Abstract
Obstructive sleep apnea syndrome (OSAS) in children includes a spectrum of respiratory disorders with significant morbidities. Diagnosis of OSAS is based on clinical suspicion, history, and physical findings, and confirmation is made by polysomnography. There has been significant progress in recent years in technologies available for diagnosis of OSAS since the consensus statement of the American Thoracic Society in 1996. The current review describes methodologies that are available today for assessment and diagnosis of OSAS in children and summarizes the most recent recommendations of the American Academy of Sleep Medicine Task Force regarding scoring sleep-related respiratory events in children.
Keywords: diagnosis, obstructive sleep apnea syndrome, pediatrics, polysomnography, snoring
The term “sleep-disordered breathing” (SDB) in children refers to a group of respiratory disorders that occur or are exacerbated during sleep. These include the following: central apnea, apnea of prematurity, hypoventilation, and the spectrum of obstructive hypoventilation disorders. The focus of this article is on the diagnosis of obstructive hypoventilation disorders. The diagnostic approaches to central apnea, apnea of prematurity, and hypoventilation disorders are outside the scope of this review.
Obstructive hypoventilation disorders have a clinical range from the relatively benign condition known as primary snoring, through upper airway resistance syndrome (UARS) associated with sleep fragmentation and excessive daytime sleepiness, to obstructive sleep apnea syndrome (OSAS). OSAS in children is characterized by recurrent events of partial or complete upper airway obstruction during sleep, resulting in disruption of normal ventilation and sleep patterns (1), neurocognitive deficits, and cardiovascular morbidities (2).
The incidence of OSAS in children is estimated to be 2% (3, 4), whereas primary snoring is more common and is estimated to occur in 6 to 9% of children (5). The peak incidence of OSAS occurs between 2 to 8 years of age and parallels the prominent growth of lymphoid tissue around the airway during these years. However, OSAS occurs in children of all ages. As early as the neonatal period, underlying conditions, such as craniofacial anomalies affecting upper airway structure and neurologic disorders affecting upper airway neuromotor tone, may lead to airway obstruction during sleep. Later onset of symptoms, particularly when associated with obesity, is common during school-age and adolescent years (6). The process of diagnosing childhood OSAS continues to evolve as more morbidities are recognized and more precise diagnostic methodologies become available.
HISTORY AND PHYSICAL EVALUATION
Nocturnal Symptoms
Snoring and difficulty breathing during sleep are the most common complaints of parents of children with OSAS, with reports of such symptoms in more than 96% of cases (7, 8). However, the history of snoring alone cannot distinguish between children with OSAS and children with primary snoring (9).
With the exception of young infants, children with OSAS often snore loudly and continuously. Parents often describe episodes of retractions with increased respiratory effort. In the presence of complete or partial upper airway obstruction, inspiratory downward motion of the diaphragm will expand the abdominal wall; however, the sudden increase in negative intrathoracic pressure will cause a paradoxical inward movement of the highly compliant ribcage of the young child. These episodes may be terminated by gasping, movement, or frequent awakenings.
Children appear to be very restless during the night, frequently changing sleep positions to those promoting airway patency and hyperextending the neck (10). Obese children with significant OSAS may prefer sleeping while sitting upright or propped upon pillows. Other common nocturnal findings include increased diaphoresis and enuresis.
Daytime Symptoms
Although respiration in children with OSAS is typically unremarkable during wakefulness, some children with severe OSAS may manifest difficulty in breathing when awake, albeit less so than when asleep. These symptoms are most likely related to adenoid and tonsillar hypertrophy. The most common complaints reported by parents of children with OSAS are mouth breathing, frequent upper respiratory tract infections, recurrent ear infections, as well as hearing and speech problems. In addition, nausea, vomiting, and difficulty swallowing are commonly reported in young children with OSAS and tonsillar hypertrophy.
Excessive daytime somnolence in children has been shown to correlate with severity of OSAS and with increased body mass index (11). However, in contrast to reports in adults with OSAS, excessive daytime somnolence is less common in children with OSAS, being present in 7 to 10% of children with OSAS (11, 12). The multiple sleep latency test and the Epworth sleepiness scale in children with OSAS were shown to be significantly different than in unaffected children, although these values are not considered abnormal by adult criteria (11, 13). These findings suggest that children with OSAS may have a threshold for sleepiness that differs from that of adults.
Neurocognitive deficits and behavioral manifestations are also common and are believed to be the result of chronic exposure to intermittent hypoxemia and sleep disruption due to sleep fragmentation. Behavioral manifestations of children with OSAS may be very similar to those with attention deficit hyperactivity disorder (7, 8, 14). Both disorders may present with symptoms of hyperactivity, inattentiveness, and poor academic performance. Interestingly, many of these symptoms, and particularly academic performance, have been shown to be reversible once OSAS has been treated in these children (15, 16). It should be noted that, in children with underlying genetic disorders and/or developmental delay such as Down's syndrome, OSAS may contribute to additional impairment in intellectual and behavioral performance (17).
Physical Examination
The physical examination of the child with OSAS is variable. In most cases, children appear to only have mildly to moderately enlarged tonsils and adenoids and do not necessarily demonstrate breathing difficulties during the examination. Significant overgrowth of lymphoid tissue in the upper airway, particularly in the retropalatal region, may frequently not be appreciated by direct oral examination. Therefore, a normal physical examination does not exclude OSAS. Physical examination should include an assessment of the child's growth pattern. Children with OSAS are frequently reported to have delayed growth and impaired weight gain (18). On the other hand, obesity in children increases the risk for OSAS 4.5-fold (4).
The physical examination begins with a general observation of the patient. Mouth breathing and adenoidal facies should be noted. Hyponasal voice is a clue of nasal obstruction and a muffled voice is suggestive of adenotonsillar enlargement. The lateral facial profile should be inspected for retrognathia, micrognathia, or midfacial hypoplasia. All can affect the nasopharyngeal and oropharyngeal passages and are key findings for diagnosis. The nose is assessed for septal deviation, mucosal thickening, polyps, and patency of either vestibule with the opposite naris occluded. The oral cavity should be observed for tongue and soft palate size and appearance: a large tongue and/or a high-arched or elongated palate, or a low dependent palate may predispose to SDB. The Mallampati classification is useful in this assessment (19), particularly for older and obese children. Integrity of the hard palate should be evaluated: a bifid uvula may be associated with submucosal cleft palate. The size of the tonsils should be assessed. A scale from 0 to a maximum of +4 when the tonsils meet the midline is commonly used (20). Some simply describe the tonsils' appearance as minimally visible, visible to the pillars, visible beyond the pillars, and visible to the uvula.
The cardiac examination is usually normal; however, in advanced cases, evidence of pulmonary hypertension manifested by a loud second pulmonary heart sound and systemic hypertension has been reported (21–23). In children with OSAS and a noncontributory physical examination, and in young infants, a neurologic examination is required to exclude any neurologic impairment, such as spinal muscular atrophy affecting upper airway muscle tone that may contribute to the disorder.
UPPER AIRWAY EVALUATION
Endoscopy/Laryngoscopy
This technique provides dynamic evaluation of the airway and localization of the region of obstruction and is usually performed under sedation or general anesthesia. Isono and coauthors used endoscopy to localize the region of maximal airway restriction in children with OSAS (24). The region was identified at the level of the adenoid and the soft palate. In clinical practice, endoscopy of the upper airway is often reserved for children with complicated airway structure and altered collapsibility. The nasal passages as well as the oral cavity of children with craniofacial anomalies who previously underwent repair of these defects should be evaluated carefully in the same manner, because these children may be at risk for recurrent OSAS (25, 26).
Pharyngometry
Acoustic pharyngometry, a noninvasive method using sound waves to evaluate upper airway cross-sectional area, has been used successfully in adults, but application in children is limited (27, 28).
Radiographic Evaluation
To evaluate the upper airway in children with OSAS, several radiologic techniques are available, including lateral neck radiographs, cephalometrics, fluoroscopy, computerized tomography, and magnetic resonance imaging (MRI) (29–31). The above modalities have all demonstrated that the upper airway of children with OSAS is smaller on average than compared with that of normal children. MRI is particularly powerful because it may be used for three-dimensional reconstruction of the entire upper airway (including soft tissues and skeletal structure) (31, 32), as well for evaluating upper airway respiratory dynamics (33).
The utility of these techniques varies, and as diagnostic tools they should be tailored to the child's condition. A lateral neck radiograph is simple and may be obtained in clinic to evaluate the size of the adenoid and upper airway patency. Other radiographic techniques should be reserved for more complicated conditions, such as those involving alterations in craniofacial growth and neurologic conditions affecting upper airway collapsibility. In such cases, it would be helpful to discuss the best imaging modality with the radiologist and the specialist involved in the care of the child.
Upper Airway Collapsibility
Functional studies evaluating airway collapsibility suggest that the upper airway in children with OSAS is more collapsible compared with that of control subjects (24, 34). Modeling of the upper airway as a Starling resistor has given some insight regarding the mechanical properties of the upper airway as a collapsible tube. By measuring changes in peak inspiratory flow during continuous external application of positive/negative pressure, the propensity for the upper airway to collapse can be measured. This measurement is known as the critical closing pressure of the pharynx (Pcrit) and is affected by both anatomic and nonanatomic mechanisms (e.g., neuromotor tone, tissue properties) (35). Adult subjects with OSAS often have a positive Pcrit, indicating that the airway would collapse during sleep due to even mild inspiratory negative pressure (36) if it were not “protected” by the action of dilator muscles. Similar results have been found in children, where Pcrit correlated with the severity of SDB (34, 37, 38). In addition to Pcrit measurement, radiographic measurements could be used for evaluating upper airway collapsibility and include fluoroscopy and respiratory-gated MRI (33, 39). At present, however, except for fluoroscopy, these techniques are mostly reserved for clinical research and are not routinely being used as a diagnostic tool for OSAS in children.
SCREENING STUDIES FOR OSAS
Questionnaires
Simple and convenient, screening questionnaires for children with OSAS based on clinical history (8, 40) have been studied for their ability to identify OSAS (2), but have not been able to precisely distinguish between OSAS and primary snoring (9). More recently, more comprehensive questionnaires developed by Chervin and colleagues (41) and Montgomery-Downs and coworkers (42) have shown better predictive values for OSAS. However, these are more useful for research purposes and are not accurate enough for individual subjects. Moreover, separate thresholds scores may be necessary to predict OSAS in children from different age groups and socioeconomic backgrounds (42).
Audio Recording
Overnight audio recording can be used to document the presence of snoring, but it is not able to reliably distinguish primary snoring from snoring associated with UARS and OSAS (43, 44). Therefore, children with positive findings should undergo a full polysomnographic evaluation to evaluate for the severity of OSAS, whereas children with negative results probably do not require further workup (44).
Video Recording
A study correlating home video recordings with polysomnography has demonstrated that a short home video recording of 30 minutes during sleep can reliably screen for OSAS in children (45), with a sensitivity of 94% and specificity of 68%. This study is encouraging, but future research is necessary to validate the utility of this tool for clinical practice.
Continuous Pulse Oximetry Recording
The finding of intermittent oxygen desaturations during sleep in children is highly suggestive of OSAS (10, 46). However, considering difficulties with the technical application of this method at home and understanding that many children with UARS have snoring and arousals without hypoxemia, this technique has significant limitations. Overall, it cannot be used as a reliable tool for the diagnosis of variable presentations of OSAS in children (47). It is possible that these limitations will be circumvented in the future with combined pulse oximetry and arousal recordings using new methodologies, such as pulse transit time (see below) (48).
Electrocardiography
Surface electrocardiography recordings have been used to screen for OSAS in children based on heart rate variability changes with respiratory events and respiration-related changes in electrocardiography (49). This technique has potential as an easy screening tool, but has not been validated in larger studies across the spectrum of OSAS.
Home Monitoring
Unattended home studies in children with OSAS have been improving in quality. In one study using a comprehensive methodology including cardiorespiratory and 8 hours of video recording, results were very similar to those obtained by polysomnography in the laboratory (50). Goodwin and colleagues reported results comparable to laboratory polysomnography in 5- to 12-year-old children in a research protocol with sleep staging and cardiorespiratory monitoring (51). The utility of unattended home studies in children to assess OSAS in a nonresearch setting and across all age groups has not yet been established.
Laboratory Tests
Polycythemia and compensatory metabolic alkalosis may support the diagnosis of longstanding and severe OSAS in children. In addition, obese children at risk for OSAS may have dyslipidemias and insulin resistance. More recently, attention has been directed toward understanding the association between OSAS and the underlying metabolic and genetic risk factors, particularly for cardiovascular and neurocognitive morbidities (52, 53).
OSAS is associated with a group of proinflammatory and prothrombotic factors that have been identified as important in the development of vasculopathy. Such studies in children note endothelial dysfunction, and increase in C-reactive protein, IL-6, fibrinogen, and plasminogen activator inhibitor (54–56). It is possible that biological markers, such as urinary or serum protein patterns (57), will identify children at risk for developing cardiovascular or neurocognitive morbidity in the presence of OSAS. Similarly, knowledge of such genetic susceptibility may provide direction to new modes of therapy to protect against the deleterious consequences of OSAS in children.
POLYSOMNOGRAPHY
Polysomnography has been recommended by an expert consensus panel assigned by the American Academy of Pediatrics (AAP) as the gold-standard test for establishing the presence and severity of SDB in children (58). The AAP does accept the use of audiovisual taping and pulse oximetry recording as screening studies for OSAS. However, polysomnography should follow if these screening studies in suspected children do not support OSAS.
Guidelines for performing laboratory-based polysomnography in children were published by the American Thoracic Society (ATS) (1). These include measures of electroencephalography (EEG) during sleep required for sleep staging, and of respiration for scoring of respiratory events. The American Academy of Sleep Medicine (AASM) recently set up task forces that reviewed the evidence for staging of sleep and the scoring of respiratory evens and arousals (59–61), and published guidelines on staging sleep and scoring events based on these reviews (62).
The polysomnographic variables monitored and recorded during polysomnography include but are not limited to the following:
EEG activity: current AASM recommendations are F4-M1, C4-M1 and O2-M1 with backup (F3-M2, C4-M1, O2-M1) (62), a change from the previous recommendation of C3 or C4 referenced to A1 or A2 (1).
Eye movements (electrooculogram) from electrodes placed near the outer canthus of each eye.
Submental electromyographic (EMG) activity from electrodes placed over the mentalis, submentalis muscle, and/or masseter regions.
Rhythm electrocardiogram (ECG) with one lead II electrode or more chest leads at the discretion of the provider (62).
Respiratory effort, by chest-wall and abdominal movement via strain gauges, piezoelectric belts, inductive plethysmography, impedance or inductance pneumography, endoesophageal pressure (1). The AASM does not recommend strain gauges or piezoelectric belts (62).
Nasal and/or oral airflow via thermistor, nasal pressure transducer, or pneumotachograph or inductance plethysmography (62).
Oxygen saturation (SpO2) via pulse oximetry including waveform (1), with an averaging time of no more than 3 seconds (62).
End-tidal CO2 (PetCO2) or transcutaneous CO2 (PtcCO2).
Body position via sensor and by direct observation.
Limb movements (right and left legs) via EMG.
Snoring recording or vibration (frequency and/or volume).
Audio/video recording by infrared or low-light equipment.
Definition of Arousal
Arousal.
An arousal is an abrupt shift in EEG frequency, which may include θ, α, and/or frequencies greater than 16 Hz, but not spindles, that lasts at least 3 seconds, with at least 10 seconds of stable sleep preceding the change. Scoring of arousals during REM requires an accompanying increase in submental EEG for at least 1 second (62). Arousals may be spontaneous or may be related to events such as technician interventions, respiratory events, and limb movements.
Measures of EEG and Arousals during Sleep
The standard for arousals reported most often in pediatric studies is that defined by the American Sleep Disorders Association in 1992 (63). The updated recommendations have not changed significantly (62). Normative data on arousal indices in children have been published (Table 1).
TABLE 1.
POLYSOMNOGRAPHY VALUES IN NORMAL CHILDREN
| First Author (Reference)
|
||||||
|---|---|---|---|---|---|---|
| Marcus (83) and Witmans (113) | Uliel (85) | Traeger (78) | Montgomery-Downs (84) | Verhulst (86) | ||
| No. of subjects | 45 | 70 | 66 | 542 | 60 | |
| Age range, yr | 1.1–17.4 | 1–15 | 2.5–9.4 | 3.2–8.6 | 6–16 | |
| 3–5, n = 173 | ⩾6, n = 369 | |||||
| HI*, events/h | 0.1 ± 0.1 | 0 | 0.3 ± 0.5 | 0.03 ± 0.07 | 0.10 ± 0.18 | |
| Apnea index, /h | 0.1 ± 0.5 | 0.86 ± 0.75 | 0.5 ± 0.52 | |||
| Obstructive apnea index, events/h | 0.02/0.1† | 0.1 ± 0.03 | 0.03 ± 0.10 | 0.05 ± 0.11 | 0.06 ± 0.16 | |
| AHI‡, events/h | 0.2 ± 0.6 | 0.4 ± 0.6 | 0.9 ± 0.78 | 0.68 ± 0.75 | 1.98 ± 1.39 | |
| Obstructive AHI, events/h | 0.08 ± 0.16 | 0.14 ± 0.22 | 0.08 ± 0.17 | |||
| %TST SpO2 > 95% | 99.6 ± 0.95 | |||||
| SpO2 nadir, % | 96 ± 2 | 94.6 ± 2.2 | 92 ± 3 | 92.7 ± 4.5 | 92.6 ± 3.6 | 91.8 ± 2.7 |
| SpO2 lower limit, % | 92 | 90 | 86 | 84 | 85 | 86 |
| ODI, events/h | 0.3 ± 0.7 | 0.29 ± 0.35 | 0.47 ± 0.96 | 0.8 ± 0.9 | ||
| PetCO2% TST > 50 mm Hg | 0.5 ± 4.0 | 0.29 ± 0.24 | 4.0 ± 15.3 | 2.0 ± 7.1 | ||
| Sleep latency, min | 24.1 ± 25.6 | 23 ± 25.3 | 45.6 ± 29.4 | |||
| Sleep efficiency, % | 90.8 ± 6.5 | 89 ± 8 | 90 ± 7 | 89.3 ± 7.5 | 80.5 ± 8.5 | |
| Arousals, events/h | 8.8 ± 3.8 | 9.3 ± 4.8 | 6.1 ± 1.8 | |||
| RERAs, events/h | 0.92 ± 2.0 | 1.2 ± 1.0 | ||||
Definition of abbreviations: AHI = apnea–hypopnea index; PetCO2 = end-tidal CO2; HI = hypopnea index; ODI = oxygen desaturation index; RERAs = respiratory event–related arousals; SpO2= oxygen saturation; TST = total sleep time.
Data are presented as mean ± SD.
Hypopnea with desaturation (3–4%) and/or arousal.
97.5 percentile.
All types of apneas and hypopneas.
Respiratory events during sleep can potentially lead to an increase in number and duration of arousals that may cause sleep fragmentation and disruption of normal sleep architecture, leading to neurocognitive and neurobehavioral consequences, such as increased sleepiness and decreased vigilance (61). However, in children, this has not always been demonstrated. Children with mild to moderate OSAS may not have changes in arousal index (15), but greater degrees of sleep apnea are associated with increased arousals in children (64–66). Sleep architecture may show greater slow-wave sleep and reduced REM sleep as compared with control subjects (66), but is grossly normal (64, 65) unless there is severe sleep apnea (67). In a more recent controlled study, Tauman and colleagues demonstrated that a surrogate measure for disrupted sleep homeostasis derived from the respiratory arousal index: the sleep pressure score correlated with severity of OSAS in children (66). In a subsequent study, using the same methodology, they found that the sleep pressure score was negatively correlated with neurocognitive abilities in children with OSAS (68).
In efforts to improve the predictive value of arousals, smaller durations of shift in EEG frequency have been studied, but these lead to a significant drop in interscorer reliability (69). Another avenue of progress may be computer-based analysis of frequency shifts, which is easier than manual scoring and may become the standard of the future (61). In addition to EEG changes in response to respiratory events in sleep, changes in autonomic function, such as changes in heart rate and blood pressure, have also been noted. In fact, these are more frequent than EEG arousals and may be more sensitive measures to detect subcortical arousals and predict neurocognitive and/or cardiovascular outcomes in children with OSAS (48, 70).
Pulse transit time.
Pulse transit time (PTT) estimates the time interval between the generation of pulse pressure at the aortic root to its arrival at the periphery. This is measured by the time between the R wave on the ECG and the detection of the pulse at the finger by photoplethysmography. PTT is inversely proportional to the blood pressure. Blood pressure elevation, associated with respiratory arousal from sleep, results in a drop in the PTT (71). Katz and coauthors reported that the number of PTT-measured arousals (6.8/h) was higher than that by EEG (2.2/h) in children with UARS (48). The significance of this measure in children with SDB and OSAS for predicting clinical outcomes has not yet been established.
Peripheral arterial tonometry.
Peripheral arterial tonometry (PAT) measures the pulse waveform in the finger connected to a plethysmograph that envelops the finger with a uniform pressure (72). Increased vasoconstriction, such as with sympathetic output, leads to a decrease in pulse amplitude. PAT provides a measure of autonomic change with arousals during sleep. Similar to PTT, PAT may be more sensitive in detecting arousals in children than EEG changes (70, 73).
Cyclic alternating pattern.
Cyclic alternating pattern (CAP) has been described as a physiologic EEG phenomenon of non-REM sleep. Changes in CAP as an indicator of sleep fragmentation have been suggested as an alternative to the conventional arousal defined by EEG changes (74). CAP is characterized by cyclic patterns consisting of activating “A” phases alternating with baseline “B” phases devoid of this phasic activity. The A phases are of three types: A1, reflecting slower, synchronized activity, and A2 and A3, reflecting progressively less synchronized activity (75). Portions of non-REM sleep without CAP are described as non-CAP. In children, two studies have shown greater sleep stability with CAP analysis in normal children in comparison to children with snoring and those with sleep apnea (76, 77). Further studies are needed to establish the role of CAP in clinical use.
Definitions of Respiratory Events during Sleep
The ATS published consensus guidelines in 1996 for scoring of respiratory phenomena, including apnea (absence of oronasal airflow) and hypopnea (reduction in oronasal airflow), respiratory effort (for obstructive and central events), hypoventilation, and oxygen desaturation (1). These guidelines have been supplanted by more recent recommendations (“rules”) for measuring and scoring respiratory events in children (infants to 18 yr of age) by the AASM (62). These are reviewed here.
Measurements.
Apnea detection.
The recommended sensor for apnea detection is an oronasal thermal sensor. However, alternatively, nasal air pressure transducer, PetCO2, and summed calibrated inductance plethysmography may be used when the recommended sensor is not reliable or, presumably, cannot be used (62).
Hypopnea detection.
The recommended sensor for hypopnea identification is a nasal pressure transducer without square root transformation of the signal. An oronasal thermal sensor may be used when the nasal pressure transducer is not reliable (62).
Respiratory effort.
Acceptable sensors for detection of respiratory effort are esophageal manometry, calibrated inductance plethysmography, or uncalibrated inductance plethysmography (62).
Blood oxygen saturation.
The recommended modality for evaluation of blood oxygen is pulse oximetry with a signal averaging time of no more than 3 seconds (62).
Alveolar ventilation.
Alveolar ventilation may be acceptably assessed by PtcCO2 or PetCO2 monitoring (62).
Definitions.
Obstructive apnea: An obstructive apnea is scored when there is a ⩾90% drop in the signal amplitude of airflow for ⩾90% of the entire event, compared with the preevent baseline amplitude, and the event lasts for at least two breaths (or the duration of two baseline breaths) with continued inspiratory effort throughout the entire period of decreased airflow. The duration of the apnea is measured from the end of the last normal breath to the beginning of the first breath that achieves the preevent baseline inspiratory excursion (62).
Mixed apnea: An event is scored as a mixed apnea if the airflow signal meets duration and amplitude criteria for obstructive apnea, and the event is associated with an absent inspiratory effort in the initial portion of the effort, followed by respiratory effort before the end of the event (62).
Central apnea: A central apnea is scored if the respiratory event is associated with absent inspiratory effort throughout the duration of the event and one of the following is present: (1) the event lasts >20 seconds or (2) the event lasts at least two missed breaths (or the duration of two baseline breaths) and is associated with an arousal, an awakening, or a ⩾3% desaturation (62).
Hypopnea: An event may be scored as a hypopnea if there is a ⩾50% drop in airflow signal amplitude compared with the preevent baseline amplitude for at least 90% of the duration of the event. In addition, the event must last at least two missed breaths (or a duration of two baseline breaths) and should be associated with an arousal, awakening, or a ⩾3% desaturation (62).
Respiratory effort–related arousal: A respiratory effort–related arousal can be scored when an event is accompanied by snoring, noisy breathing, increase in PetCO2/PtcCO2, or visual evidence of increased work of breathing, and the event lasts at least two breath cycles (or the duration of two baseline breaths) if one of the following is present: (1) a discernable reduction in amplitude of the nasal air pressure sensor that is less than 50% in comparison to the baseline level with a flattening of the nasal pressure waveform or (2) there is a progressive increase in inspiratory effort during the event on an esophageal pressure sensor tracing (62).
Hypoventilation: Sleep-related hypoventilation may be scored when >25% of the total sleep time (TST) is spent with a CO2 >50 mm Hg, measured by PtcCO2 and/or PetCO2 sensors (62).
Apnea index: Number of obstructive and/or central apneic events per hour of sleep.
Obstructive apnea index: Number of obstructive apneic events per hour of sleep.
Hypopnea index: Number of hypopneas per hour of sleep.
Apnea–hypopnea index (AHI): The summation of apnea index and hypopnea index.
Obstructive AHI: The summation of obstructive apneic events and hypopneic events per hour of sleep.
Upper airway resistance syndrome: A respiratory disorder of sleep associated with snoring, causing excessive daytime sleepiness due to arousals and sleep fragmentation.
Measures of Respiration during Sleep
Respiratory effort.
Measures of respiratory effort are important to differentiate between central and obstructive events and to categorize hypopneas and respiratory effort–related arousal. Various techniques to measure respiratory effort are available for clinical use.
Piezoelectric belts.
Piezoelectric belts placed around the chest and abdomen measure change in belt tension by the voltage generated in a piezoelectric crystal in the belt. They are similar in principle to strain gauges and cannot be calibrated; therefore, they display qualitative data rather than quantitative data. They have been extensively used in pediatrics and may be associated with overestimation of paradoxical breathing as compared with respiratory inductance plethysmography (RIP) (78). This technique is not recommended in the current standards of the AASM (60).
Impedance pneumography.
This modality uses two or three electrodes attached to the patient in a configuration similar to that of a three-lead ECG to detect impedance changes as the cross-sectional area of thorax/abdomen as it expands and contracts using a weak, alternating electrical current. Impedance pneumography allows only qualitative estimates of thoracoabdominal movements and is susceptible to cardiogenic artifact.
RIP.
RIP measures the change in cross-sectional area of a coil of wire placed around the thorax and abdomen on the basis of the change in impedance of the coil. RIP is able to measure movement in the thorax and abdomen in a quantitative manner when calibrated and can reveal in phase and out-of-phase or paradoxical movements of the thorax and abdomen. Displacement of the belt may lead to poor signal quality, often seen in restless or obese children.
Esophageal manometry.
Esophageal manometry measures intrathoracic pressure by a liquid-filled cannula (79), solid-state transducer, or balloon introduced into the lower esophagus through the nose. Inadequate filling of the balloon or patient movement may render the pressure measurements inaccurate. Esophageal manometry is difficult to use in clinical practice and is not widely used because of patient discomfort and awakenings leading to alteration in sleep architecture. Chervin and coworkers reported that, among 54 children, 51 allowed insertion and 33 maintained the catheters throughout the night (80). Esophageal manometry is recommended as the gold standard for evaluation of respiratory effort in UARS and in the differentiation of central and obstructive apnea (60). The cutoff for abnormal respiratory effort is not clearly defined. However, abnormal inspiratory pressures are considered between −10 and −20 cm H2O or less (41, 81).
PTT.
PTT is inversely proportional to the blood pressure, and increases as the blood pressure drops with inspiration (71), thus serving as an index of respiratory effort and flow. PTT had the ability to differentiate obstructive from central events with a sensitivity of 91 and 94% and specificity of 95 and 97% for two observers (82). The sources of error using PTT are mostly related to pulse waveform artifact and variation in baseline PTT. Definitive comparisons between esophageal manometry and PTT in the detection of apnea–hypopnea events have not been reported in children.
Recording of airflow.
In the individual child, it is prudent to use or have available more than one method to assess airflow because any one method may not be usable or may fail during the study. For example, children with pervasive developmental disorder may not allow thermistors and cannulas to be used.
Pneumotachography.
Pneumotachography is considered the gold standard for airflow measurement (60), against which other modalities are evaluated. The commonest variety of pneumotachometer used is the differential pressure flow transducer in which respired air passes through a resistive element and airflow is proportional to the pressure drop across the resistance. The pneumotachometer is attached to a face mask that must have a leak-free seal over the face. Despite its accuracy for flow and volume measurement, this technique is restricted to clinical research purposes, mostly due to inconvenience to the patient, alterations in breathing pattern, and alterations in sleep architecture.
Thermistors and thermocouples.
Thermistors are resistors that have variable resistances in response to change in temperature. Thermocouples are conductors that generate voltage when subjected to a thermal gradient. Both are small devices that are easily taped between the nose and mouth and provide a qualitative estimate of airflow on the basis of temperature differences between ambient air and respired air. They can simultaneously assess nasal and oral flow, which can be an advantage when studying children who breathe through the mouth. Thermistors are widely used in pediatric polysomnography and have the benefit of being the most widely used method of airflow estimation in studies of normative data in children (78, 83–86). However, thermistors have inherent limitations in their ability to detect airflow and underestimate flow reductions. Thermal devices have a long-time constant response (87), do not reproduce flow limitation with fidelity (88), and are inadequate for assessment of possible UARS (81). Other limitations include poor signal quality with condensation on the sensor or when the sensor is dislodged.
Nasal pressure transducer.
A nasal pressure transducer consists of a nasal cannula connected to a pressure transducer that provides a semiquantitative estimate of airflow based on the measurement of pressure. Nasal pressure tends to overestimate the degree of reduction in flow. Validation studies of nasal pressure in adults have shown good agreement with the pneumotachograph and good intraobserver and interobserver agreement (89, 90). In most clinical situations, using the square root correction to minimize overestimation of hypopnea may not be necessary (89) or could be remediated by using a higher (75% reduction) threshold for identifying hypopneas (60, 90).
Studies comparing nasal cannula pressure with thermistors consistently show higher rates of detection of events by nasal cannula even when liberal criteria, such as any discernable reduction in amplitude, are used for the thermistor (91, 92). Nasal pressure tracings can demonstrate both the flattened contour of flow limitation (93) that is considered a feature of UARS, and snore-related vibrations (94). The relevance of flow limitation in children in relation to clinical outcomes has not been defined. Published studies of nasal pressure usage in children are more limited than in adults (92, 95, 96).
Failure rates in children and infants with nasal cannulas (4 and 2% of sleep time, respectively) were higher compared with thermistors (0 and 0% of sleep time, respectively) in one study (92), and another study reported a failure rate of 28%. Failure is related to displacement of the cannula, obstruction by the nasal wall, nasal secretions, and mouth breathing. There may be difficulty in simultaneously obtaining adequate end-tidal CO2 and nasal pressure signals (96), and concern has been raised regarding a possible increase in resistance to airflow in the smaller nares of children (92, 97). The use of a thermistor has been suggested over nasal pressure in the detection of central apnea because of the tendency of a pressure transducer to misclassify hypopnea as apnea (60).
End-tidal CO2.
End-tidal CO2 tracings may provide a qualitative estimate of nasal flow using variation in the amplitude of the CO2 waveform. These have been used in conjunction with other modalities in children to detect apnea–hypopnea (83, 84). This modality has not been extensively reported on as a means of flow assessment in the diagnosis of sleep apnea.
RIP.
RIP allows a good estimate of tidal volume and has been widely used in the evaluation of OSAS. It provides measurement of thoracoabdominal synchrony, and detection of apnea and hypopnea. Percentage changes in baseline tidal volume at the mouth can be made on the basis of the sum of the changes in thoracic and abdominal volume (98). The calibration coefficients that describe the volume–motion relationship may change significantly during varying sleep stages, when subjects change position, such as supine to prone, and when they are allowed to flex the trunk freely (99). Thoracoabdominal asynchrony and changes in tidal volume with sleep stage may also contribute to errors in estimation of tidal volume. Estimates of tidal volume from calibrated RIP differed in clinically significant amounts when compared with pneumotachography (100). Nevertheless, comparison of RIP to pneumotachography in adults has shown modest to good agreement for definition of apnea–hypopnea (89, 90). In children, paradoxical movements can be present normally up to the age of 30 months (101), presenting a problem in the use of RIP as the sole method of assessment of apnea–hypopnea (60). Problems with RIP include inadequate signal due to belt slippage, which affects the quality of the signal.
Gas exchange
Measures of oxygenation.
Pulse oximetry gives an estimate of arterial oxygen saturation (SpO2) rather than the partial pressure of oxygen (PaO2), but has become the standard for assessment of oxygenation based on ease of use, reliability, and reproducibility. It is based on the differential absorption of light at 850–1,000 and 600–750 nm wavelengths by oxyhemoglobin and deoxyhemoglobin, respectively, and differentiation of the “pulse-added absorbance” with arterial pulsation from the background tissue absorption levels. The reliability of the output is affected by motion, perfusion pressure, ambient light, nail polish, methemoglobin, carboxyhemoglobin, sickle hemoglobin, and improper probe placement with optical shunting (102, 103). Display of the pulse waveform helps to corroborate the accuracy of the reading and is recommended in pediatric studies (1). The displayed value represents blood from the lung delayed by the circulation time from the lung to the tissue that is about 5 seconds in children (102). Averaging time is an important consideration in sleep studies: increasing the time over which the saturation value is averaged reduces error, but masks brief desaturations. In pediatric sleep studies in which the number of desaturations may be important, shorter averaging times are desirable (102, 104). The difference in averaging time and the use of proprietary algorithms by different manufacturers to minimize artifact make comparison of values between studies difficult.
Hypoxemia and repetitive significant oxygen desaturation are frequent in children with OSAS. Stradling and colleagues found that, of 61 children referred for adenotonsillectomy, 61% were noted to be hypoxemic during sleep before surgery (10). Rosen and colleagues found significant oxygen desaturations to less than 85% in 19 of 20 children referred for evaluation of OSAS with a relative low apnea index of 1.9 ± 3.2 (105), suggesting that significant and severe upper airway obstruction in children may not be identified by the apnea index alone. The same authors showed that hypopneas can be responsible for gas exchange abnormalities in this group, as reported previously by Brouillette and coworkers (14).
Published normative data allow a description of the range of various oxygen-related indices seen in children (78, 83–86). Basal oxygen saturation values range from 95 to 100%, oxygen saturation nadir can normally be as low as 84 to 86% saturation, and the number of desaturations of 4% or more per hour can range from 0 to 2.6 per hour. Even though mild desaturation (90–93%) is relatively common in children during sleep, it may be associated with poorer academic function (106).
Measures of ventilation.
The technique of sampling CO2 tension in respired air is capnometry and its graphical display is capnography. The terminal expired concentration of CO2 reaches a plateau and reflects alveolar gas concentration. This measurement, known as end-tidal CO2 (PetCO2), can provide an estimate of ventilation during sleep (1).
Exhaled air is sampled via a sidestream capnometer and the CO2 levels are estimated by infrared spectroscopy or mass spectroscopy. Poor signal may be obtained with poor positioning of the cannula, rapid respiratory rates without stable exhaled CO2 levels, mouth breathing, simultaneous delivery of oxygen, and blockage of the cannula with secretions or humidity (84, 107). Capnography can be unreliable in the presence of lung disease. Discomfort from the cannula may lead to refusal or disturbed sleep. The expiratory PetCO2 tracing is also affected by the amount of expiratory flow and the rate of withdrawal into the sidestream, and the amount of room air diluting the expired gas flow (108).
There are several groups who are at high risk for developing hypercarbia and hypoventilation. Hypoventilation is the most common polysomnographic abnormality in children with Down's syndrome and OSAS (109). Obese children with OSAS were also found to develop hypoventilation during sleep by Silvestri and colleagues, who noted hypercarbia in 75% of these children (110). Those who were morbidly obese were at greatest risk for the development of this abnormality. In the ATS consensus statement, based on data in 50 healthy infants and adolescents, hypoventilation was defined by the following criteria: peak PetCO2 > 53 mm Hg, or PetCO2 > 50 mm Hg more than 8% of sleep time, or when duration of hypoventilation (PetCO2 > 45 mm Hg) exceeds more than 60% of TST (83). However, a recent study of 543 healthy children reported that 20% of the children spent 50% or more of TST at 45 mm Hg or greater and 2.2% spent 50% or more of TST at 50 mm Hg or greater (84). The AASM guidelines recommend that obstructive hypoventilation can be diagnosed when more than 25% of TST is spent with a PetCO2 of more than 50 mm Hg (62).
At times, the nasal sampling catheter required for the measuring of PetCO2 may be difficult to maintain, especially in young children. In these cases, PtcCO2 monitoring can be useful. Transcutaneous CO2 measurements are made by warming the surface of the skin so that capillary blood is arterialized and closely matches arterial CO2 tension. Transcutaneous measurements have been used in pediatric studies to estimate hypoventilation (107) and provide interpretable data in the majority of children (111). However, normative data in sleeping children are not available. This technique has received mixed reviews in the assessment of hypoventilation (60).
Normal Polysomnography Values
Normative polysomnography values derived from several studies on a total of more than 700 children have been published so far (78, 84–86, 106, 113). Some of these studies used different methodologies, particularly for measurements of respiratory effort and airflow, and definitions of hypopnea and arousal slightly varied; therefore, data for some values are not directly comparable (Table 1). Extrapolated threshold values from these studies, such as the apnea index, AHI, and the nadir of SpO2, are commonly used to make the diagnosis of OSAS in children (1, 112, 113). In clinical practice, one should be familiar with these datasets and the methodologies in which these were obtained when applying to patients studied in one's own laboratory.
DIAGNOSTIC APPROACH TO CHILDREN WITH SUSPECTED OSAS
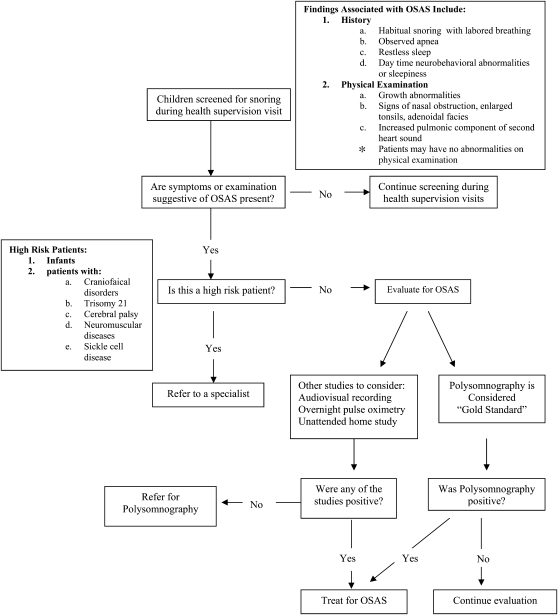
The goals of diagnosis of OSAS in children are as follows: (1) identify patients who are at risk for adverse outcomes and (2) avoid unnecessary intervention in patients who are not at risk for adverse outcomes. As suggested by the AAP (58), all children should be screened for snoring during a health supervision visit (Figure 1). The screening should include direct questions obtained by history, such as the presence of snoring with labored breathing, observed apnea, restless sleep, neurobehavioral abnormalities or sleepiness, and evaluation for alterations in growth or physical findings that may support the diagnosis of OSAS. If any of the above is present, further consideration should be made regarding additional risk factors for OSAS, beyond adenotonsillar hypertrophy and obesity. These include craniofacial or syndromic conditions affecting upper airway anatomy or neurologic conditions affecting upper airway motor control. Children without identifiable additional risk factors should be referred for polysomnography, whereas children with risk factors should be referred to a sleep specialist for further evaluation before polysomnography.
Figure 1.
Algorithm for evaluation of obstructive sleep apnea syndrome (OSAS) in children. Modified by permission from Reference 58.
At present, polysomnography provides the gold-standard tool for evaluating the presence and severity of OSAS in children (58). Studies with relatively small numbers of children show only slight variation of polysomnography parameters from night to night, and polysomnography should not miss significant OSAS (114, 115). Obese children may have a lower number of obstructive events in the prone position that may contribute to night-to-night variability (116). The diagnosis of OSAS should be made when a significant deviation (2 SD > mean) from normal polysomnographic values are present. Particular attention should be paid to the following measures: apnea and apnea–hypopnea indices, gas exchange abnormalities, and the number of arousals related to respiratory events. Thus, when any of these are abnormal, the diagnosis of OSAS can be made and a clinical treatment plan can be outlined on the basis of polysomnography results, history, and physical findings.
Clinical sequelae for children with OSAS may be dependent on the type and severity of polysomnographic abnormalities. Recent studies in school-aged children support this concept: While an abnormal AHI with hypopneas not defined by desaturation was associated with lower cognitive scores (104), AHI defined by hypopnea with desaturation was associated with hypertension in the same cohort (104, 117). Measures derived from EEG changes with respiration (118) or from sleep fragmentation related to respiratory events (68) may be associated with deficits in neurobehavioral functions that are independent of respiratory disturbance and hypoxemia. Thus, as research progresses, discriminate evaluation of the various indices derived from polysomnography appears to be increasingly useful for making specific interventional decisions.
The pathophysiologic role of UARS in children is not clear and may be related to some morbidities seen in children with snoring but without demonstrated sleep apnea. For diagnosis of UARS, the use of esophageal manometry is usually recommended to provide assessment of intrathoracic negative pressure effort leading to arousal (119). RIP and nasal flow measurements may provide a surrogate marker for increased effort and decreased flow by identifying changes in contour or duration of breaths, at least in a subset of patients, such as older children (93, 120). PTT is another modality that has promise because it appears to be correlated to arousal with respiratory effort (48), but its role in the diagnosis of UARS is not yet clear.
In children with the very mild forms of SDB, such as primary snoring and mild OSAS, when marginal or no abnormalities in polysomnography can be detected, there is evidence of association with behavioral abnormalities, such as hyperactivity, inattentiveness, and emotional lability (15, 121, 122). In addition, significant reductions in cognitive function have been noted in these conditions (122–124). Suratt and coworkers reported that AHI and persistent snoring both independently predict lower cognitive function scores (123), and Urschitz and colleagues found that snoring alone without oxygen desaturations was associated with poorer performance in mathematics and spelling in school-aged children. (124). However, mechanisms linking these conditions to behavior and neurocognitive deficits are still unknown, as are diagnostic tests with adequate specificity and normative thresholds.
CONCLUSIONS
SDB and OSAS include a spectrum of disorders. Although diagnosis is based on history, physical findings, and the gold standard, laboratory polysomnography, there is a need for a better understanding of the underlying causal mechanisms related to morbidity, particularly in regard to neurocognitive, behavioral, metabolic, and cardiovascular outcomes.
Similarly, studies that establish the functional outcomes associated with polysomnographic parameters are necessary to provide evidence-based guidelines for the management of children with SDB. Future studies that explore the linkage between biomarkers such as cytokines (125, 126) or urinary proteins (57) may allow a more selective approach to the management of these disorders. In addition, the development of more efficient home-based studies could reduce the cost of diagnosis and allow children greater access to appropriate therapy.
Supported by grant HD-053693 (to R.A.) from the National Institutes of Health.
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878. [DOI] [PubMed] [Google Scholar]
- 2.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:e69. [DOI] [PubMed] [Google Scholar]
- 3.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4–5 year olds. Arch Dis Child 1993;68:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 1999;159:1527–1532. [DOI] [PubMed] [Google Scholar]
- 5.Corbo GM, Fuciarelli F, Foresi A, De Benedetto F. Snoring in children: association with respiratory symptoms and passive smoking. BMJ 1989;299:1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep 2004;27:997–1019. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung 1981;159:275–287. [DOI] [PubMed] [Google Scholar]
- 8.Brouilette R, Hanson D, David R, Klemka L, Szatkowski A, Fernbach S, Hunt C. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr 1984;105:10–14. [DOI] [PubMed] [Google Scholar]
- 9.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest 1995;108:610–618. [DOI] [PubMed] [Google Scholar]
- 10.Stradling JR, Thomas G, Warley AR, Williams P, Freeland A. Effect of adenotonsillectomy on nocturnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet 1990;335:249–253. [DOI] [PubMed] [Google Scholar]
- 11.Gozal D, Wang M, Pope DW Jr. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics 2001;108:693–697. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CL. Clinical features of obstructive sleep apnea hypoventilation syndrome in otherwise healthy children. Pediatr Pulmonol 1999;27:403–409. [DOI] [PubMed] [Google Scholar]
- 13.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics 2004;114:768–775. [DOI] [PubMed] [Google Scholar]
- 14.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr 1982;100:31–40. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Ruzicka DL, Giordani BJ, Weatherly RA, Dillon JE, Hodges EK, Marcus CL, Guire KE. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006;117:e769–e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616–620. [DOI] [PubMed] [Google Scholar]
- 17.Andreou G, Galanopoulou C, Gourgoulianis K, Karapetsas A, Molyvdas P. Cognitive status in Down syndrome individuals with sleep disordered breathing deficits (SDB). Brain Cogn 2002;50:145–149. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Carroll JL, Koerner CB, Hamer A, Lutz J, Loughlin GM. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr 1994;125:556–562. [DOI] [PubMed] [Google Scholar]
- 19.Mallampati SR, Gatt SP, Gugino LD, Desai SP, Waraksa B, Freiberger D, Liu PL. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32:429–434. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989;36:1551–1569. [DOI] [PubMed] [Google Scholar]
- 21.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med 1998;157:1098–1103. [DOI] [PubMed] [Google Scholar]
- 22.Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Witt SA, Glascock BJ, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:1395–1399. [DOI] [PubMed] [Google Scholar]
- 23.Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, Bokulic R, Daniels SR. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med 2004;169:950–956. [DOI] [PubMed] [Google Scholar]
- 24.Isono S, Shimada A, Utsugi M, Konno A, Nishino T. Comparison of static mechanical properties of the passive pharynx between normal children and children with sleep-disordered breathing. Am J Respir Crit Care Med 1998;157:1204–1212. [DOI] [PubMed] [Google Scholar]
- 25.McColley SA, April MM, Carroll JL, Naclerio RM, Loughlin GM. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 1992;118:940–943. [DOI] [PubMed] [Google Scholar]
- 26.Rosen GM, Muckle RP, Mahowald MW, Goding GS, Ullevig C. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics 1994;93:784–788. [PubMed] [Google Scholar]
- 27.Monahan KJ, Larkin EK, Rosen CL, Graham G, Redline S. Utility of noninvasive pharyngometry in epidemiologic studies of childhood sleep-disordered breathing. Am J Respir Crit Care Med 2002;165:1499–1503. [DOI] [PubMed] [Google Scholar]
- 28.Gozal D, Burnside MM. Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med 2004;169:163–167. [DOI] [PubMed] [Google Scholar]
- 29.Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 1979;133:401–404. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima S, Niikuni N, Chia-hung L, Takahasi Y, Kohno M, Nakajima I, Akasaka M, Sakata H, Akashi S. Cephalometric comparisons of craniofacial and upper airway structures in young children with obstructive sleep apnea syndrome. Ear Nose Throat J 2000;79:499–502, 505–496. [PubMed] [Google Scholar]
- 31.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2001;164:698–703. [DOI] [PubMed] [Google Scholar]
- 32.Fregosi RF, Quan SF, Kaemingk KL, Morgan WJ, Goodwin JL, Cabrera R, Gmitro A. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol 2003;95:2030–2038. [DOI] [PubMed] [Google Scholar]
- 33.Arens R, Sin S, McDonough JM, Palmer JM, Dominguez T, Meyer H, Wootton DM, Pack AI. Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2005;171:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol 1994;77:918–924. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 1988;64:535–542. [DOI] [PubMed] [Google Scholar]
- 36.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991;143:1300–1303. [DOI] [PubMed] [Google Scholar]
- 37.Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, Galster P, Carson KA. Developmental changes in upper airway dynamics. J Appl Physiol 2004;97:98–108. [DOI] [PubMed] [Google Scholar]
- 38.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol 1999;87:626–633. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly LF, Strife JL, Myer CM III. Is sedation safe during dynamic sleep fluoroscopy of children with obstructive sleep apnea? AJR Am J Roentgenol 2001;177:1031–1034. [DOI] [PubMed] [Google Scholar]
- 40.Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg 1995;121:525–530. [DOI] [PubMed] [Google Scholar]
- 41.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000;1:21–32. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87–94. [DOI] [PubMed] [Google Scholar]
- 43.Lamm C, Mandeli J, Kattan M. Evaluation of home audiotapes as an abbreviated test for obstructive sleep apnea syndrome (OSAS) in children. Pediatr Pulmonol 1999;27:267–272. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein NA, Sculerati N, Walsleben JA, Bhatia N, Friedman DM, Rapoport DM. Clinical diagnosis of pediatric obstructive sleep apnea validated by polysomnography. Otolaryngol Head Neck Surg 1994;111:611–617. [DOI] [PubMed] [Google Scholar]
- 45.Sivan Y, Kornecki A, Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J 1996;9:2127–2131. [DOI] [PubMed] [Google Scholar]
- 46.Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics 2000;105:405–412. [DOI] [PubMed] [Google Scholar]
- 47.Owen GO, Canter RJ. Overnight pulse oximetry in normal children and in children undergoing adenotonsillectomy. Clin Otolaryngol 1996;21:59–65. [DOI] [PubMed] [Google Scholar]
- 48.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res 2003;53:580–588. [DOI] [PubMed] [Google Scholar]
- 49.Shouldice RB, O'Brien LM, O'Brien C, de Chazal P, Gozal D, Heneghan C. Detection of obstructive sleep apnea in pediatric subjects using surface lead electrocardiogram features. Sleep 2004;27:784–792. [DOI] [PubMed] [Google Scholar]
- 50.Jacob SV, Morielli A, Mograss MA, Ducharme FM, Schloss MD, Brouillette RT. Home testing for pediatric obstructive sleep apnea syndrome secondary to adenotonsillar hypertrophy. Pediatr Pulmonol 1995;20:241–252. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin JL, Enright PL, Kaemingk KL, Rosen GM, Morgan WJ, Fregosi RF, Quan SF. Feasibility of using unattended polysomnography in children for research–report of the Tucson Children's Assessment of Sleep Apnea study (TuCASA). Sleep 2001;24:937–944. [DOI] [PubMed] [Google Scholar]
- 52.Kalra M, Chakraborty R. Genetic susceptibility to obstructive sleep apnea in the obese child. Sleep Med 2007;8:169–175. [DOI] [PubMed] [Google Scholar]
- 53.Verhulst SL, Schrauwen N, Haentjens D, Rooman RP, Van Gaal L, De Backer WA, Desager KN. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. J Pediatr 2007;150:608–612. [DOI] [PubMed] [Google Scholar]
- 54.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest 2006;129:947–953. [DOI] [PubMed] [Google Scholar]
- 55.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med 2007;176:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bravo MD, Serpero LD, Barcelo A, Barbe F, Agusti A, Gozal D. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath 2007;11:177–185. [DOI] [PubMed]
- 57.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med 2006;7:221–227. [DOI] [PubMed] [Google Scholar]
- 58.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704–712. [DOI] [PubMed] [Google Scholar]
- 59.Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, Wise M, Picchietti DL, Sheldon SH, Iber C. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med 2007;3:201–240. [PubMed] [Google Scholar]
- 60.Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, Parthasarthy S, Somers VK, Strohl KP, Sulit LG, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med 2007;3:169–200. [PubMed] [Google Scholar]
- 61.Bonnet MH, Doghramji K, Roehrs T, Stepanski EJ, Sheldon SH, Walters AS, Wise M, Chesson AL Jr. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med 2007;3:133–145. [PubMed] [Google Scholar]
- 62.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
- 63.American Sleep Disorders Association Sleep Disorders Atlas Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 64.Scholle S, Zwacka G. Arousals and obstructive sleep apnea syndrome in children. Clin Neurophysiol 2001;112:984–991. [DOI] [PubMed] [Google Scholar]
- 65.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med 2000;162:682–686. [DOI] [PubMed] [Google Scholar]
- 66.Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep 2004;27:274–278. [DOI] [PubMed] [Google Scholar]
- 67.Guilleminault C, Eldridge FL, Simmons FB, Dement WC. Sleep apnea in eight children. Pediatrics 1976;58:23–30. [PubMed] [Google Scholar]
- 68.O'Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep 2004;27:279–282. [DOI] [PubMed] [Google Scholar]
- 69.Wong TK, Galster P, Lau TS, Lutz JM, Marcus CL. Reliability of scoring arousals in normal children and children with obstructive sleep apnea syndrome. Sleep 2004;27:1139–1145. [DOI] [PubMed] [Google Scholar]
- 70.Tauman R, O'Brien LM, Mast BT, Holbrook CR, Gozal D. Peripheral arterial tonometry events and electroencephalographic arousals in children. Sleep 2004;27:502–506. [DOI] [PubMed] [Google Scholar]
- 71.Pitson DJ, Sandell A, van den Hout R, Stradling JR. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur Respir J 1995;8:1669–1674. [DOI] [PubMed] [Google Scholar]
- 72.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction:–a new marker of obstructive sleep apnea. Sleep 1999;22:939–946. [PubMed] [Google Scholar]
- 73.O'Brien LM, Gozal D. Potential usefulness of noninvasive autonomic monitoring in recognition of arousals in normal healthy children. J Clin Sleep Med 2007;3:41–47. [PubMed] [Google Scholar]
- 74.Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep 1985;8:137–145. [DOI] [PubMed] [Google Scholar]
- 75.Thomas RJ. Cyclic alternating pattern in the electroencephalogram: what is its clinical utility? Sleep 2007;30:553–555. [DOI] [PubMed] [Google Scholar]
- 76.Lopes MC, Guilleminault C. Chronic snoring and sleep in children: a demonstration of sleep disruption. Pediatrics 2006;118:e741–e746. [DOI] [PubMed] [Google Scholar]
- 77.Kheirandish-Gozal L, Miano S, Bruni O, Ferri R, Pagani J, Villa MP, Gozal D. Reduced NREM sleep instability in children with sleep disordered breathing. Sleep 2007;30:450–457. [DOI] [PubMed] [Google Scholar]
- 78.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatr Pulmonol 2005;40:22–30. [DOI] [PubMed] [Google Scholar]
- 79.Kushida CA, Giacomini A, Lee MK, Guilleminault C, Dement WC. Technical protocol for the use of esophageal manometry in the diagnosis of sleep-related breathing disorders. Sleep Med 2002;3:163–173. [DOI] [PubMed] [Google Scholar]
- 80.Chervin RD, Ruzicka DL, Wiebelhaus JL, Hegeman GL III, Marriott DJ, Marcus CL, Giordani BJ, Weatherly RA, Dillon JE. Tolerance of esophageal pressure monitoring during polysomnography in children. Sleep 2003;26:1022–1026. [DOI] [PubMed] [Google Scholar]
- 81.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest 1993;104:781–787. [DOI] [PubMed] [Google Scholar]
- 82.Argod J, Pepin JL, Levy P. Differentiating obstructive and central sleep respiratory events through pulse transit time. Am J Respir Crit Care Med 1998;158:1778–1783. [DOI] [PubMed] [Google Scholar]
- 83.Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis 1992;146:1235–1239. [DOI] [PubMed] [Google Scholar]
- 84.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–753. [DOI] [PubMed] [Google Scholar]
- 85.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest 2004;125:872–878. [DOI] [PubMed] [Google Scholar]
- 86.Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol 2007;42:159–167. [DOI] [PubMed] [Google Scholar]
- 87.Xiong C, Sjoberg BJ, Sveider P, Ask P, Loyd D, Wranne B. Problems in timing of respiration with the nasal thermistor technique. J Am Soc Echocardiogr 1993;6:210–216. [DOI] [PubMed] [Google Scholar]
- 88.Farre R, Montserrat JM, Rotger M, Ballester E, Navajas D. Accuracy of thermistors and thermocouples as flow-measuring devices for detecting hypopnoeas. Eur Respir J 1998;11:179–182. [DOI] [PubMed] [Google Scholar]
- 89.Heitman SJ, Atkar RS, Hajduk EA, Wanner RA, Flemons WW. Validation of nasal pressure for the identification of apneas/hypopneas during sleep. Am J Respir Crit Care Med 2002;166:386–391. [DOI] [PubMed] [Google Scholar]
- 90.Thurnheer R, Xie X, Bloch KE. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am J Respir Crit Care Med 2001;164:1914–1919. [DOI] [PubMed] [Google Scholar]
- 91.Norman RG, Ahmed MM, Walsleben JA, Rapoport DM. Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistor. Sleep 1997;20:1175–1184. [PubMed] [Google Scholar]
- 92.Trang H, Leske V, Gaultier C. Use of nasal cannula for detecting sleep apneas and hypopneas in infants and children. Am J Respir Crit Care Med 2002;166:464–468. [DOI] [PubMed] [Google Scholar]
- 93.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med 1998;157:1461–1467. [DOI] [PubMed] [Google Scholar]
- 94.Ballester E, Badia JR, Hernandez L, Farre R, Navajas D, Montserrat JM. Nasal prongs in the detection of sleep-related disordered breathing in the sleep apnoea/hypopnoea syndrome. Eur Respir J 1998;11:880–883. [DOI] [PubMed] [Google Scholar]
- 95.Budhiraja R, Goodwin JL, Parthasarathy S, Quan SF. Comparison of nasal pressure transducer and thermistor for detection of respiratory events during polysomnography in children. Sleep 2005;28:1117–1121. [DOI] [PubMed] [Google Scholar]
- 96.Serebrisky D, Cordero R, Mandeli J, Kattan M, Lamm C. Assessment of inspiratory flow limitation in children with sleep-disordered breathing by a nasal cannula pressure transducer system. Pediatr Pulmonol 2002;33:380–387. [DOI] [PubMed] [Google Scholar]
- 97.Lorino AM, Lorino H, Dahan E, d'Ortho MP, Coste A, Harf A, Lofaso F. Effects of nasal prongs on nasal airflow resistance. Chest 2000;118:366–371. [DOI] [PubMed] [Google Scholar]
- 98.Tabachnik E, Muller N, Toye B, Levison H. Measurement of ventilation in children using the respiratory inductive plethysmograph. J Pediatr 1981;99:895–899. [DOI] [PubMed] [Google Scholar]
- 99.Whyte KF, Gugger M, Gould GA, Molloy J, Wraith PK, Douglas NJ. Accuracy of respiratory inductive plethysmograph in measuring tidal volume during sleep. J Appl Physiol 1991;71:1866–1871. [DOI] [PubMed] [Google Scholar]
- 100.Cantineau JP, Escourrou P, Sartene R, Gaultier C, Goldman M. Accuracy of respiratory inductive plethysmography during wakefulness and sleep in patients with obstructive sleep apnea. Chest 1992;102:1145–1151. [DOI] [PubMed] [Google Scholar]
- 101.Gaultier C, Praud JP, Canet E, Delaperche MF, D'Allest AM. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. J Dev Physiol 1987;9:391–397. [PubMed] [Google Scholar]
- 102.Poets CF, Southall DP. Noninvasive monitoring of oxygenation in infants and children: practical considerations and areas of concern. Pediatrics 1994;93:737–746. [PubMed] [Google Scholar]
- 103.McMorrow RC, Mythen MG. Pulse oximetry. Curr Opin Crit Care 2006;12:269–271. [DOI] [PubMed] [Google Scholar]
- 104.Kaemingk KL, Pasvogel AE, Goodwin JL, Mulvaney SA, Martinez F, Enright PL, Rosen GM, Morgan WJ, Fregosi RF, Quan SF. Learning in children and sleep disordered breathing: findings of the Tucson Children's Assessment of Sleep Apnea (TuCASA) prospective cohort study. J Int Neuropsychol Soc 2003;9:1016–1026. [DOI] [PubMed] [Google Scholar]
- 105.Rosen CL, D'Andrea L, Haddad GG. Adult criteria for obstructive sleep apnea do not identify children with serious obstruction. Am Rev Respir Dis 1992;146:1231–1234. [DOI] [PubMed] [Google Scholar]
- 106.Urschitz MS, Wolff J, Sokollik C, Eggebrecht E, Urschitz-Duprat PM, Schlaud M, Poets CF. Nocturnal arterial oxygen saturation and academic performance in a community sample of children. Pediatrics 2005;115:e204–e209. [DOI] [PubMed] [Google Scholar]
- 107.Morielli A, Desjardins D, Brouillette RT. Transcutaneous and end-tidal carbon dioxide pressures should be measured during pediatric polysomnography. Am Rev Respir Dis 1993;148:1599–1604. [DOI] [PubMed] [Google Scholar]
- 108.Weese-Mayer DE, Corwin MJ, Peucker MR, Di Fiore JM, Hufford DR, Tinsley LR, Neuman MR, Martin RJ, Brooks LJ, Davidson Ward SL, et al. Comparison of apnea identified by respiratory inductance plethysmography with that detected by end-tidal CO2 or thermistor. The CHIME study group. Am J Respir Crit Care Med 2000;162:471–480. [DOI] [PubMed] [Google Scholar]
- 109.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with down syndrome. Pediatrics 1991;88:132–139. [PubMed] [Google Scholar]
- 110.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol 1993;16:124–129. [DOI] [PubMed] [Google Scholar]
- 111.Kirk VG, Batuyong ED, Bohn SG. Transcutaneous carbon dioxide monitoring and capnography during pediatric polysomnography. Sleep 2006;29:1601–1608. [DOI] [PubMed] [Google Scholar]
- 112.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med 2003;168:1540. [DOI] [PubMed] [Google Scholar]
- 113.American Thoracic Society. Cardiorespiratory sleep studies in children: establishment of normative data and polysomnographic predictors of morbidity. Am J Respir Crit Care Med 1999;160:1381–1387. [DOI] [PubMed] [Google Scholar]
- 114.Katz ES, Greene MG, Carson KA, Galster P, Loughlin GM, Carroll J, Marcus CL. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr 2002;140:589–594. [DOI] [PubMed] [Google Scholar]
- 115.Li AM, Wing YK, Cheung A, Chan D, Ho C, Hui S, Fok TF. Is a 2-night polysomnographic study necessary in childhood sleep-related disordered breathing? Chest 2004;126:1467–1472. [DOI] [PubMed] [Google Scholar]
- 116.Dayyat E, Maarafeya MM, Capdevila OS, Kheirandish-Gozal L, Montgomery-Downs HE, Gozal D. Nocturnal body position in sleeping children with and without obstructive sleep apnea. Pediatr Pulmonol 2007;42:374–379. [DOI] [PubMed] [Google Scholar]
- 117.Enright PL, Goodwin JL, Sherrill DL, Quan JR, Quan SF. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea study. Arch Pediatr Adolesc Med 2003;157:901–904. [DOI] [PubMed] [Google Scholar]
- 118.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep 2004;27:116–121. [DOI] [PubMed] [Google Scholar]
- 119.Guilleminault C, Pelayo R, Leger D, Clerk A, Bocian RC. Recognition of sleep-disordered breathing in children. Pediatrics 1996;98:871–882. [PubMed] [Google Scholar]
- 120.Masa JF, Corral J, Martin MJ, Riesco JA, Sojo A, Hernandez M, Douglas NJ. Assessment of thoracoabdominal bands to detect respiratory effort-related arousal. Eur Respir J 2003;22:661–667. [DOI] [PubMed] [Google Scholar]
- 121.Rosen CL, Storfer-Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics 2004;114:1640–1648. [DOI] [PubMed] [Google Scholar]
- 122.O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, Raffield TJ, Gozal D. Neurobehavioral implications of habitual snoring in children. Pediatrics 2004;114:44–49. [DOI] [PubMed] [Google Scholar]
- 123.Suratt PM, Peruggia M, D'Andrea L, Diamond R, Barth JT, Nikova M, Perriello VA Jr, Johnson ML. Cognitive function and behavior of children with adenotonsillar hypertrophy suspected of having obstructive sleep-disordered breathing. Pediatrics 2006;118:e771–e781. [DOI] [PubMed] [Google Scholar]
- 124.Urschitz MS, Guenther A, Eggebrecht E, Wolff J, Urschitz-Duprat PM, Schlaud M, Poets CF. Snoring, intermittent hypoxia and academic performance in primary school children. Am J Respir Crit Care Med 2003;168:464–468. [DOI] [PubMed] [Google Scholar]
- 125.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. [Epub ahead of print 5 Sep 2007]. Sleep Med 2007. [DOI] [PMC free article] [PubMed]
- 126.Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, Redline S. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006;166:1725–1731. [DOI] [PubMed] [Google Scholar]