Abstract
Despite the high efficacy of continuous positive airway pressure (CPAP) to reverse upper airway obstruction in sleep apnea, treatment effectiveness is limited by variable adherence to prescribed therapy. When adherence is defined as greater than 4 hours of nightly use, 46 to 83% of patients with obstructive sleep apnea have been reported to be nonadherent to treatment. Evidence suggests that use of CPAP for longer than 6 hours decreases sleepiness, improves daily functioning, and restores memory to normal levels. The decision to embrace CPAP occurs during the first few days of treatment. Although many strategies in patient interface with CPAP or machine modality are marketed to improve CPAP usage, there are few data to support this. No single factor has been consistently identified as predictive of adherence. Patient perception of symptoms and improvement in sleepiness and daily functioning may be more important in determining patterns of use than physiologic aspects of disease severity. Emerging data suggest that various behavioral interventions may be effective in improving CPAP adherence.
Keywords: sleep apnea, obstructive sleep apnea, compliance, continuous positive airway pressure
One of the most dramatic immediate effects of any medical treatment is the ability of continuous positive airway pressure (CPAP) treatment to reverse the repetitive upper airway obstruction of sleep apnea and associated daytime sleepiness. Patients will describe the effect as emerging from a daytime fog and being able to live a productive and healthy life. CPAP, the primary treatment for obstructive sleep apnea (OSA), has been shown to normalize sleep architecture, reduce daytime sleepiness, enhance daily function, elevate mood, reduce automobile accidents, and decrease blood pressure and other cardiovascular events (1). Despite the efficacy of CPAP in reversing sleep apnea, of those studies using the cut point of at least 4 hours per night to define adherence, 29 to 83% of patients were nonadherent (1–12). This article will review the nature of CPAP adherence, review the evidence regarding salient predictors, and describe interventions that have been tested to improve adherence.
PATTERNS OF CPAP ADHERENCE
Compared with the extensive research in maximizing adherence to treatments in such chronic diseases as asthma and diabetes, research into increasing patient use of positive pressure devices is in its infancy. In addition, adherence research is generally focused on pharmaceutical treatments, not cumbersome devices such as CPAP machines and associated mask types. However, the measurement of treatment adherence in CPAP therapy is more precise than metrics used with most pharmaceutical interventions. Smart cards, modem, or web-based methodology can be used to obtain data regarding the nightly duration of therapy at effective pressure—that is, the amount of time the mask is applied directly to the patient.
Using this technology, we know that the pattern of adherence is established early, within the first week of treatment, and predicts long-term use (13–19). Those who skip nights of treatment also use CPAP for shorter nightly durations—on average, 3 hours per night (13). Failure to use CPAP on a nightly basis permits the reemergence of daytime sleepiness and neurobehavioral deficits, even with one skipped night of treatment (20, 21).
However, an important limitation to evaluating individual adherence to CPAP and developing interventions to promote its use is knowing the exact implications of greater or fewer hours of effective use. Do greater hours of CPAP use improve cardiovascular, neurobehavioral, and cognitive outcomes? Is there interindividual variation in the relationship between hours of CPAP use and outcomes? Large-scale studies on this question are currently being implemented. It is also important to recognize that there are many patients who refuse to consider treatment for sleep apnea because of the nature of CPAP as a mechanical mask– and machine-based therapy. This nonacceptance of therapy is therefore a crucial cause of nonadherence.
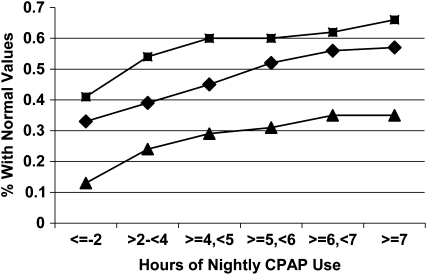
Whether using CPAP all night every night provides the best clinical outcomes has recently become the focus of several studies. It has been observed that improvements in symptoms, daytime sleepiness, neurological behavior, blood pressure, and quality of life occur with greater use (22–26). Some studies suggest that even low levels of application provide some benefit (24, 27, 28). For example, an examination of the effect of nightly duration on 5-year survival rates found that use greater than 1 hour per night significantly lowered mortality (28). However, this study was unable to differentiate benefit with nightly CPAP use of between 1 and 6 hours and use of more than 6 hours per night. Other studies, however, have demonstrated a dose–response relationship (see Figure 1) (24). Normal levels of subjective sleepiness, objective sleepiness, memory, and daily functioning have been achieved with more than 4, 6, more than 6, and 7.5 hours per night, respectively (24–26). Moreover, patients with sleep apnea are eight times more likely to obtain normal scores on a visual memory task with CPAP use of more than 6 hours per night (26) compared with those using it for shorter durations. Using a variety of clinical outcomes, this evidence suggests that any use is better than no use, but greater gains in clinical outcomes may be obtained with longer nightly durations of CPAP therapy. It also indicates that the definition of optimal use may be outcome specific (24). Although it remains unclear why some patients benefit with less CPAP use than others, independent of the baseline apnea–hypopnea index or degree of obesity, it is important to interpret adequate use in terms of benefits achieved (24).
Figure 1.
Cumulative proportion of participants obtaining normal threshold values on the Epworth Sleepiness Scale (squares), Multiple Sleep Latency Test (triangles), and Functional Outcomes of Sleep Questionnaire (diamonds). CPAP = continuous positive airway pressure. Reprinted by permission from Reference 24.
PREDICTORS OF CPAP USE
Interface/Masks
The intrusive nature of CPAP therapy into the sanctity of the bedroom and the natural aversion to wearing unattractive headgear to bed have often been espoused as the reasons for nonadherence. The original CPAP machines involved mask attachment using silastic glue or rudimentary straps. Subsequently, there have been progressive changes in mask technology with greater diversity of interfaces available. However, it is unclear whether these progressive changes in mask type improve adherence or are simply a reflection of marketing strategies. Given the numbers of CPAP masks used worldwide, it is surprising to note the lack of properly powered, randomized superiority or equivalence studies comparing interfaces. Regarding usage of CPAP devices, there is no evidence of superiority of oral masks or nasal pillows over conventional nasal masks (29–32).
If not the interface, then what are the salient predictors of nonadherence to CPAP therapy? Although considerable progress has been made in understanding the nature of CPAP adherence, specific predictors have not been consistently isolated. In a comprehensive review of studies that have examined predictors of adherence to CPAP, Engleman and Wild reported that, collectively, identified variables explained only 4 to 25% of the variance in CPAP use (33). As indicated in Table 1, variables explored to date include patient characteristics, parameters of disease severity, aspects of the technological interface, factors related to the initial exposure to CPAP, and psychological and social variables.
TABLE 1.
CRITICAL ELEMENTS ASSOCIATED WITH CONTINUOUS POSITIVE AIRWAY PRESSURE ADHERENCE
| Pretreatment | With Treatment Initiation |
|---|---|
| Referral source: patient, bed partner, other physician | Heated humidification |
| Assessment of knowledge of OSA and perception of CPAP treatment | Phone call/follow-up first wk of treatment |
| Involvement of bed partner in education and treatment initiation | Assessment of CPAP use and associated outcomes |
| Evaluation of patient awareness and assessment of symptoms | Assessment of patient perception of treatment and symptom-related treatment response |
| How does patient handle challenges in life—active or passive problem solving? | Evaluation of bed partner perception of treatment |
| Assessment of claustrophobic tendencies | Troubleshoot problems immediately—especially during the first week of treatment |
| Evaluation of nasal resistance | Evaluate for the presence of residual sleepiness and, if present, initiate treatment |
| Patient-centered mask and device selection | Retitration if presence of residual events suspected |
| Exposure to CPAP before initiation of therapy |
Definition of abbreviations: CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea.
Reprinted by permission from Reference 61.
Patient Characteristics
Age, sex, marital status, and socioeconomic status have not consistently been associated with CPAP adherence. The issue of whether race plays a role remains to be determined. However, in retrospective studies, African Americans were five-and-a-half times more likely to be nonadherent than whites, after controlling for sex and body mass index, with a nightly duration of CPAP use of 1 to 2 hours less (19, 34, 35). It is undetermined whether these observed differences are solely related to race or reflect aspects of socioeconomic status. There is a dearth of data regarding patterns of use in other ethnic groups. Thus, the effect of race and ethnicity on the inclination to accept and regularly use CPAP is an important area for further investigation.
Disease Severity
Disease severity, as measured by the apnea–hypopnea index, has been shown to have a weak relationship with CPAP adherence (1, 19). There is also a lack of evidence indicating that level of nocturnal hypoxemia is instrumental in determining CPAP adherence (2, 15, 17, 19). In contrast, there is stronger support for symptomatic severity to influence adherence. Self-reported daytime sleepiness, as indicated by a score of greater than 10 on the Epworth Sleepiness Scale, has been shown to be associated with long-term CPAP use (14). Usage of CPAP is low among patients with severe sleep apnea but little sleepiness (36).
There is emerging evidence that increased nasal resistance affects CPAP use and initial acceptance of this treatment (37–39). Using acoustic rhinometry to measure the internal dimensions of the airway, those patients with smaller nasal cross-sectional area and reduced volume were much less likely to be adherent (37). Age-adjusted minimum cross-sectional area explained 22% of the variance in CPAP adherence (37). Interestingly, self-reported nasal stuffiness was not associated with nasal dimensions. Nasal resistance/obstruction also seems to influence the initial acceptance of CPAP treatment, with increased nasal pressure resulting in a 50% greater chance of rejecting CPAP as a treatment (38, 39). Acceptance of CPAP was improved with nasal surgery, suggesting that the nasal cavity should be thoroughly evaluated before treatment, and surgery initiated for patients presenting with either total nasal resistance of more than 0.38 mm Hg/cm3 per second, nasal obstruction that would not be decreased with medical treatment, nasal septum deviation, or inferior turbinate hypertrophy.
Side Effects
As indicated above, there have been considerable advancements in CPAP technology principally aimed at reducing the side effects experienced by most CPAP users. However, although approximately two-thirds of CPAP users report side effects, the evidence does not support side effects as a deterrent to CPAP use (1, 33, 40). Indeed, there are no side effects if the treatment isn't applied.
Method of CPAP Initiation
Beyond technological advances in the treatment of OSA, some investigators have suggested that initial presentation of CPAP in a supportive, controlled environment may influence adherence outcomes (41). Indeed, it has been hypothesized that the actual initial exposure to CPAP in the laboratory may be an influential variable on treatment success (41). Whether the patient received a 2-night or split-night polysomnography for CPAP pressure titration does not appear to be a factor in determining adherence (1). With the availability of more sophisticated home sleep study equipment and autotitrating devices, the question arises whether an unattended study makes a difference in CPAP adherence. In one study, participants undergoing technologist-attended polysomnography used CPAP 1 hour longer and applied it one more night than those whose initial CPAP exposure was unattended (42). Indeed, using semistructured interviews after in-laboratory autotitration, another study found that the experience during the titration night was predictive of initial problems on the first night of CPAP, significantly contributing to CPAP adherence. Although untested, these data suggest that having someone available to reinforce the important benefits of this treatment, to immediately troubleshoot any interface-related problems, and to provide education may enhance CPAP adherence.
Claustrophobia
Although, in general, side effects have not consistently deterred use of CPAP, there have been reports that the sensation of claustrophobia may interfere with use (2, 11). In a prospective study, participants who used CPAP for more than 5 hours per night had a significant decrease in claustrophobic tendencies compared with those using it for shorter periods. Those using CPAP for fewer than 2 hours per night had the greatest variability in CPAP use and a higher level of self-reported claustrophobia on a measure of claustrophobic tendencies. A score greater than 25 on the modified Fear and Avoidance Scale predicted that the patient was twice as likely to use CPAP for fewer than 2 hours per night.
Psychological Factors
Given the absence of any easily identifiable and reliable demographic or technologic predictor of CPAP adherence, there has been increased investigation of psychological factors. Many of these studies have incorporated different theories of behavioral change and health maintenance, including Bandura's social cognitive theory (43), Prochaska and DiClementes' transtheoretical model of behavior (44), and Lazarus and Folkman's stress and coping model (45). Each study has provided significant insight with regard to early decisions to accept, adapt to, and adhere to treatment in patients with sleep apnea.
Mood, such as anxiety and depression, as well as stress, anger, and social desirability, did not influence CPAP adherence (46, 47). Patients' perception of the benefit in symptoms following CPAP and their view of this treatment in terms of health value have been shown to be related to better adherence (33, 48). Patients who experienced greater improvements in daily functioning had higher levels of CPAP adherence (47).
As discussed above, initial perception of CPAP as a desirable and effective treatment may be a critical factor in a patient's acceptance of CPAP. Components of social cognitive theory, such as patient perception of the risk of the illness, benefit of treatment, and volition to use the therapy, formed during the first week of treatment, but not pretreatment, also affect subsequent adherence (49, 50). The strength of the relationship between behavioral change and adherence increased with continued experience on treatment (50) with social cognitive theory constructs and variables of the transtheoretical model accounting for greater than 30% of variance in CPAP adherence rates at 1 month (49). The way in which individuals cope also appears to affect persistence with this treatment. The utilization of active coping rather than passive coping was robustly associated with increased adherence (46). Twenty percent of the variance in CPAP adherence could be explained by the way in which patients troubleshot CPAP problems beyond that explained by the apnea–hypopnea index or excessive daytime sleepiness (46). Those patients who tackled obstacles associated with CPAP use employing an aggressive and problem-solving approach were more successful users.
Finally, social variables, such as social support, partner interaction, and partner sleep quality, have been explored in several investigations to determine their impact on CPAP adherence behavior. Social support was found to have a positive influence on adherence in the few studies that examined this factor (35, 51). If the idea to seek medical attention was not the patient's, and was, in particular, the bed partner's, this adversely affected the patient's decision to adhere (52). The partner's post-treatment sleep quality and overall quality of life were also instrumental in the decision to adhere to treatment (53).
INTERVENTIONS TO IMPROVE CPAP ADHERENCE
Humidification of the Airway
As mentioned above, nasal stuffiness is commonly associated with CPAP treatment. Although some nasal stuffiness may have a potential allergic component, experimental studies using normal volunteers showed that mouth leak was also an important cause of these nasal symptoms (54). Moreover, nasal resistance increases could be attenuated using heated humidification but not cold-water systems (55). However, these experimental data have not been translated into consistent randomized controlled clinical data. An initial three-way randomized crossover study comparing heated and cold humidification and control showed use of CPAP about 30 minutes longer and fewer complaints of nasal side effects with heated humidification, but no difference in sleepiness post-treatment (56). One weakness of this study was the lack of a proper placebo form of humidification. Using a form of placebo, another group demonstrated that the addition of heated humidification reduced the frequency of adverse upper airway symptoms, and modestly (∼20 min) improved initial CPAP use over 3 weeks. Again, there was no improvement in daytime sleepiness or treatment satisfaction with humidification. There was no clear preference by patients for humidification.
Subsequent work using real and placebo humidification showed no real benefit for using humidification on the initial CPAP titration night (57). In addition, another randomized study has found that the initial night and continued humidification showed no advantage in compliance over an approach using humidification as needed according to side effects (58). Although the American Academy of Sleep Medicine has recommended the use of heated humidification as a standard of practice (59), conflicting reports regarding the value of humidification (60) would indicate that its application should be evaluated on an individual basis. However, all existing research investigating the value of humidification is limited by inability to measure true compliance with humidification instructions and proper blinding in long-term studies.
Machine Design
Whether there is historically increased use of CPAP related to major advances in CPAP design (and cost), such as device miniaturization, pressure ramp settings, or provision of objective compliance data to health professionals, is anecdotal and untested. Given the potential cost to health systems, particularly in developing countries, it is important that such advances are placed in a scientific and not simply a marketing context.
Most research has focused on various different CPAP modalities that have been developed on the basis of improving adherence to therapy. These modalities include bilevel CPAP, designed with a lower expiratory pressure to reduce expiratory work of breathing and increase patient comfort; auto-CPAP, developed to vary and optimize the level of CPAP through the night, reducing mean pressure and minimizing local side effects; and pressure-relief or flexible CPAP (e.g., C-Flex [Respironics, Pittsburgh, PA]), which alternates airway pressure between exhalation and inhalation on a breath-by-breath basis to improve patient comfort. It is important to recognize that many of these new modalities were developed or marketed as devices to lower average CPAP level during sleep based on belief that higher CPAP levels are strongly associated with reduced adherence. The reality is that there is little evidence that the level of fixed-pressure CPAP influences adherence (for review, see Reference 61). However, a number of studies have now addressed whether these new modalities of CPAP influence adherence.
There are very limited data on the value of bilevel CPAP on improving CPAP adherence instead of its more common role in sleep-related ventilatory failure. Data are inconclusive and, given the much higher cost of bilevel CPAP, there seems little rationale for its use except in a “rescue” role after poor adherence is observed with standard CPAP (60, 62, 63).
Most studies suggest that patients prefer to use auto-CPAP to either fixed CPAP or neither treatment, but such studies are not double-blinded. However, there is no significant increase in patient quality of life or decrease in symptoms of OSA in comparison to fixed CPAP. Moreover, meta-analyses either find that auto-CPAP does not improve compliance with treatment compared with fixed CPAP in unselected patients or that there is a very modest improvement of 15–20 minutes per night (64, 65). Bench testing has questioned the efficacy of auto-CPAP compared with fixed-pressure devices, suggesting that, in some patients, auto-CPAP “undertreats” with inadequate pressure (66). Alternatively, pressure changes may disturb sleep (67).
Data on the effect of flexible CPAP on improving CPAP adherence are limited to a few studies without consistent results (68, 69). Another modality of CPAP, adaptive servoventilation, has been recently promoted to improve adherence in a subgroup of patients with OSA labeled as “complex sleep apnea.” Such patients are characterized by development of central apnea and sleep disturbance on CPAP (70). However, there are no prospective randomized controlled data reported for adaptive servoventilation, and recent studies seriously question the need for such expensive intervention because most cases of complex sleep apnea seem to resolve with standard CPAP (71).
Future research with different modalities of CPAP will need to focus on patient selection so that work is targeted on clinically meaningful patient subgroups who may benefit from these devices or groups in whom it may be inappropriate to use such treatments. Information on comparative long-term efficacy and effectiveness will need to be provided.
Behavioral Interventions
It is logical to assume that mechanical treatments such as CPAP require patient instruction and support. In a sense, this is little different than setting up a computer or cell phone. However, what is not known is how much education, instruction, and support is required, and whether the way such behavioral treatments are “packaged” is important. There are no meta-analyses of the various CPAP behavioral strategies designed to increase adherence due to study heterogeneity of interventions (65). This was demonstrated by the observation (65) that the equivalent amount of education and support employed as the control in one study (52) served as the intervention in another (72).
One of the earliest studies examining a strategy to improve CPAP adherence used positive reinforcement with nurse contact by telephone without appreciable improvement in CPAP usage. In contrast, the very intensive (and therefore potentially expensive) intervention to improve CPAP adherence used by the Edinburgh group (52) was shown to increase hours of CPAP use in the intervention compared with the control groups (5.4 vs. 3.9 h). Interestingly, adherence was better when the patient was self-referred rather than partner-referred.
In a small, randomized trial, others showed that giving patients pretreatment information and some feedback about their usage on treatment did lead to improvement in adherence at 12 weeks of use (72). Behavioral approaches based on social cognitive therapy have recently been studied to improve adherence to a range of therapies, including CPAP (68, 74–76). The value of this has been emphasized by a recent randomized controlled trial comparing a group cognitive behavioral therapy (CBT) intervention plus standard information package versus the standard package alone (77). This study revealed that the additional CBT resulted in marked improvement in CPAP usage by 2.9 hours daily over the first month of treatment. Importantly, the program was commenced before CPAP initiation and resulted in much fewer dropouts before CPAP initiation. Although this type of study needs to be replicated to infer generalizability across settings, it strongly suggests that it is important to pursue behavioral interventions for improving CPAP adherence. If one compares the greater improvement in CPAP adherence by a group CBT program compared with expensive CPAP modalities, such as auto-CPAP or flexible CPAP, then there is a strong rationale to preferentially prescribe and fund such a behavioral strategy. This is especially important because there is evidence that many patients refuse to consider CPAP or drop out after initial exposure to therapy (12, 35, 78).
Behavioral interventions show promise as an approach aimed at improving CPAP users' adaptation to CPAP through guided troubleshooting and problem solving that is initiated early in the treatment period. Future research should evaluate approaches incorporating such behavioral approaches successfully within routine clinical practice. Recent studies reporting that selected technologic factors, initial experience with CPAP, and psychological factors are important predictors of CPAP adherence, suggest, as proposed by Engleman and Wild (33), that interventions to improve CPAP use will likely be multidimensional.
Supported by NHLBI HL076101-01 and HL60287 (T.E.W.). R.R.G. was supported by NHMRC (Australia) grants 457355, 457094, and 264598; GlaxoSmithKline, Sanofi-Aventis, Respironics, ResMed, Cypress Bioscience, Cephalon, and DiagnoseIT.
Conflict of Interest Statement: T.E.W. has received royalty fees for the use of the Functional Outcomes of Sleep Questionnaire from the following commercial entities: 2005: Jazz Pharmaceuticals, $3,000; Sleep Solutions, $3,000; InfluENT Medical, $3,000; N.V. Organon, $3,000; 2006: Jazz Pharmaceuticals, Inc., $3,000; Merck & Co., $3,176; 2007: Aspire Medical, $3,100. She also received a $300,000 research grant from the Respironics Sleep and Respiratory Research Foundation. R.R.G.'s department has received free equipment for research from Respironics, ResMed, and Somnomed.
References
- 1.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006;29:381–401. [DOI] [PubMed] [Google Scholar]
- 2.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887–895. [DOI] [PubMed] [Google Scholar]
- 3.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest 1993;103:1675–1680. [DOI] [PubMed] [Google Scholar]
- 4.Meurice JC, Dore P, Paquereau J, Neau JP, Ingrand P, Chavagnat JJ, Patte F. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in sleep apnea syndrome. Chest 1994;105:429–433. [DOI] [PubMed] [Google Scholar]
- 5.Hui DS, Choy DK, Li TS, Ko FW, Wong KK, Chan JK, Lai CK. Determinants of continuous positive airway pressure compliance in a group of Chinese patients with obstructive sleep apnea. Chest 2001;120:170–176. [DOI] [PubMed] [Google Scholar]
- 6.Russo-Magno P, O'Brien A, Panciera T, Rounds S. Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc 2001;49:1205–1211. [DOI] [PubMed] [Google Scholar]
- 7.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest 2002;121:430–435. [DOI] [PubMed] [Google Scholar]
- 8.Massie CA, McArdle N, Hart RW, Schmidt-Nowara WW, Lankford A, Hudgel DW, Gordon N, Douglas NJ. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med 2003;167:20–23. [DOI] [PubMed] [Google Scholar]
- 9.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res 2003;54:71–76. [DOI] [PubMed] [Google Scholar]
- 10.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest 2004;126:1248–1254. [DOI] [PubMed] [Google Scholar]
- 11.Chasens ER, Pack AI, Maislin G, Dinges DF, Weaver TE. Claustrophobia and adherence to CPAP treatment. West J Nurs Res 2005;27:307–321. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample: what are the benefits and the treatment compliance? Sleep Med 2006;7:553–560. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep 1997;20:278–283. [DOI] [PubMed] [Google Scholar]
- 14.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108–1114. [DOI] [PubMed] [Google Scholar]
- 15.Krieger J. Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep 1992;15(6, Suppl):S42–S46. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal L, Gerhardstein R, Lumley A, Guido P, Day R, Syron ML, Roth T. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med 2000;1:215–220. [DOI] [PubMed] [Google Scholar]
- 17.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med 1994;149:149–154. [DOI] [PubMed] [Google Scholar]
- 18.Sanders MH, Gruendl CA, Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest 1986;90:330–333. [DOI] [PubMed] [Google Scholar]
- 19.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V, Hudgel DW. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep 2007;30:320–324. [PubMed] [Google Scholar]
- 20.Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, Maislin G, Dinges DF. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:1162–1168. [DOI] [PubMed] [Google Scholar]
- 21.Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep 1996;19:774–782. [DOI] [PubMed] [Google Scholar]
- 22.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:461–467. [DOI] [PubMed] [Google Scholar]
- 23.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 2001;163:344–348. [DOI] [PubMed] [Google Scholar]
- 24.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stradling JR, Davies RJ. Is more NCPAP better? Sleep 2000;23:S150–S153. [PubMed] [Google Scholar]
- 26.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest 2006;130:1772–1778. [DOI] [PubMed] [Google Scholar]
- 27.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:656–664. [DOI] [PubMed] [Google Scholar]
- 28.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005;128:624–633. [DOI] [PubMed] [Google Scholar]
- 29.Beecroft J, Zanon S, Lukic D, Hanly P. Oral continuous positive airway pressure for sleep apnea: effectiveness, patient preference, and adherence. Chest 2003;124:2200–2208. [DOI] [PubMed] [Google Scholar]
- 30.Anderson FE, Kingshott RN, Taylor DR, Jones DR, Kline LR, Whyte KF. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep 2003;26:721–726. [DOI] [PubMed] [Google Scholar]
- 31.Khanna R, Kline LR. A prospective 8 week trial of nasal interfaces vs. a novel oral interface (Oracle) for treatment of obstructive sleep apnea hypopnea syndrome. Sleep Med 2003;4:333–338. [DOI] [PubMed] [Google Scholar]
- 32.Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst Rev 2006;4:CD005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med Rev 2003;7:81–99. [DOI] [PubMed] [Google Scholar]
- 34.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath 2004;8:173–183. [DOI] [PubMed] [Google Scholar]
- 35.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med 2007;3:285–288. [PMC free article] [PubMed] [Google Scholar]
- 36.Barbe F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, Monasterio C, Bosch M, Ladaria A, Rubio M, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 2001;134:1015–1023. [DOI] [PubMed] [Google Scholar]
- 37.Li HY, Engleman H, Hsu CY, Izci B, Vennelle M, Cross M, Douglas NJ. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep 2005;28:1554–1559. [DOI] [PubMed] [Google Scholar]
- 38.Nakata S, Noda A, Yagi H, Yanagi E, Mimura T, Okada T, Misawa H, Nakashima T. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology 2005;43:296–299. [PubMed] [Google Scholar]
- 39.Sugiura T, Noda A, Nakata S, Yasuda Y, Soga T, Miyata S, Nakai S, Koike Y. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration 2007;74:56–60. [DOI] [PubMed] [Google Scholar]
- 40.Weaver TE. Adherence to CPAP treatment and functional status in adult obstructive sleep apnea. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis and treatment. New York: Marcel Decker; 2001; pp. 523–554.
- 41.Popescu G, Latham M, Allgar V, Elliott MW. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax 2001;56:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Means MK, Edinger JD, Husain AM. CPAP compliance in sleep apnea patients with and without laboratory CPAP titration. Sleep Breath 2004;8:7–14. [DOI] [PubMed] [Google Scholar]
- 43.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. [DOI] [PubMed] [Google Scholar]
- 44.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–395. [DOI] [PubMed] [Google Scholar]
- 45.Lazarus R, Folkman S. Coping and adaptation. In: Gentry W, editor. The handbook of behavioral medicine. New York: Guilford; 1984. pp. 282–325.
- 46.Stepnowsky CJ Jr, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep 2002;25:758–762. [DOI] [PubMed] [Google Scholar]
- 47.Wells RD, Freedland KE, Carney RM, Duntley SP, Stepanski EJ. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom Med 2007;69:449–454. [DOI] [PubMed] [Google Scholar]
- 48.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J 2004;24:461–465. [DOI] [PubMed] [Google Scholar]
- 49.Stepnowsky CJ Jr, Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med 2002;3:239–247. [DOI] [PubMed] [Google Scholar]
- 50.Aloia M. Predicting treatment adherence in obstructive sleep apnea using principles of behavioral change. J Clin Sleep Med 2005;1:346–353. [PubMed] [Google Scholar]
- 51.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004;27:134–138. [DOI] [PubMed] [Google Scholar]
- 52.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med 1999;159:1096–1100. [DOI] [PubMed] [Google Scholar]
- 53.McArdle N, Kingshott R, Engleman HM, Mackay TW, Douglas NJ. Partners of patients with sleep apnoea/hypopnoea syndrome: effect of CPAP treatment on sleep quality and quality of life. Thorax 2001;56:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes MJ, McGregor FB, Roberts DN, Schroter RC, Pride NB. Continuous nasal positive airway pressure with a mouth leak: effect on nasal mucosal blood flux and nasal geometry. Thorax 1995;50:1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE. Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med 1996;154:182–186. [DOI] [PubMed] [Google Scholar]
- 56.Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999;116:403–408. [DOI] [PubMed] [Google Scholar]
- 57.Duong M, Jayaram L, Camfferman D, Catcheside P, Mykytyn I, McEvoy RD. Use of heated humidification during nasal CPAP titration in obstructive sleep apnoea syndrome. Eur Respir J 2005;26:679–685. [DOI] [PubMed] [Google Scholar]
- 58.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest 2005;128:2151–2158. [DOI] [PubMed] [Google Scholar]
- 59.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J Jr, Friedman L, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006;29:375–380. [DOI] [PubMed] [Google Scholar]
- 60.Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003;26:864–869. [DOI] [PubMed] [Google Scholar]
- 61.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med 2006;12:409–413. [DOI] [PubMed] [Google Scholar]
- 62.Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 1995;151:443–449. [DOI] [PubMed] [Google Scholar]
- 63.Muir JF, Cuvelier A, Bota S, Portier F, Benhamou D, Onea G. Modalities of ventilation in obesity. Monaldi Arch Chest Dis 1998;53:556–559. [PubMed] [Google Scholar]
- 64.Ayas NT, Patel SR, Malhotra A, Schulzer M, Malhotra M, Jung D, Fleetham J, White DP. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004;27:249–253. [DOI] [PubMed] [Google Scholar]
- 65.Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database Syst Rev 2004;4:CD003531. [DOI] [PubMed] [Google Scholar]
- 66.Abdenbi F, Chambille B, Escourrou P. Bench testing of auto-adjusting positive airway pressure devices. Eur Respir J 2004;24:649–658. [DOI] [PubMed] [Google Scholar]
- 67.Marrone O, Insalaco G, Bonsignore MR, Romano S, Salvaggio A, Bonsignore G. Sleep structure correlates of continuous positive airway pressure variations during application of an autotitrating continuous positive airway pressure machine in patients with obstructive sleep apnea syndrome. Chest 2002;121:759–767. [DOI] [PubMed] [Google Scholar]
- 68.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005;127:2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest 2006;130:1018–1024. [DOI] [PubMed] [Google Scholar]
- 70.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007;30:468–475. [DOI] [PubMed] [Google Scholar]
- 71.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest 2007;132:81–87. [DOI] [PubMed] [Google Scholar]
- 72.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep 1997;20:284–289. [DOI] [PubMed] [Google Scholar]
- 73.Aloia MS, Dio LD, Ilniczky N, Perlis ML, Greenblatt DW, Giles DE. Improving compliance with nasal CPAP and vigilance in older adults with OSAHS. Sleep Breath 2001;5:13–22. [DOI] [PubMed] [Google Scholar]
- 74.Aloia MS, Arnedt JT, Riggs RL, Hecht J, Borrelli B. Clinical management of poor adherence to CPAP: motivational enhancement. Behav Sleep Med 2004;2:205–222. [DOI] [PubMed] [Google Scholar]
- 75.Weaver TE, Maislin G, Dinges DF, Younger J, Cantor C, McCloskey S, Pack AI. Self-efficacy in sleep apnea: instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep 2003;26:727–732. [DOI] [PubMed] [Google Scholar]
- 76.Weaver TE. Predicting adherence to continuous positive airway pressure–the role of patient perception. J Clin Sleep Med 2005;1:354–356. [PubMed] [Google Scholar]
- 77.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007;30:635–640. [DOI] [PubMed] [Google Scholar]
- 78.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J 2000;16:921–927. [DOI] [PubMed] [Google Scholar]