Abstract
The current enthusiasm for stem cell research stems from the hope that damaged or diseased tissues may one day be repaired through the manipulation of endogenous or exogenous stem cells. The postnatal human respiratory system is highly accessible and provides unique opportunities for the application of such techniques. Several putative adult lung epithelial stem cells have been identified in the mouse model system. However, their in vivo capabilities to contribute to different lineages, and their control mechanisms, remain unclear. If stem cell–based therapies are to be successful in the lung, it is vitally important that we understand the normal behavior of adult lung stem cells, and how this is regulated. Lung embryonic progenitor cells are much better defined and characterized than their adult counterparts. Moreover, experiments on a variety of developing tissues are beginning to uncover general mechanisms by which embryonic progenitors influence final organ size and structure. This provides a framework for the study of lung embryonic progenitor cells, facilitating experimental design and interpretation. A similar approach to investigating adult lung stem cells could produce rapid advances in the field.
Keywords: lung embryonic progenitor, lung stem cell, lineage tracing
The adult organism is maintained by the actions of tissue-specific stem and progenitor cells that divide throughout life to replace postmitotic or damaged cells (Table 1). Adult stem cells have been well characterized in some organs, such as the gut, hematopoietic system, skin, and hair follicle. It is clear that they are maintained under very tight regulatory control; either excessive, or insufficient, stem cell proliferation can lead to abnormal phenotypes. However, adult stem cells are still ill defined in the lung, and the mechanisms that control their proliferation and differentiation are almost completely unknown. Nevertheless, the possibility that lung disorders may one day be treated by manipulating endogenous lung stem cells, or with exogenously applied stem cells, is the focus of much research effort. If this approach is to be successful it is necessary to understand the normal behavior of endogenous lung stem cells, and how they are controlled by their environment.
TABLE 1.
STEM AND PROGENITOR CELL DEFINITIONS
| Name | Additional Names | Description |
|---|---|---|
| Adult stem cell | Tissue-specific stem cell | Self-renews throughout the lifetime of the mature animal giving rise to one or more different differentiated cell types within a particular organ. Individual organs may have more than one stem cell population. Typically considered to be “less differentiated” than other mature cells and to divide infrequently. However, these features are not universal characteristics of all adult stem cells; presumably each tissue has evolved a system to suit its own unique requirements. |
| Transiently amplifying | Transit amplifying, TA, or progenitor | The immediate daughter of an adult stem cell. Divides and gives rise to the same differentiated cell types as the adult stem cell. However, it is distinguished from the adult stem cell by its limited ability to self-renew. |
| Self-renewing differentiated | Differentiated cell | Population of differentiated cells, any of which can self-renew to maintain the overall population. This cell type does not contribute to other differentiated cell lineages. It does not need to be replenished by a stem cell. |
| Terminally differentiated cell | Postmitotic cell | Cannot enter the cell cycle and is replenished by a stem cell population. |
| Embryonic progenitor | Self-renews during development and can contribute to one or more differentiated cell lineages within one organ. Gives rise to adult stem cells. However, adult stem cells are distinct from embryonic progenitors: they usually give rise to a more restricted number of different cell types and are regulated differently. | |
| Multipotent cell | Stem or progenitor cell that is capable of dividing to give rise to all of the different epithelial or mesenchymal cells in one organ. | |
| Self-renewing | Division of a stem or progenitor cell that results in a new stem or progenitor cell being born. Symmetric self-renewing divisions result in two progenitors. Asymmetric self-renewing divisions result in one new progenitor and one cell that will differentiate. |
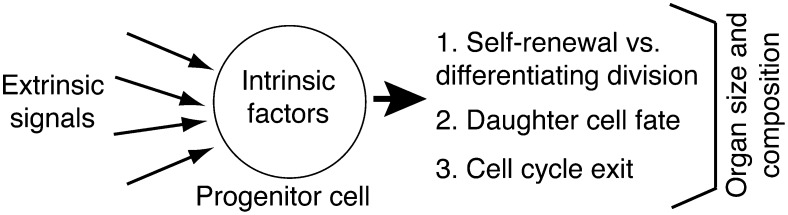
In general, progenitor cells in the embryo are much better characterized than adult stem cells. During development, individual organs are constructed from transient populations of organ-specific progenitor cells that reside in either epithelial or mesenchymal compartments. These cells self-renew only during development, although they do give rise to adult tissue–specific stem cells (see Table 1 for definitions). Organ morphogenesis is controlled by cell–cell signaling between the epithelium, mesenchyme, and vasculature, both between progenitors and differentiating cells. The behavior of embryonic progenitors can influence organ size, shape, and cellular composition (Figure 1). Final organ size depends both on the number of cell divisions that the progenitors undergo and on the type of these divisions. Progenitors can divide symmetrically to give rise to two identical daughters, either to two new progenitors or to two cells that will differentiate, or asymmetrically to give rise to one progenitor and one cell that will differentiate. The balance between symmetric and asymmetric division of progenitors can obviously have a tremendous effect on organ size and is consequently tightly regulated during development. For example, in the developing nervous system, the ratio of these different types of divisions changes in time and space in a highly reproducible fashion (1). Gene expression in the progenitors influences the fate (also known as identity) of their differentiating daughters. This has also been well characterized in the developing nervous system in which progenitors express a sequence of transcription factors that control both the fate and the number of rounds of division of their daughter cells (2). Similarly, in the developing pancreas, expression of the transcription factor ngn3 (neurogenin 3) has been demonstrated to control progenitor cell “competence” (3). By competence, we mean which cell lineages the progenitors can give rise to. Therefore, both organ size and cell composition are controlled during development in part by regulating progenitor cell behavior (Figure 1). Current evidence suggests that adult stem cells have a similar ability to influence organ size and morphology during homeostasis and repair.
Figure 1.
Aspects of tissue morphogenesis potentially controlled by regulation of progenitor cell behavior. Progenitor cells can control various aspects of tissue morphogenesis and hence influence organ size and structure. These include (1) the number of progenitor cell divisions and whether these are symmetric or asymmetric, (2) the final differentiated fate that daughter cells acquire, (3) whether daughter cells exit the cell cycle or continue to proliferate. Progenitor cells themselves are regulated both by extrinsic signaling and intrinsic factors determined by their developmental history and in turn signal back to their neighbors. These characteristics are common to both embryonic progenitors and adult stem cells.
Embryonic progenitors have been well defined for a few tissues, including the retina, central nervous system, and pancreas. Over the past few years, progress has also been made toward defining embryonic lung epithelial progenitors, and how they interact with their surrounding environment. This knowledge has direct clinical relevance to the long-term respiratory disorders that are a consequence of premature birth. The study of embryonic progenitors also serves to illustrate important questions that need to be answered for adult lung stem and progenitor cells. For example, does stem cell gene expression determine which differentiated cell types can be produced? Or is this determined by signaling between the differentiating progeny of the stem cell and their environment? Does the balance between symmetric and asymmetric cell divisions change in response to injury compared with homeostasis? Which signals initiate, or inhibit, stem cell division? Powerful genetic techniques are being applied in model organisms to investigate these issues in embryonic lung epithelial progenitors. This article describes the current state of this research and outlines the next steps that need to be taken to advance the study of both embryonic and adult lung progenitor cells.
IDENTIFYING THE EMBRYONIC EPITHELIAL PROGENITOR CELL POPULATIONS
The most definitive lung progenitor cell studies have been performed in the mouse. Mouse lung embryonic development has been extensively reviewed (e.g., see Reference 4). Briefly, the lung arises from the foregut endoderm at Embryonic Day 9.5 (E9.5). At this stage, the primary buds consist of an inner, apparently unpatterned, epithelium surrounded by loosely packed mesenchyme and a thin mesothelial layer. The lung buds undergo repeated rounds of branching and outgrowth. During this stage (pseudoglandular, E10.5–E16.5), the conducting airways are formed and lined with a mixture of secretory (Clara), ciliated, and neuroendocrine (NE) cells. The unpatterned distal tips of the lungs then elongate (canalicular stage, E16.5–E17.5) and ultimately give rise to the terminal sacs containing type 1 and type 2 epithelial cells. Postnatally, the sacs enlarge and outgrowth of alveoli septae occurs. The cells of the conducting airways proliferate and the tubes continue to increase in length and diameter. The abilities of different cells to act as embryonic epithelial progenitors during each of these different morphogenetic phases remain to be formally tested.
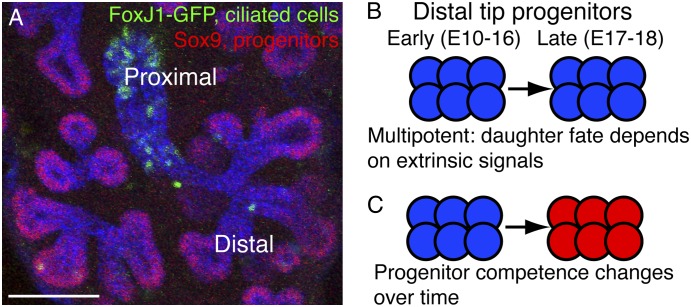
During branching morphogenesis of the lung, the distal-most epithelial cells are hypothesized to be a multipotent progenitor cell population (Figure 2). One current model suggests that, as the lung branches, descendents of the distal tip progenitors are left behind in the stalks, where they begin to differentiate, whereas the self-renewing progenitors remain within the epithelial budding tips. There are several lines of evidence that strongly support this hypothesis. First, distal epithelial cells have different cell cycle kinetics compared with the rest of the epithelium; a higher proportion of them incorporate the thymidine analog bromodeoxyuridine (BrdU) in a short pulse (5). Second, these cells have a unique pattern of gene expression (Table 2), including high levels of the transcription factors etv5 (ets variant gene 5, also known as ERM), nmyc (v-myc myeloctomatosis viral related oncogene, neuroblastoma-derived), id2 (inhibitor of differentiation 2), and sox 9 (sry box containing gene 9), and high levels of activity of the Wnt, Bmp, Fgf, and Shh signaling pathways (6–8). Many of these genes and pathways including etv5, sox9, and fibroblast growth factor (Fgf) signaling are associated with progenitor cells in other endodermally-derived organs such as the pancreas (e.g., see References 9 and 10). Third, the phenotypes that result from loss or overexpression of the distal epithelial cell–specific transcription factor nmyc can be interpreted as specific effects on progenitor cells (5). Conditional deletion of nmyc results in a smaller lung with decreased proliferation, increased apoptosis, and a decrease in the number of sox9+ distal epithelial cells, suggesting that the progenitor cell population has been depleted. In contrast, overexpression of nmyc results in increased overall proliferation and an increase in the number of sox9+ distal epithelial cells, suggesting that the progenitor pool is expanded. Both of these manipulations result in lungs that are normally patterned along the proximal–distal axis. This implies that the role of nmyc is restricted to promoting proliferation of the progenitor cell population. Taken together, these data provide convincing evidence that the distal epithelial population contains the epithelial progenitor cells during branching morphogenesis when the conducting airways are being laid down.
Figure 2.
A multipotent epithelial progenitor cell population is localized within the distal epithelial buds throughout lung development. (A) Confocal image of Embryonic Day 14.5 (E14.5) FoxJ1-GFP lung. E-cadherin (blue) labels the epithelium. Progenitor cells (Sox9+, red) are located at the budding tips of the epithelium. As the lung branches and cells exit the distal tips, they turn off Sox9 and other distal epithelium-specific genes, become committed to a specific lineage, and begin to differentiate. Differentiating cells (in this example, green fluorescent protein [GFP+] ciliated cells; green) are located proximally to the tips. Bar = 50 μm. (B, C) The conducting airways are laid down between E10 and E16 and the alveoli between E17 and E18. A multipotent progenitor population is located at the distal epithelial tips throughout lung development and therefore must transition from generating conducting airway cells to generating alveolar cells. There are several possible mechanisms by which this may occur. (B) Progenitors are multipotent throughout lung development and the fate of their progeny is completely dependent on extrinsic signaling. (C) There is only one population of progenitor cells, but its competence to give rise to different cell types changes over the course of development.
TABLE 2.
GENES HIGHLY EXPRESSED IN THE DISTAL LUNG EPITHELIAL PROGENITOR CELLS
| Gene | Description | Roles in Progenitor Cells | References |
|---|---|---|---|
| Foxp1, Foxp2 | Transcription factors | Partially redundant; possibly required for progenitor cell self-renewal through regulation of Nmyc. | (21) |
| Id2 | Transcription factor | No reported lung phenotype. | (7) |
| Nmyc | Transcription factor | Promotes distal epithelial cell self-renewal and inhibits differentiation. | (5) |
| Sox9 | Transcription factor | Lung epithelial specific knockouts have no phenotype. | (7, 22) |
| BMP4 | Signaling molecule | Required to promote distal epithelial cell proliferation and maintain cell morphology. | (23) |
| Elf5 | Transcription factor | Downstream of Fgf signaling. Mutants have no reported lung phenotype. | (46) |
| Etv5 | Transcription factor | Downstream of Fgf signaling. Promotes epithelial cell differentiation and inhibits proliferation. | (7, 47) |
| Spry2 | Inhibitor of Fgf signaling | Overexpression inhibits branching morphogenesis and epithelial proliferation. | (48) |
| Shh | Signaling molecule | Required for epithelial and mesenchymal proliferation, branching morphogenesis, and mesenchymal differentiation. | (25) |
| Wnt7b | Signaling molecule | Promotes mesenchymal proliferation. | (49) |
| Wnt5a | Signaling molecule | Mutants have one extra branch of conducting airways; effect on progenitors unclear. | (26) |
| Dkk1 | Inhibitor of Wnt signaling | Inhibits branching in in vitro culture. | (50) |
| Notch1 | Signaling molecule | Not investigated in vivo. | (33) |
| Thbs1 | Extracellular calcium binding protein | No embryonic lung phenotype. | (7) |
The epithelial progenitors of the alveolar compartment of the lung have yet to be identified. An attractive model is that the alveolar progenitor population is located within the distal epithelial tips during the canalicular stage of lung development. However, there is currently no published evidence to support, or refute, this hypothesis.
The lungs continue to grow in size during the early postnatal period. In the conducting airways at this stage, based on the kinetics of cell labeling after a short pulse of tritiated [3H]thymidine, Clara cells both self-renew and act as progenitors for ciliated cells (11, 12). This is supported by more recent lineage-labeling data (13). However, it is not clear whether all Clara cells have this capacity. In the adult alveoli, type II cells have been observed to proliferate and give rise to type I cells after injury (14). It is generally assumed, but not yet tested, that this process also occurs during postnatal growth.
The exact cellular identity of the embryonic lung epithelial progenitors will aid the investigation of their control mechanisms and the roles they play in determining final organ size and structure. In the developing pancreas, lineage tracing has been used to demonstrate that individual distal epithelial tip cells are multipotent progenitors (10). Lineage tracing, or lineage labeling, is the permanent labeling of a cell in such a way that all of its descendents inherit the label. The most commonly used genetic method of lineage tracing takes advantage of the enzyme known as Cre DNA recombinase. This enzyme catalyzes site-specific DNA recombination between LoxP sequences, removing the intervening DNA. Several mouse strains have been engineered to express a reporter gene from a ubiquitously expressed promoter only in response to Cre activity. For lineage tracing, the Cre enzyme is placed under the control of a cell type–specific promoter and the reporter is activated in the Cre-expressing cells and all of their descendents. A temporal component of control can be added to the system by making Cre activity dependent on exposure of the mice to either tamoxifen (using a Cre-ER [estrogen receptor] fusion protein) or doxycycline (using a tet-operator to control Cre expression) (reviewed in Reference 15). If given at particularly high doses, tamoxifen, doxycycline, and Cre have all been reported to potentially cause developmental abnormalities in various tissues. Therefore, it is important to always perform the appropriate controls to ensure that normal development is being studied. Nevertheless, these lineage-tracing systems have already been successfully used in the adult mouse trachea and lung to demonstrate that keratin-14–positive cells can act as progenitors and that ciliated cells cannot (16, 17). In the developing lung, the human SPC (also known as SFTPC) and rat Ccsp (also known as CC10, CCA, and Scgb1a1) promoters have been used for lineage tracing (13, 18). These transgenic mice have proven to be highly useful tools for genetically manipulating different compartments of the developing lung. However, their promoter sequences do not specifically drive expression in only one cell type, limiting their ability to definitively identify specific subsets of progenitor cells.
To lineage trace a defined population of epithelial cells from early stages of lung development, I have targeted Cre-ER to the Id2 locus. The resulting mice specifically express the tamoxifen-inducible Cre in the putative multipotent distal epithelial progenitor population, allowing me to test the ability of these cells to act as progenitors during development. My unpublished data show that there is indeed a multipotent, Id2+ progenitor population at the distal epithelial tips and that this gives rise to all conducting airway (NE, ciliated, Clara) cells and all alveolar epithelial (type I, type II) cells (E.L. Rawlins, unpublished data). This work raises the question of how the distal progenitors give rise to conducting airway cells during the pseudoglandular stage and then switch to generating alveolar cells during the canalicular stage? There are several possible mechanisms that could account for this. These include the following:
A homogenous population of multipotent progenitor cells is located at the distal tips throughout lung development. Its descendents are always capable of differentiating into any of the different mature lung epithelial cell types and this choice is determined by extrinsic signaling rather than progenitor gene expression (Figure 2B).
There is one population of self-renewing progenitors located at the distal tips. It is initially competent to give rise to conducting airway cells only, but at the canalicular stage it switches in competence to give rise to alveolar cells (Figure 2C). This model would be similar to that predicted for the embryonic pancreatic progenitors (3).
The relative roles of progenitor cell competence and extrinsic signaling in controlling the cell types produced by the multipotent progenitor remain to be tested; the in vivo situation may require a balance between the two. By demonstrating that there is indeed a single multipotent epithelial progenitor population located in the distal epithelial buds throughout embryogenesis, this work provides a framework for investigating the control of lung epithelial progenitor behavior.
WHAT FACTORS CONTROL PROGENITOR CELL BEHAVIOR DURING DEVELOPMENT?
Premature infants are frequently born with their lungs in the canalicular or terminal sac (rather than alveolar) stage of development. In these children, all alveologenesis must occur postnatally, often in the abnormal context of injury and inflammation resulting from infection or the treatment necessary for their survival. Work on the mouse pancreas suggests that final organ size is limited by the number of progenitor cells available at early developmental stages and that “catch up” growth is not possible (19). Is lung development similarly limited if the progenitors are thrust into an abnormal environment and cannot function properly? Could we one day treat premature infants who are at risk of developing bronchopulmonary dysplasia, or other lung disorders, by manipulating progenitor cell behavior? For the promise of eventual therapeutic use of progenitor cells to be realized, we need to understand not only which cells are the progenitors but also how they are regulated during normal development.
Previously, uncertainty over progenitor cell identity has made investigating their regulation difficult. The genetic tools that are becoming available for labeling epithelial progenitors in vivo, combined with improved live cell–imaging techniques, will help to solve this problem. Given the importance of epithelial–mesenchymal interactions in the overall patterning of the lung, it is likely that branching morphogenesis and progenitor cell proliferation and competence are coordinately regulated by the same signals. Moreover, the behavior of lung progenitors is hypothesized to be controlled both by extrinsic signals and intrinsic factors, such as the effects of their developmental history on chromatin structure. The intrinsic aspects of progenitor cell control provide the context in which extrinsic signals are interpreted. The published genetic data can be split into two categories: manipulations that affect progenitor cell proliferation and self-renewal and those that affect the patterning of their daughters.
REGULATION OF LUNG PROGENITOR CELL PROLIFERATION
Embryonic progenitor cells must undergo a mixture of symmetric and asymmetric divisions during development. One current challenge is to distinguish between these types of divisions at the cellular level. In time, it may be possible to do this by looking at differences in spindle orientation or differential inheritance of cytoplasmic or membrane-bound proteins. At the moment, we can only infer that certain molecules normally act to promote self-renewal or differentiation of progenitors indirectly by comparing the number of progenitor cells identified in mutant, and sibling control, lungs.
A network of transcription factors and signaling molecules are known to affect lung growth and therefore presumably progenitor cell proliferation. The transcription factor Nmyc is both necessary and sufficient for the division of lung epithelial progenitor cells and may promote self-renewing divisions (5). Several forkhead/winged helix (fox) family transcription factors have mutant phenotypes, which suggests that one of their functions is to promote lung epithelial progenitor cell proliferation. For example, mice with both of the related genes foxa1 and foxa2 conditionally deleted have small lungs with decreased rates of cell division (20). Foxa1 and foxa2 are not localized exclusively to the distal epithelial cells, and the mutant lungs also have a general epithelial differentiation defect that may be independent of the progenitor cell phenotype. Similarly, in foxp2−/−; foxp1+/− mutants, the lungs are smaller than normal, with decreased levels of proliferation and Nmyc expression, but normal proximal–distal patterning (21). In this case, foxp1 and foxp2 are enriched in the distal epithelial progenitors, suggesting that their primary role is to promote the maintenance of the progenitor cell compartment by promoting self-renewing divisions. Sox9 is highly expressed in distal epithelial cells, but lung-specific conditional deletion has no effect on progenitor cell behavior (22). Sox9 may act redundantly with other, as yet unknown, regulators of progenitor cell proliferation. However, not all distal epithelium-specific genes are predicted to have a role in regulating progenitor cell function. Others may regulate the cell shape changes that occur during branching morphogenesis, or pattern the mesenchyme.
Lung epithelium–specific deletion of Bmp receptor 1a (Bmpr1a) or the distal epithelium-specific Bmp ligand Bmp4 results in smaller lungs, with reduced rates of proliferation and decreased nmyc and foxa2 expression (23). This suggests that autocrine Bmp signaling is required for proliferation of the distal epithelial progenitor cell population. In contrast, Fgf10 is expressed in the distal mesenchyme, but is required in a paracrine fashion for the proliferation of the distal epithelium (24). Shh in the distal epithelium jointly regulates lung proliferation and branching morphogenesis, but not proximal–distal patterning or differentiation, and also likely promotes progenitor cell proliferation (25). Wnt5a, believed to be a noncanonical Wnt, is enriched in and around the distal epithelial tips. The null mutant lungs appear to have an additional branch of the conducting airways and a general increase in levels of cell proliferation (26). The role of progenitor cells in the development of this phenotype is unknown. These signaling pathways likely act cooperatively to control distal epithelial progenitor cell proliferation. However, the specific details of pathway integration—for example, common regulation of transcriptional targets, a signaling hierarchy, cross-talk at the cell surface or between second messengers—are still to be determined.
Proliferation of the distal epithelial progenitor cell population has also been shown to be regulated by micro-RNAs (27). These are a class of small, noncoding RNAs that regulate gene expression at a post-transcriptional level. Overexpression of the miR-17–92 cluster throughout the developing lung epithelium results in the accumulation of large numbers of highly proliferative epithelial cells expressing sox9 (multipotent progenitor cells) and a delay in differentiation (27). This suggests that miR-17–92 promotes self-renewal of progenitors at the expense of differentiative divisions. This phenotype may be partly mediated by a decrease in the levels of the tumor suppressor rbl2, which is one of potentially many targets of mir-17–92. However, neither mir-17–92 nor rbl2 is specifically expressed in the distal epithelial progenitor cells.
LUNG EMBRYONIC PROGENITORS AND PROXIMAL–DISTAL PATTERNING: ROLE OF Wnt AND Bmp SIGNALING
In some systems, such as the developing pancreas, gene expression in the progenitor cells determines their competence to give rise to different differentiated cell types (3). It is not known if the lung multipotent progenitors also transition through different competence states, or if the specific cellular identity of the differentiated epithelial cells is determined by signals received from the mesenchyme (Figure 2).
Current evidence suggests that Wnt and Bmp signaling are important regulators of proximal–distal patterning in the lung, but it is not clear if their effects are mediated through progenitor cells. Reporters of Wnt pathway activity are highly active in the distal epithelial tip cells at early stages of lung development. Reducing levels of Wnt pathway activity by overexpression of Dickkopf-1, a Wnt inhibitor, throughout the developing lung epithelium expands the proximal (conducting) airways at the expense of the distal. This has no effect on total levels of proliferation, suggesting that Wnt signaling regulates proximal–distal patterning and progenitor cell proliferation independently (6). Similarly, lung-specific deletion of β-catenin, required both for Wnt signaling and cell adhesion, prevented differentiation of distal airway epithelium (28). These data suggest that Wnt signaling functions to promote distal airway fate at the expense of the proximal airways. Reducing levels of Bmp signaling by overexpression of the Bmp antagonists Noggin or Gremlin, or a dominant-negative Bmp receptor, also results in a proximalization of the lung epithelium, suggesting that the normal function of the Bmp pathway is to promote distal and repress proximal cell fate (29, 30). This is in contrast to the effects on progenitor cell proliferation reported from lung-specific Bmpr1a or Bmp4 deletion. These lung proximalization phenotypes result from a general reduction of Wnt or Bmp signaling, whereas disruption of individual ligands or receptors has yet to result in a gross proximal–distal patterning phenotype (e.g., see References 23 and 26). Further genetic studies will no doubt resolve these discrepancies. Moreover, manipulating pathway activity using more cell type–specific promoters may provide insight into whether Wnt and Bmp signaling influences proximal–distal lung patterning via their actions in progenitor, or differentiating, cells.
In the developing retina and neural cortex, Notch signaling has been found to promote progenitor cell identity at the expense of differentiated cell phenotypes (31, 32). This possibility has yet to be directly tested in vivo in the lung. Notch1 is expressed at high levels in the distal epithelial progenitors during the pseudoglandular stage of lung development (33). Expression of a constitutively active form of Notch3 throughout the developing lung epithelium prevents cell differentiation (34). However, this study did not distinguish whether in the presence of excessive Notch signaling the cells remained as multipotent epithelial progenitors, or whether differentiation of committed precursors was inhibited.
COMMITMENT OF DAUGHTER CELLS TO SPECIFIC DIFFERENTIATED LINEAGES: NEUROENDOCRINE CELL DEVELOPMENT
The exit of cells from the progenitor pool and their gradual acquisition of a differentiated fate is an important aspect of progenitor cell regulation. In common with other organs, differentiation in the embryonic lung is likely to be a multistep process (Figure 3). First, a cell becomes specified. It expresses transcription factors that promote cell lineage identity, although at this stage it can be redirected to an alternative fate in response to signaling. Second, the cell expresses genes that promote commitment of its genome (including epigenetic changes) and overall structure to the differentiated lineage. It is now a precursor of a specific differentiated cell type and cannot be redirected to another fate, except by experimental manipulation. Third, it becomes fully differentiated and expresses the proteins required for its function—for example, secreted factors or structural molecules. Cell differentiation does not necessarily require cell cycle exit and there are various examples of differentiated cells that self-renew, such as β cells in the pancreas and hepatocytes in the liver (35, 36).
Figure 3.
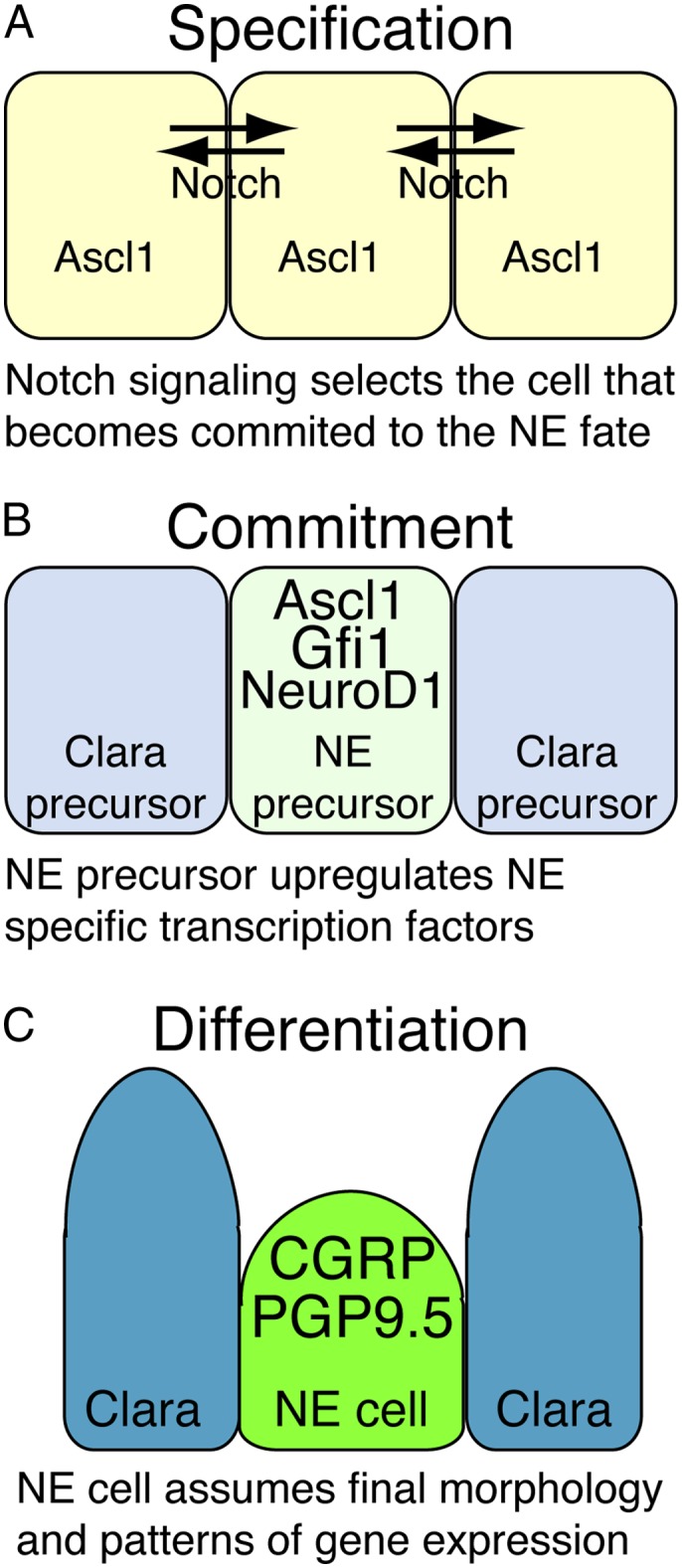
Neuroendocrine (NE) cell fate specification. During development, cells are believed to acquire a specific differentiated identity via a multistep process. (A) Expression of transcription factors and local signaling result in a cell becoming specified to differentiate along a particular lineage. For example, cells that express high levels of Ascl1 and do not respond to Notch signaling become the NE cell precursors. (B) The precursor expresses genes, often transcription factors, that commit its architecture to its final differentiated fate. High levels of ascl1, neuroD1, and gfi1 appear to be important at this stage of NE cell differentiation. (C) The final differentiated cell maintains a specific morphology and pattern of gene expression appropriate for its role in the tissue. At this stage the NE cells express high levels of calcitonin gene-related peptide (CGRP) and uchl1 (ubiquitin carboxy-terminal hydrolase 1, also known as PGP9.5).
The NE lineage is the only lung epithelial cell type for which we are beginning to develop a detailed understanding of the cellular events leading to specification, commitment, and subsequent differentiation (Figure 3). The basic helix–loop–helix (bHLH) transcription factor achaete-scute complex homolog 1 (ascl1) (also known as mash1) begins to be expressed in small groups of epithelial cells at E13.5. Notch pathway signaling, mediated by the bHLH transcription factor hairy and enhanced of split 1 (hes 1), between the ascl1+ cells results in many cells turning off ascl1 expression. Hes1 null mutants have increased numbers of NE cells at the expense of the Clara cell population, showing that the cells that down-regulate ascl1 subsequently differentiate as Clara cells. The remaining Ascl1+ cells appear to be committed to differentiating as NE cells (37). They up-regulate the transcription factors growth factor independent 1 (gfi1) and neurogenic differentiation 1 (neurod1) and subsequently the NE cell differentiation markers (calcitonin gene related peptide; also known as Calca) and uchl1 (ubiquitin carboxy-terminal hydrolase 1, also known as PGP9.5) (38). The retinoblastoma family proteins function to restrict the number of NE cells, although their exact role is currently ill defined (27, 39).
Much less is known about the commitment and differentiation of other lung epithelial cell types. There are several mutant conditions in which the mutant cells are unable to fully differentiate: for example, foxa1−/−; foxa2−/− and nkx2.1+/−; gata6+/− mutant lung epithelia, which both seem to have a general block in epithelial cell differentiation rather than defects in particular lineages (20, 40). However, given the lack of available markers for the continuum of epithelial cell specification, commitment, and differentiation, we cannot determine at which stage the epithelial cells in these lungs are arrested.
ADULT LUNG EPITHELIAL STEM CELLS AND EMBRYONIC LUNG PROGENITORS: DISTINCT LINEAGE-RELATED POPULATIONS
A key unanswered question is whether the adult lung epithelial stem cells are a distinct cell population, or whether some of the multipotent embryonic progenitors persist to adulthood? Accumulating evidence from studies in several organs suggests that, apart from their ability to proliferate, the embryonic cells that build a tissue are different from the adult cells that maintain and repair it (reviewed in Reference 41). This still needs to be confirmed for the lung epithelium. Nevertheless, two lines of evidence suggest that lung embryonic progenitors and adult stem cells are separate, although lineage-related, populations. First, the majority of the genes that are expressed in the multipotent distal epithelial embryonic progenitors are not believed to be expressed in the adult lung, and by extension the adult lung stem cells. This is not unexpected because the two cell types have different behaviors. The distal epithelial embryonic cells undergo shape changes to mediate branching morphogenesis and send signals to pattern the mesenchyme, as well as proliferating rapidly and acting as epithelial progenitors. In contrast, during homeostasis, the adult stem cells proliferate rarely and are not predicted to undergo shape changes or to actively pattern the mesenchyme. Whether adult lung stem cells up-regulate distal embryonic progenitor specific genes in response to injury still remains to be investigated. Second, there is no evidence that a multipotent stem cell population exists in the adult lung. By contrast, each region of the adult organ—upper and lower trachea, bronchi, bronchioles, terminal bronchioles, alveoli—appears to be maintained by its own stem or progenitor cell population (reviewed in References 42 and 43).
A more systematic approach to both identifying and studying adult lung stem cells is required. Specifically, this needs to be rooted in the various mechanisms by which progenitors can influence organ size and structure (Figure 1). Moreover, many experiments are still performed in vitro and their relevance to the in vivo situation has not yet been established. For example, one cell type that has recently received considerable attention is the dual-positive (CCSP+, SpC+) cells located in the terminal bronchioles. It has been suggested that these can contribute to both bronchiolar and alveolar lineages and may be the “true stem cell” for both compartments (44). This is based on three lines of evidence. First, that these cells are the first to divide after naphthalene or bleomycin-mediated injury in the mouse, although it should be remembered that both type 2 and Clara cells have also been shown to divide in response to these injures. Second, if these cells are isolated by flow cytometry (as Sca1+, CD34+ cells) and cultured in vitro, they can both self-renew and give rise to bronchiolar and alveolar cell types. Third, these cells are a target of activated Ras-mediated neoplastic transformation in the mouse. More recently, deletion of p38α MAP kinase in the adult lung was shown to cause increased levels of proliferation in the alveoli at steady state, to cause an increase in the number of dual-positive (CCSP+, SpC+) cells, and to make the lung more susceptible to activated Ras-mediated oncogenic transformation (45). Together, these data support the idea that the dual-positive (CCSP+, SpC+) cells may be a population that is particularly susceptible to oncogenic transformation. However, the current evidence that these cells are stem cells relies on prospective isolation and in vitro culture and needs to be confirmed in vivo.
CONCLUSIONS
The deployment of safe, effective cell-based therapies for the treatment of lung conditions remains a tantalizing prospect rather than a practicable possibility. For cell-based treatments to be safe, it will be imperative to ensure that exogenous cells cannot contribute to tumor formation either directly by proliferation or, more indirectly, by triggering self-renewal of endogenous stem and progenitor cells. This article highlights recent progress in the identification of lung embryonic epithelial progenitor cells and their control mechanisms. This work illustrates general questions about the regulation of adult stem cells that must be answered before cell-based therapies can be developed (Figure 1). The progress that has been made in the embryonic lung has resulted from improved in vivo genetic analysis of lung development (13, 18). To achieve a clearer understanding of the regulation of stem cell function in the adult lung, it will be important to use similar in vivo genetic techniques to investigate the following specific questions. First, which cells are the stem cells during homeostasis and repair? As in the embryonic lung, it will be necessary to lineage trace different cell types to determine the relative contributions of the various putative progenitor populations to all of the different airway lineages. In the adult, different cells may function as stem or progenitors under different conditions. Consequently, the lineage tracing should be performed under conditions of both homeostasis and repair. The genetic tools generated for lineage tracing will themselves be invaluable for conditional gene deletion and activation in the stem cells. Second, which signals promote proliferation after an epithelial injury and how is proliferation normally inhibited during homeostasis? To answer this, the interactions between stem cells and their local environment, including adjacent epithelial and mesenchymal cells, basement membrane, blood vessels, and bone marrow–derived cells need to be dissected both after injury and at steady state. Third, how do cells leave the stem cell compartment and begin to be specified or to differentiate along a particular lineage? Does this require a balance between asymmetric and symmetric cell divisions? And, are there intrinsic limitations on the number of times a stem cell can divide, or the different lineages it can contribute to due to its developmental history? The answers to these questions will not only improve fundamental knowledge but also may convert the concept of treating lung disorders using stem cells from a desirable idea to a realistic possibility.
Acknowledgments
The author thanks Brigid L. M. Hogan for critical reading of the manuscript.
Supported by National Institutes of Health grant NIH-3036424 and the Parker B. Francis Family Foundation.
Conflict of Interest Statement: E.L.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004;7:136–144. [DOI] [PubMed] [Google Scholar]
- 2.Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development 2007;134:3771–3780. [DOI] [PubMed] [Google Scholar]
- 3.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624. [DOI] [PubMed] [Google Scholar]
- 5.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 2005;132:1363–1374. [DOI] [PubMed] [Google Scholar]
- 6.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, et al. Wnt/beta-catenin signaling acts upstream of n-myc, bmp4, and fgf signaling to regulate proximal-distal patterning in the lung. Dev Biol 2005;283:226–239. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Hogan BL. Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns 2002;2:229–233. [DOI] [PubMed] [Google Scholar]
- 8.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996;122:1693–1702. [DOI] [PubMed] [Google Scholar]
- 9.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. Sox9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA 2007;104:1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 2007;13:103–114. [DOI] [PubMed] [Google Scholar]
- 11.McDowell EM, Newkirk C, Coleman B. Development of hamster tracheal epithelium: II. Cell proliferation in the fetus. Anat Rec 1985;213:448–456. [DOI] [PubMed] [Google Scholar]
- 12.Plopper CG, Nishio SJ, Alley JL, Kass P, Hyde DM. The role of the nonciliated bronchiolar epithelial (Clara) cell as the progenitor cell during bronchiolar epithelial differentiation in the perinatal rabbit lung. Am J Respir Cell Mol Biol 1992;7:606–613. [DOI] [PubMed] [Google Scholar]
- 13.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150. [DOI] [PubMed] [Google Scholar]
- 15.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn 2006;235:2376–2385. [DOI] [PubMed] [Google Scholar]
- 16.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA 2007;104:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 2002;99:10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007;445:886–891. [DOI] [PubMed] [Google Scholar]
- 20.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of foxa1 and foxa2 during lung morphogenesis. J Biol Chem 2005;280:13809–13816. [DOI] [PubMed] [Google Scholar]
- 21.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and foxp1 cooperatively regulate lung and esophagus development. Development 2007;134:1991–2000. [DOI] [PubMed] [Google Scholar]
- 22.Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after sox9 inactivation in the respiratory epithelium. Genesis 2005;41:23–32. [DOI] [PubMed] [Google Scholar]
- 23.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol 2006;291:67–82. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 2007;307:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998;8:1083–1086. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol 2002;248:68–81. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microrna mir-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol 2007;310:442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238. [DOI] [PubMed] [Google Scholar]
- 29.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999;126:4005–4015. [DOI] [PubMed] [Google Scholar]
- 30.Lu MM, Yang H, Zhang L, Shu W, Blair DG, Morrisey EE. The bone morphogenic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev Dyn 2001;222:667–680. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 2007;449:351–355. [DOI] [PubMed] [Google Scholar]
- 32.Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci USA 2006;103:18998–19003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post LC, Ternet M, Hogan BL. Notch/delta expression in the developing mouse lung. Mech Dev 2000;98:95–98. [DOI] [PubMed] [Google Scholar]
- 34.Dang TP, Eichenberger S, Gonzalez A, Olson S, Carbone DP. Constitutive activation of notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene 2003;22:1988–1997. [DOI] [PubMed] [Google Scholar]
- 35.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46. [DOI] [PubMed] [Google Scholar]
- 36.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology (Baltimore) 2006;43:S45–S53. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913–3921. [DOI] [PubMed] [Google Scholar]
- 38.Linnoila RI, Jensen-Taubman S, Kazanjian A, Grimes HL. Loss of gfi1 impairs pulmonary neuroendorine cell proliferation, but the neuroendocrine phenotype has limited impact on post-naphthalene airway repair. Lab Invest 2007;87:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development 2004;131:4299–4310. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. Gata and nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development 2007;134:189–198. [DOI] [PubMed] [Google Scholar]
- 41.Dor Y, Stanger BZ. Regeneration in liver and pancreas: time to cut the umbilical cord? Sci STKE 2007;414:pe66. [DOI] [PubMed] [Google Scholar]
- 42.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465. [DOI] [PubMed] [Google Scholar]
- 43.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 45.Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR. P38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet 2007;39:750–758. [DOI] [PubMed] [Google Scholar]
- 46.Metzger DE, Xu Y, Shannon JM. Elf5 is an epithelium-specific, fibroblast growth factor-sensitive transcription factor in the embryonic lung. Dev Dyn 2007;236:1175–1192. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors pea3/erm in mouse lung development. Dev Biol 2003;261:10–24. [DOI] [PubMed] [Google Scholar]
- 48.Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that sprouty2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev 2001;102:81–94. [DOI] [PubMed] [Google Scholar]
- 49.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002;129:4831–4842. [DOI] [PubMed] [Google Scholar]
- 50.De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (dkk1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol 2005;277:316–331. [DOI] [PubMed] [Google Scholar]