Abstract
Dynamic changes to the developing lung endoderm during the process of lung development result in the establishment of functionally distinct epithelial compartments that vary both in their cellular composition and mechanisms contributing to their maintenance in adulthood. This focused review compares the hierarchical organization of cells within slowly and rapidly renewing tissues as a basis to better understand cellular and molecular mechanisms regulating epithelial maintenance and repair in the lung.
Keywords: progenitor, stem cell, lung repair
GLOSSARY*.
Progenitor cell: A collective term used to describe any cell that has the capacity to proliferate. Terminology that takes into account the functional distinctions between progenitor cells of the same tissue and between tissue types is suggested below.
Adult tissue stem cell: A relatively undifferentiated cell that has the capacity for unlimited self-renewal. Adult tissue stem cells have a differentiation potential equivalent to the cellular diversity of the tissue in which they reside. The hematopoietic stem cell is a prototypical adult tissue stem cell.
Transit-amplifying cell: The progeny of a tissue stem cell that retain relatively undifferentiated character and have a finite capacity for proliferation. The sole function of transit-amplifying cells is generation of sufficient specialized progeny for tissue maintenance. Examples of transit-amplifying cells include lineage committed progenitor cells such as the myeloid and lymphoid progenitors of the hematopoietic system.
Facultative progenitor cell: A cell with proliferative capacity that has functional properties of differentiated cell types in its quiescent state. Functions of facultative progenitor cells in their quiescent state can render them susceptible to environmental or nutritional stress. Examples of facultative progenitor cells include bronchiolar Clara cells, hepatocytes, and pancreatic beta cells. Facultative progenitor cells in their active proliferative state exhibit many of the properties of a transit-amplifying cell.
Classical stem cell hierarchy: A stem cell hierarchy in which the adult tissue stem cell is required for normal tissue maintenance and gives rise to a transit-amplifying cell. Within this type of hierarchy, renewal potential resides in cells at the top of the hierarchy (i.e., stem and transit-amplifying cells).
Nonclassical stem cell hierarchy: A stem cell hierarchy in which the adult tissue stem cell does not typically participate in normal tissue maintenance but can be activated to participate in repair after progenitor cell depletion. Nonclassical stem cell hierarchies are typical of slowly renewing tissues such as the lung.
* Adapted from Reference 26.
The lung develops as a result of dynamic interactions between endoderm and mesoderm (1). These interactions result in the establishment of functionally distinct epithelial compartments that vary both in their cellular composition and mechanisms contributing to their maintenance in adulthood. This focused review compares the hierarchical organization of cells within slowly and rapidly renewing tissues as a basis to better understand cellular and molecular mechanisms regulating epithelial maintenance and repair in the lung.
DIFFERENCES BETWEEN TISSUES IN THE RATE OF EPITHELIAL REPLACEMENT: CONTINUOUS VERSUS INTERMITTENT
The life span of epithelial cells varies considerably between tissues, suggesting that distinct cellular and molecular mechanisms must operate to regulate their maintenance during normal and diseased states. The epithelium of the small intestine and colon is replaced every 5 days, whereas that of the lung and other foregut-derived organs (pancreas, liver, and thymus) turns over with very slow kinetics in the normal adult state. These distinctions can be used to classify epithelia as either rapidly renewing (e.g., intestinal epithelium) or slowly renewing (e.g., lung, pancreas, liver, and thymus). A second distinction between rapidly and slowly renewing epithelia is their response to acute or chronic injury. Since the intestinal epithelium is in a state of constitutive renewal due to the rapid rate of epithelial turnover, perturbations to the epithelium are not associated with dramatic changes in cell cycle frequency. However, injury to slowly renewing tissues results in their acquisition of a rapidly renewing state, the duration of which is dependent upon the magnitude and persistence of the injured condition. Based upon these tissue-specific differences between normal and injured conditions, distinct regulatory mechanisms must exist in which signaling pathways involved in regulation of cell proliferation, self-renewal, and differentiation are differentially used to accommodate the unique requirements for tissue maintenance and repair.
PROGENITOR CELLS IN TISSUE MAINTENANCE AND RENEWAL
Progenitor cells are broadly defined as a population of proliferative cells that either directly or indirectly give rise to specialized cell types important for organ function. Tissue-specific differences are observed in the relative contribution of endogenous versus recruited progenitor cells. In the lung, despite earlier reports of circulating progenitor cells contributing to epithelial renewal (2–5), it is now generally accepted that if circulating cells can give rise to lung epithelium, these events are extremely rare and unlikely to be of physiological relevance (6–8). Progenitor cells differ in their capacity for long-term self-renewal, their cell cycle frequency, their differentiation potential, and their molecular phenotype relative to other cell types within the tissue. The application of such functional criteria to stratify progenitor cells has formed the basis for their hierarchical organization and the concept of tissue stem cell hierarchies.
ORGANIZATION OF PROGENITOR CELLS AND THEIR DIFFERENTIATED PROGENY INTO A CLASSICAL STEM CELL HIERARCHY
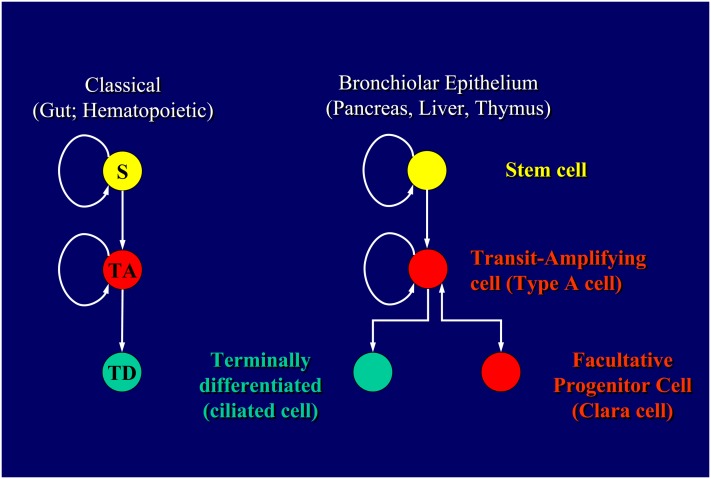
The prototypical stem cell hierarchy is that described for maintenance of the diffuse hematopoietic system. This hierarchy was defined by a combination of fractionation methods involving use of cell surface markers coupled with in vitro and/or in vivo assays to assess the capacity for self-renewal and differentiation (9, 10). Using these approaches, a progenitor cell type was defined that retained the capacity for long-term reconstitution of hematopoiesis in vivo when introduced back into the circulation of myeloablated (ablation of the hematopoietic lineage using either chemical or radiation treatments) syngeneic hosts. The basis for this finding was that this progenitor cell, referred to as the hematopoietic stem cell, had the capacity for unlimited self-renewal in addition to having the capacity to generate all differentiated cell types of the hematopoietic system. Other progenitor cell types defined using these assays were shown to exhibit finite capacity for self-renewal and in most cases a differentiation potential that represented a subset of that possessed by the hematopoietic stem cell. These progenitor cells were referred to as lineage-committed progenitor cells, such as those capable of giving rise to either lymphoid or myeloid derivatives. As such, hierarchical ordering of these cell types according to their capacity for self-renewal and differentiation potential led to the development of the classical stem cell hierarchy (Figure 1).
Figure 1.
Hierarchical organization of progenitor cells and their differentiated progeny within rapidly (classical stem cell hierarchy; left) and slowly (nonclassical stem cell hierarchy; right) renewing tissues. S, adult tissue stem cell; TA, transit-amplifying cell; TD, terminally differentiated cells.
The epithelium lining the intestine is also maintained through the action of a classical stem cell hierarchy (11, 12). Isolation and transplantation assays analogous to those developed for characterization of the hematopoietic system are lacking for the intestine. However, other approaches have been used to define the hierarchical organization of intestinal progenitor cells and their differentiated progeny that take advantage of differences in their spatial distribution, gene expression, and capacity for self-renewal. Epithelial progenitor cells of both the small intestine and colon are localized exclusively within protected invaginations that in the small intestine are referred to as crypts of Lieberkuhn. Specialized cell types are derived from the rapidly cycling pool of progenitor cells referred to as transit-amplifying cells. Transit-amplifying cells are termed as such due to their finite capacity to proliferate; up to six to eight cell divisions. In the small intestine, these specialized cell types either migrate out of the crypt and give rise to the absorptive and secretory cell types of the villus epithelium, or migrate to the crypt base in the case of paneth cells. Progenitor cells of the colon differ from those of the small intestine with respect to their differentiation potential; lacking the capacity to generate Paneth cells. As such, regional specialization of functions within the intestine results in distinct populations of progenitor cells that differ not only by their spatial localization along the proximal to distal axis of the intestine but also by their differentiation potential. This property is shared by other organs such as the lung, for which regional differences in epithelial cell function are maintained through the action of region-specific stem cell hierarchies (13–16).
Even though it is generally accepted that the intestinal epithelium is maintained through the action of a classical stem cell hierarchy, the identity of the progenitor cell thought to represent the stem cell has changed with improving technologies. The physical location of the stem cell within the crypt of the small intestine was initially defined based primarily upon their proximal location relative to paneth cells and use of DNA-label retention assays to identify the infrequently cycling fraction of progenitor cells. Using these criteria, the stem cell of the small intestinal epithelium was predicted to reside approximately four cell diameters proximal to the crypt base. However, a new candidate stem cell has been proposed based on identification of novel genes expressed within subpopulations of intestinal stem cells and on use of this knowledge to generate new tools to investigate roles for these cell types in epithelial maintenance (17). Barker and colleagues found that epithelia of both the small intestine and colon harbor a population of progenitor cells that are each unique in their expression of Lgr5. They were able to show that Lgr5-expressing progenitor cells were located at the base of intestinal crypts and interspersed by Paneth cells. Moreover, through use of lineage tracing approaches they were able to demonstrate that these cells were capable of long-term self-renewal and gave rise to all other progenitor and specialized cell types of the epithelium.
PROGENITOR CELLS OF SLOWLY RENEWING TISSUES
Differences in steady-state proliferative kinetics between progenitor cells of rapidly and slowly renewing tissues make it difficult to appropriately apply the terminology developed to define the cellular components of a classical hierarchy to those cells of slowly renewing tissues. The most readily apparent difference can be observed in the normal adult state when progenitor cell proliferation in slowly renewing tissues is infrequent. This is in dramatic contrast to the continuously proliferating population of progenitor cells within the intestinal crypt. A second important distinction is the capacity of cells that fulfill critical differentiated functions in the normal tissue to undergo the transition to an actively proliferating progenitor cell after injury. In the bronchiolar airways of the lung, now classical studies of Evans and colleagues demonstrated that Clara cells have the functional capacity to transition to a bi-potential progenitor cell following selective injury to ciliated cells (Figure 1B) (18). In their study, ozone exposure resulted in the appearance of a nonciliated cell in bronchiolar airways that lacked ultrastructural features of a mature nonciliated cell and could be labeled with the DNA precursor [3H]-thymidine deoxyribose ([3H]-TdR). These [3H]-TdR-labeled cells, referred to as Type A cells, were shown to have the capacity to generate mature Clara cells and ciliated cells based upon the ability to chase labeled DNA into these cell types during a post-labeling recovery period. They were subsequently able to demonstrate that Clara cells labeled with [3H]-TdR during recovery from initial ozone exposure gave rise to immature nonciliated cells and underwent another round of the cell cycle with a second exposure to ozone (19).
The capacity of the bronchiolar progenitor to transition from a “differentiated” to an “undifferentiated” state between normal and repairing conditions represents a clear distinction from the constitutively undifferentiated phenotype of progenitor cells observed within rapidly renewing tissues such as the small intestine (Figure 1). Based on this distinction, Clara cells are more appropriately termed a facultative progenitor cell to reflect their changing roles in the normal and injured airway (Stripp and Reynolds, unpublished data). Equivalent facultative progenitor cells can be found in other slowly renewing tissues, including the liver, pancreas, and thymus, suggesting that this change in progenitor cell function is an important adaptation to balance their contrasting functions in the normal and repairing states. When activated to proliferate, these cells behave in a fashion that is equivalent to the transit-amplifying cells of rapidly renewing tissues. However, whereas the lifespan of the intestinal transit-amplifying cell can be accurately determined due to the rapid rate of epithelial turnover, this is not the case for the facultative progenitor cell pools of slowly renewing tissues.
With the recent demonstration that intestinal stem and transit cells cycle with similar frequency (17), the relative lifespan of progenitor cell types is the only functional characteristic that can be equally applied to the hematopoietic and intestinal hierarchies to distinguish stem cells from transit cells. This more limited criterion for distinguishing stem and transit-amplifying cells of rapidly renewing tissues cannot easily be applied to the hierarchical organization of progenitor cells in slowly renewing tissues due to the longevity of the facultative progenitor cell. Accordingly, the role and even existence of adult tissue stem cells has been questioned in the pancreas and by inference in other slowly renewing tissues (20, 21). Particularly in the liver and lung, the normal functions of the facultative progenitor cell render them susceptible to environmental agents. Clara cells of the bronchiole and hepatocytes of the liver are the primary sites for xenobiotic metabolism within their respective organ. This functional property of the facultative progenitor cell renders it susceptible to pollutants (such as naphthalene in the case of mouse Clara cells) whose toxicity is related to their metabolic bioactivation. Furthermore, Clara cells have been shown to undergo phenotypic changes in the setting of allergic inflammation that may compromise their capacity to function as a progenitor cell (22). As such, the functions of the facultative progenitor in the normal state suggest that its lifespan may vary considerably depending upon environmental and nutritional status.
The finding in rodent models of a population of pollutant-resistant cells capable of contributing to either lung or liver regeneration suggests that this criterion may be effectively used to hierarchically organize progenitor cell types in a manner that is of functional importance to slowly renewing tissues. Adult tissue stem cells may not be a required participant in normal epithelial maintenance but represent a critical reserve pool of cells that can contribute to repair when environmental or nutritional factors lead to depletion of the abundant facultative progenitor. Bronchiolar airways harbor a population of progenitor cells that can be distinguished from Clara cells based upon their resistance to naphthalene (23). Naphthalene-resistant cells are located within discrete microenvironments within bronchioles that include the neuroepithelial body (NEB) and bronchoalveolar duct junction (BADJ) (14, 16). The unique spatial distribution of naphthalene-resistant bronchiolar progenitor cells coupled with evidence indicating that they possess a unique molecular phenotype (13) suggest that these cells are intrinsically naphthalene-resistant and that this property is most likely conferred by the microenvironment in which they reside. Both NEB- and BADJ-associated naphthalene-resistant cells share the property of CCSP expression with the abundant population of Clara cells (16). More recently, naphthalene-resistant cells that localize to the BADJ were shown to co-express both CCSP and surfactant protein C, a gene expressed within the early lung endoderm and restricted primarily to alveolar type 2 cells of the adult lung (24, 25). Kim and colleagues were able to define a unique cell surface phenotype, Sca1pos/CD34pos/CD45neg/CD31neg, associated with SPC/CCSP dual positive cells isolated using methods developed for enrichment of type 2 alveolar epithelial cells (24). Collectively, these studies define distinct members of the bronchiolar progenitor cell pool. If organized according to their relative susceptibility to naphthalene, these cells can be hierarchically stratified. We propose that this strategy for stratification of the bronchiolar progenitor cell pool defines a nonclassical stem cell hierarchy in which the naphthalene-resistant bronchiolar epithelial cell is analogous to the adult tissue stem cell of a classical stem cell hierarchy (Figure 1B).
SUMMARY AND FUTURE DIRECTIONS
Herein and in a related article (Stripp and Reynolds, unpublished data), a basis is provided for hierarchical organization of progenitor cells within slowly renewing tissues. We propose that relative resistance to environmental and nutritional factors is a functional basis for placing progenitor cells in these tissues into a “nonclassical” stem cell hierarchy. In the lung, rare progenitor cells that localize to discrete anatomical sites exhibit the property of pollutant resistance. These cells represent a reserve population of progenitor cells whose longevity is ensured by virtue of their resistance to environmental agents. Ongoing and future studies are aimed at:
Understanding the unique cellular and molecular regulation of progenitor cells located within distinct epithelial compartments distributed along the proximal-distal axis of the airway.
Providing a more rigorous molecular definition of distinct airway progenitor cell types.
Developing methods for prospective isolation and in vitro characterization of distinct airway progenitor cell populations.
Developing strategies for the modulation of epithelial reparative capacity through either molecular/pharmacologic or cell-based strategies.
Supported by the National Heart, Lung and Blood Institute (HL064888, HL090146).
Conflict of Interest Statement: B.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624. [DOI] [PubMed] [Google Scholar]
- 2.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 3.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood 2003;102:3483–3493. [DOI] [PubMed] [Google Scholar]
- 4.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006;173:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 2006;169:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001;17:387–403. [DOI] [PubMed] [Google Scholar]
- 11.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays 2002;24:91–98. [DOI] [PubMed] [Google Scholar]
- 12.Radtke F, Clevers H, Riccio O. From gut homeostasis to cancer. Curr Mol Med 2006;6:275–289. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670. [DOI] [PubMed] [Google Scholar]
- 16.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 18.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to no2 or o3. Lab Invest 1976;35:246–257. [PubMed] [Google Scholar]
- 19.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978;38:648–653. [PubMed] [Google Scholar]
- 20.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46. [DOI] [PubMed] [Google Scholar]
- 21.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol 2007;5:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–L799. [DOI] [PubMed] [Google Scholar]
- 24.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 25.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human sp-c gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol 1993;156:426–443. [DOI] [PubMed] [Google Scholar]
- 26.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]