAH assumes that there is an evidence-based definition of “milder” OSAH and that it requires treatment. It may reasonably be anticipated that intervention trials will provide important information concerning these issues, highlighting the overlap between studies that elucidate the nature of the disorder and those that explore its treatment.
In acknowledging the lack of universal applicability, feasibility, or supremacy of a given clinical interventional trial design it is also important to recognize the value of multiple trials examining specific issues, the results of which, when taken in aggregate, will have a high likelihood of yielding durable, clinically relevant, and useful information. By challenging the sleep medicine community to contemplate the quality of its research, Wright and coworkers performed a considerable service. The response of the sleep medicine community to this challenge has benefited our science and our patients.
Supported by NIH grants R01 AG023977 and 1 UL1 RR024153 (M.H.S. and R.J.G.), R01 HL 70301 (M.H.S.), R01 HL63767 (M.H.S.), R01 AG19362 (M.H.S.), and N01-HR-76193 (M.H.S.); and by Spanish Ministry of Science and Technology grant SAF2005-0110 (R.F.).
Conflict of Interest Statement: M.H.S. is a scientific consultant to Respironics, Inc., and from time to time has given talks at sessions sponsored by Respironics, Inc. He is coinventor of BiPAP manufactured by Respironics, Inc., and he has substantial financial interest in that brand and related technologies by Respironics, Inc. This article does not identify products as manufactured by Respironics and does not use brand names. J.M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ 1997;314:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobin MJ. The role of a journal in a scientific controversy. Am J Respir Crit Care Med 2003;168:511–515. [DOI] [PubMed] [Google Scholar]
- 4.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878–1886. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss D. Beyond randomized, controlled trials. Curr Opin Crit Care 2004;10:574–578. [DOI] [PubMed] [Google Scholar]
- 6.Petitti D. Hormone replacement therapy and coronary heart disease: results of randomized trials. Prog Cardiovasc Dis 2003;3:231–238. [DOI] [PubMed] [Google Scholar]
- 7.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000;133:933–941. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 9.Stradling JR, Davies RJO. The unacceptable face of evidence-based medicine. J Eval Clin Pract 1997;3:99–103. [DOI] [PubMed] [Google Scholar]
- 10.Pack AI, Young T. Superficial analysis ignores evidence on efficacy of treatment. BMJ 1997;315:367. [PMC free article] [PubMed] [Google Scholar]
- 11.Findley LJ, Unverzagt ME, Suratt PM. Automobile accidents involving patients with obstructive sleep apnea. Am Rev Respir Dis 1988;138:337–340. [DOI] [PubMed] [Google Scholar]
- 12.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnoea and the risk of traffic accidents. N Engl J Med 1999;340:847–851. [DOI] [PubMed] [Google Scholar]
- 13.George CF, Smiley A. Sleep apnea and automobile crashes. Sleep 1999;22:790–795. [PubMed] [Google Scholar]
- 14.Barbé F, Sunyer J, de la Pena A, Pericas J, Mayoralas LR, Anto JM, Agusti AG. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration 2007;74:44–49. [DOI] [PubMed] [Google Scholar]
- 15.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565–571. [DOI] [PubMed] [Google Scholar]
- 16.Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, von Wichert P. Risk of traffic accidents in patients with sleep-disordered breathing: reduction with nasal CPAP. Eur Respir J 1996;9:2606–2611. [DOI] [PubMed] [Google Scholar]
- 17.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med 2000;161:857–859. [DOI] [PubMed] [Google Scholar]
- 18.George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger J, Meslier N, Lebrun T, Levy P, Phillip-Joet F, Sailly JC, Racineux JL; Working Group ANTADIR, Paris and CRESGE, Lille, France; Association Nationale de Traitement a Domicile des Insuffisants Respiratoires. Accidents in obstructive sleep apnea patients treated with nasal continuous positive airway pressure: a prospective study. Chest 1997;112:1561–1566. [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36): tests of data quality, scaling assumptions, and reliability across diverse patients groups. Med Care 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson C, Davies RJO, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353:2100–2105. [DOI] [PubMed] [Google Scholar]
- 22.Montserrat JM, Ferrer M, Hernandez L, Farré R, Vilagut G, Navajas D, Badia JR, Carrasco E, De Pablo J, Ballester E. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med 2001;164:608–613. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835–843. [PubMed] [Google Scholar]
- 24.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 2001;163:344–348. [DOI] [PubMed] [Google Scholar]
- 25.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax 2005;60:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, Trinder J, Saunders NA, McEvoy RD, Pierce RJ. A randomised controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:773–780. [DOI] [PubMed] [Google Scholar]
- 27.Barbé F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, Monasterio C, Bosch M, Ladaria A, Rubio M, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med 2001;134:1015–1023. [DOI] [PubMed] [Google Scholar]
- 28.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000;320:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 30.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000;160:2289–2295. [DOI] [PubMed] [Google Scholar]
- 31.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 32.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest 1997;99:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome. Arch Intern Med 2007;167:757–765. [DOI] [PubMed] [Google Scholar]
- 34.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003;107:68–73. [DOI] [PubMed] [Google Scholar]
- 35.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, Almeida-Gonzalez C. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006;129:1459–1467. [DOI] [PubMed] [Google Scholar]
- 36.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension 2000;35:144–147. [DOI] [PubMed] [Google Scholar]
- 37.Pepperell JCT, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJO. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002;359:204–210. [DOI] [PubMed] [Google Scholar]
- 38.Robinson GV, Smith DM, Langford BA, Davies RJO, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006;27:1229–1235. [DOI] [PubMed] [Google Scholar]
- 39.Norman D, Loredo JS, Nelesen RA, Ancoli-Israel S, Mills PJ, Ziegler MG, Dimsdale JE. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension 2006;47:840–846. [DOI] [PubMed] [Google Scholar]
- 40.Arias MA, García-Río F, Alonso-Fernández A, Martínez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea—effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006;27:1106–1113. [DOI] [PubMed] [Google Scholar]
- 41.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers.” Sleep 1996;19:378–381. [DOI] [PubMed] [Google Scholar]
- 42.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:656–664. [DOI] [PubMed] [Google Scholar]
- 43.Turnbull F; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure–lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 44.Narkiewicz K, Kato M, Phillips BG, Pesek DA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999;100:2332–2335. [DOI] [PubMed] [Google Scholar]
- 45.Vasan RS, Massaro JM, Wilson PW, Seshadri S, Wolf PA, Levy D, D'Agostino RB; Framingham Heart Study. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation 2002;105:48–53. [DOI] [PubMed] [Google Scholar]
- 46.MacMahon S, Peto R, Collins R, Godwin J, MacMahon S, Cutler J, Sorlie P, Abbott R, Collins R, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 47.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 48.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea–hypopnea patients treated with positive airway pressure. Chest 2005;128:624–633. [DOI] [PubMed] [Google Scholar]
- 49.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-age men with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:159–165. [DOI] [PubMed] [Google Scholar]
- 50.Marti S, Sampol G, Muñoz X, Torres F, Roca A, Lloberes P, Sagalés T, Quesada P, Morell F. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J 2002;20:1511–1518. [DOI] [PubMed] [Google Scholar]
- 51.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol 2003;94:179–184. [DOI] [PubMed] [Google Scholar]
- 52.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005;112:2660–2667. [DOI] [PubMed] [Google Scholar]
- 53.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M. Elevated production of tumor necrosis factor-α by monocytes in patients with obstructive sleep apnea syndrome. Chest 2004;126:1473–1479. [DOI] [PubMed] [Google Scholar]
- 54.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003;107:1129–1134. [DOI] [PubMed] [Google Scholar]
- 55.Tazaki T, Minoguchi K, Yokoe T, Samson KTR, Minoguchi H, Tanaka A, Watanabe Y, Adachi M. Increased levels and activity of matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004;170:1354–1359. [DOI] [PubMed] [Google Scholar]
- 56.Minoguchi K, Yokoe T, Tanaka A, Ohta S, Hirano T, Yoshino G, O'Donnell CP, Adachi M. Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J 2006;28:378–385. [DOI] [PubMed] [Google Scholar]
- 57.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-κB–dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2006;174:824–830. [DOI] [PubMed] [Google Scholar]
- 58.Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, Somers VK. Oxidative stress in obstructive sleep apnoea. Eur Heart J 2005;26:2435–2439. [DOI] [PubMed] [Google Scholar]
- 59.Wali SO, Bahammam AS, Massaeli H, Pierce GN, Iliskovic N, Singal PK, Kryger MH. Susceptibility of LDL to oxidative stress in obstructive sleep apnea. Sleep 1998;21:290–296. [PubMed] [Google Scholar]
- 60.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 2000;162:566–570. [DOI] [PubMed] [Google Scholar]
- 61.Barceló A, Miralles C, Barbé F, Vila M, Pons S, Agusti AGN. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J 2000;16:644–647. [DOI] [PubMed] [Google Scholar]
- 62.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004;27:123–128. [PubMed] [Google Scholar]
- 63.Dyugoyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934–939. [DOI] [PubMed] [Google Scholar]
- 64.Barceló A, Barbé F, de la Peña M, Vila M, Pérez G, Piérola J, Durán J, Agustí AGN. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J 2006;27:756–760. [DOI] [PubMed] [Google Scholar]
- 65.Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 66.Simantirakis EN, Schizab SI, Marketoua ME, Chrysostomakisa SI, Chlouverakisc GI, Klapsinosa NC, Siafakasb NS, Vardasa PE. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J 2004;25:1070–1076. [DOI] [PubMed] [Google Scholar]
- 67.Harbison J, O'Reilly P, McNicholas WT. Cardiac rhythm disturbances in the obstructive sleep apnea syndrome: effects of nasal continuous positive airway pressure therapy. Chest 2000;118:591–595. [DOI] [PubMed] [Google Scholar]
- 68.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348:1233–1241. [DOI] [PubMed] [Google Scholar]
- 69.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169:361–366. [DOI] [PubMed] [Google Scholar]
- 70.Smith LA, Vennelle M, Gardner RS, McDonagh TA, Denvir MA, Douglas NJ, Newby DE. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J 2007;28:1221–1227. [DOI] [PubMed] [Google Scholar]
- 71.D'Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 72.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 73.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163:685–689. [DOI] [PubMed] [Google Scholar]
- 74.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 75.Rapoport DM. Methods to stabilize the upper airway using positive pressure. Sleep 1996;19(9 Suppl):S123–S130. [DOI] [PubMed] [Google Scholar]
- 76.Montserrat JM, Ballester E, Olivi H, Reolid A, Lloberes P, Morello A, Rodriguez-Roisin R. Time-course of stepwise CPAP titration: behavior of respiratory and neurological variables. Am J Respir Crit Care Med 1995;152:1854–1859. [DOI] [PubMed] [Google Scholar]
- 77.Farre R, Rigau J, Montserrat JM, Buscemi L, Ballester E, Navajas D. Static and dynamic upper airway obstruction in sleep apnea: role of the breathing gas properties. Am J Respir Crit Care Med 2003;168:659–663. [DOI] [PubMed] [Google Scholar]
- 78.Norman RG, Ahmed MM, Walsleben JA, Rapoport DM. Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistor. Sleep 1997;20:1175–1184. [PubMed] [Google Scholar]
- 79.Meurice JC, Paquereau J, Denjean A, Patte F, Series F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypopnoea syndrome. Eur Respir J 1998;11:1121–1127. [DOI] [PubMed] [Google Scholar]
- 80.Stradling JR, Barbour C, Glennon J, Langford BA, Crosby JH. Which aspects of breathing during sleep influence the overnight fall of blood pressure in a community population? Thorax 2000;55:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calero G, Farré R, Ballester E, Hernandez L, Daniel N, Montserrat Canal JM. Physiological consequences of prolonged periods of flow limitation in patients with sleep apnea hypopnea syndrome. Respir Med 2006;100:813–817. [DOI] [PubMed] [Google Scholar]
- 82.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest 2001;119:827–835. [DOI] [PubMed] [Google Scholar]
- 83.Farré R, Nácher M, Serrano-Mollar A, Gáldiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep 2007;30:930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bureau MP, Series F. Comparison of two in-laboratory titration methods to determine effective pressure levels in patients with obstructive sleep apnoea. Thorax 2000;55:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lloberes P, Ballester E, Montserrat JM, Botifoll E, Ramirez A, Reolid A, Gistau C, Rodriguez-Roisin R. Comparison of manual and automatic CPAP titration in patients with sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1996;154:1755–1758. [DOI] [PubMed] [Google Scholar]
- 86.Teschler H, Berthon-Jones M, Thompson AB, Henkel A, Henry J, Konietzko N. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med 1996;154:734–740. [DOI] [PubMed] [Google Scholar]
- 87.Stradling JR, Barbour C, Pitson DJ, Davies RJ. Automatic nasal continuous positive airway pressure titration in the laboratory: patient outcomes. Thorax 1997;52:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berkani M, Lofaso F, Chouaid C, Pia DM, Theret D, Grillier-Lanoir V, Harf A, Housset B. CPAP titration by an auto-CPAP device based on snoring detection: a clinical trial and economic considerations. Eur Respir J 1998;12:759–763. [DOI] [PubMed] [Google Scholar]
- 89.Masa JF, Jimenez A, Duran J, Capote F, Monasterio C, Mayos M, Teran J, Hernandez L, Barbé F, Maimo A, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med 2004;170:1218–1224. [DOI] [PubMed] [Google Scholar]
- 90.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography. Ann Intern Med 2007;146:157–166. [DOI] [PubMed] [Google Scholar]
- 91.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 92.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 1998;1598:494–503. [DOI] [PubMed] [Google Scholar]
- 93.Deutsch PA, Simmons MS, Wallace JM. Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2006;15:145–153. [PubMed] [Google Scholar]
- 94.McArdle N, Grove A, Devereux G, Mackay-Brown L, Mackay T, Douglas NJ. Split-night versus full-night studies for sleep apnoea/hypopnoea syndrome. Eur Respir J 2000;15:670–675. [DOI] [PubMed] [Google Scholar]
- 95.Sanders MH, Costantino JP, Strollo PJ, Studnicki K, Atwood CW. The impact of split-night polysomnography for diagnosis and positive pressure therapy titration on treatment acceptance and adherence in sleep apnea/hypopnea. Sleep 2000;23:17–24. [DOI] [PubMed] [Google Scholar]
- 96.Strollo PJ, Sanders MH, Costantino JP, Walsh SK, Stiller RA, Atwood CW. Split-night studies for the diagnosis and treatment of sleep-disordered breathing. Sleep 1996;19(10 Suppl):S255–S259. [DOI] [PubMed] [Google Scholar]
- 97.Rodway GW, Sanders MH. The efficacy of split-night sleep studies. Sleep Med Rev 2003;7:391–401. [DOI] [PubMed] [Google Scholar]
- 98.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005;28:499–521. [DOI] [PubMed] [Google Scholar]
- 99.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J Jr, Friedman L, et al.; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006;29:375–380. [DOI] [PubMed] [Google Scholar]
- 100.Kapur VK, Sullivan SD. More isn't always better: cost-effectiveness analysis and the case for using a split-night protocol. J. Clin. Sleep Med. 2006;2:154–155. [PubMed] [Google Scholar]
- 101.Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis 1993;147:1526–1530. [DOI] [PubMed] [Google Scholar]
- 102.West SD, Jones DR, Stradling JR. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax 2006;61:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitzpatrick MF, Alloway CE, Wakeford TM, MacLean AW, Munt PW, Day AG. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med 2003;167:716–722. [DOI] [PubMed] [Google Scholar]
- 104.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep 2004;27:1512–1517. [DOI] [PubMed] [Google Scholar]
- 105.Hukins CA. Arbitrary-pressure continuous positive airway pressure for obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2005;171:500–505. [DOI] [PubMed] [Google Scholar]
- 106.Ayas NT, Patel SR, Malhotra A, Schulzer M, Malhotra M, Jung D, Fleetham J, White DP. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004;27:249–253. [DOI] [PubMed] [Google Scholar]
- 107.Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short-term trial of two autoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med 2003;168:1506–1511. [DOI] [PubMed] [Google Scholar]
- 108.Marrone O, Resta O, Salvaggio A, Giliberti T, Stefano A, Insalaco G. Preference for fixed or automatic CPAP in patients with obstructive sleep apnea syndrome. Sleep Med 2004;5:247–251. [DOI] [PubMed] [Google Scholar]
- 109.Hussain SF, Love L, Burt H, Fleetham JA. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea–hypopnea. Respir Med 2004;98:330–333. [DOI] [PubMed] [Google Scholar]
- 110.Pevernagie DA, Proot PM, Hertegonne KB, Neyens MC, Hoornaert KP, Pauwels RA. Efficacy of flow- vs impedance-guided autoadjustable continuous positive airway pressure: a randomized cross-over trial. Chest 2004;126:25–30. [DOI] [PubMed] [Google Scholar]
- 111.Nolan GM, Ryan S, O'Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006;28:159–164. [DOI] [PubMed] [Google Scholar]
- 112.Sanders MH, Kern N. Treatment of obstructive sleep apnea by using independently adjusted inspiratory and expiratory positive pressure via nasal mask: physiologic and clinical implications. Chest 1990;98:317–324. [DOI] [PubMed] [Google Scholar]
- 113.Reeves-Hochè MK, Hudgel D, Meck R, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 1995;151:443–449. [DOI] [PubMed] [Google Scholar]
- 114.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006;29:381–401. [DOI] [PubMed] [Google Scholar]
- 115.Resta O, Guido P, Picca V, Sabato R, Rizzi M, Scarpelli F, Sergi M. Prescription of nCPAP and nBiPAP in obstructive sleep apnoea syndrome: Italian experience in 105 subjects. A prospective two centre study. Respir Med 1998;92:820–827. [DOI] [PubMed] [Google Scholar]
- 116.Schafer H, Ewig S, Hasper E, Luderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998;92:208–215. [DOI] [PubMed] [Google Scholar]
- 117.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest 2006;130:1018–1024. [DOI] [PubMed] [Google Scholar]
- 118.Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs standard CPAP: a two-night randomized crossover trial. Sleep Breath 2007;11:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bacon JP, Farney RJ, Jensen RL, Walker JM, Cloward TV. Nasal continuous positive airway pressure devices do not maintain the set pressure dynamically when tested under simulated clinical conditions. Chest 2000;118:1441–1449. [DOI] [PubMed] [Google Scholar]
- 120.Brown LK. Autotitrating CPAP: how shall we judge safety and efficacy of a “black box”? Chest 2006;130:312–314. [DOI] [PubMed] [Google Scholar]