Abstract
The crystal structure of human pancreatic cationic trypsin showed the chemical modification of Tyr-154, which was originally described as phosphorylation [Gaboriaud et al. (1996) J Mol Biol 259, 995–1010]. Here we report that Tyr-154 is sulfated, not phosphorylated. Cationic and anionic trypsinogens were purified from human pancreatic juice and subjected to alkaline hydrolysis. Modified tyrosine amino-acids were separated on a Dowex cation-exchange column and analyzed by thin-layer chromatography. Both human cationic and anionic trypsinogens contained tyrosine-sulfate, but no tyrosine-phosphate, whereas bovine trypsinogen contained neither. Furthermore, incorporation of [35S]SO4 into human cationic trypsinogen transiently expressed by human embryonic kidney 239T cells was demonstrated. Mutation of Tyr-154 to Phe abolished radioactive sulfate incorporation, confirming that Tyr-154 is the site of sulfation in cationic trypsinogen. Sulfated pancreatic cationic trypsinogen exhibited faster autoactivation than a non-sulfated recombinant form, suggesting that tyrosine-sulfation of trypsinogens might enhance intestinal digestive zymogen activation in humans. Finally, sequence alignment revealed that the sulfation motif is only conserved in primate trypsinogens, suggesting that typsinogen sulfation is absent in other vertebrates.
Keywords: human cationic trypsin, human anionic trypsin, post-translational modification, tyrosine-sulfation, hereditary pancreatitis
The human pancreas secretes two major trypsinogen isoforms, named cationic and anionic trypsinogens, which share 90 % identity in their primary structure. In addition to their physiological function in digestion, trypsinogens also play an important pathological role in pancreatitis. Mutations that stimulate activation of cationic trypsinogen cause hereditary pancreatitis, whereas a mutation that renders anionic trypsinogen sensitive to degradation was shown to protect against pancreatitis (1, 2). In contrast to other known vertebrate trypsinogens, human trypsinogens appear to undergo post-translational modification. In 1996, Gaboriaud et al. (3) solved the crystal structure of human cationic trypsin and described the presence of a phosphate group on Tyr-154 (Tyr-151 in the chymotrypsin numbering system). The authors also carried out mass spectrometry on native trypsin samples and masses consistent with phosphorylation were determined. Corroborating evidence for the presence of a modifying group on human cationic trypsinogen came from Szilágyi et al. in 2001, who confirmed the increased mass of purified native trypsin by mass spectrometry and discussed the possibility that the modifying group might be sulfate instead of phosphate, as both modifications would result in approximately 80 Da extra mass (4). This consideration was in line with an earlier study by Scheele et al., demonstrating that incubation of human pancreatic slices with radioactive sodium-sulfate resulted in the incorporation of radioactive sulfate into human trypsinogens (5).
To resolve the controversy about the nature of the modifying group on human trypsinogens, we isolated the tyrosine-sulfate amino-acid from pancreatic trypsinogens and demonstrated radioactive sulfate incorporation into Tyr-154 of cationic trypsinogen transiently expressed in human embryonic kidney (HEK) 293T cells. The experiments presented here conclusively determine that human cationic and anionic trypsinogens are tyrosine-sulfated and not phosphorylated.
RESULTS
Electrophoretic analysis of human pancreatic trypsinogens
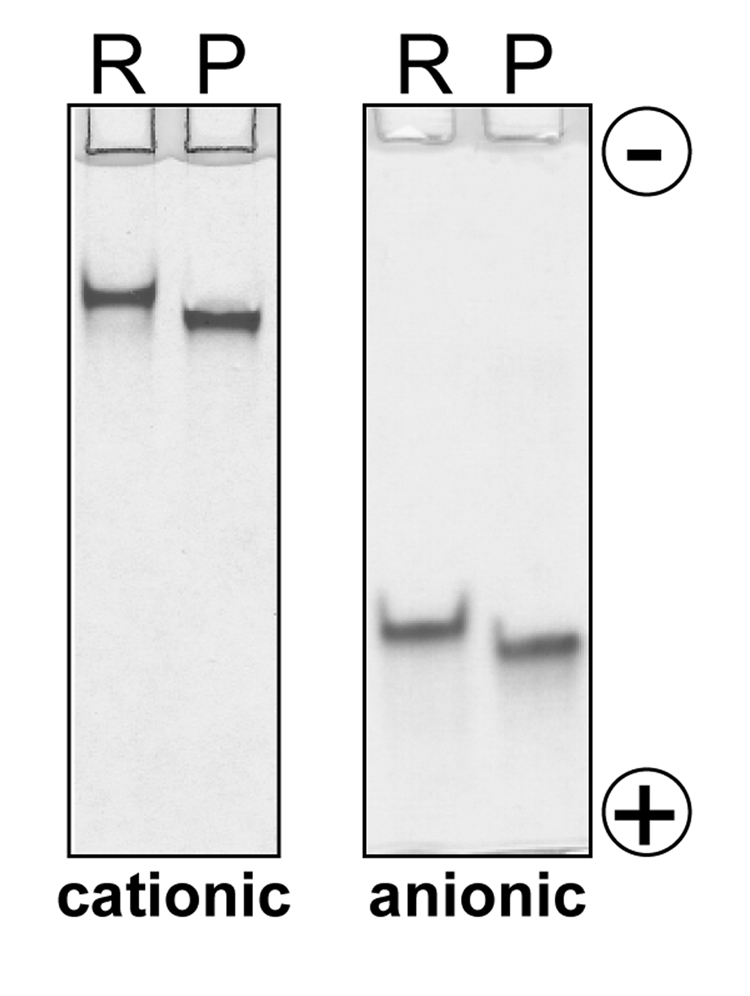
We purified human cationic and anionic trypsinogens from pancreatic juice and compared their electrophoretic behavior to recombinant trypsinogens expressed in E. coli. These recombinant preparations were previously characterized by mass spectrometry and N-terminal sequencing and contain no post-translational modifications (2, 4). Analysis on SDS-PAGE under reducing or non-reducing conditions revealed no discernible differences (not shown). On the other hand, when native and recombinant trypsinogens were electrophoresed in the absence of SDS on 13 % "native" polycarylamide gels, trypsinogens isolated from pancreatic juice migrated faster than their recombinant counterparts (Fig 1). The faster mobility of pancreatic trypsinogens on native gels indicates the presence of extra negative charge(s), which is consistent with a sulfo-tyrosine or phospho-tyrosine amino-acid.
Figure 1.
Native gel electrophoresis of pancreatic (P) and recombinant (R) human cationic and anionic trypsinogens. Pancreatic human trypsinogens were purified from pancreatic juice. Recombinant human trypsinogens were expressed in E. coli. Aliquots (~10 µg) of purified trypsinogens were lyophilized and re-suspended in native sample buffer devoid of SDS or reducing agents. Samples were resolved on 13 % polyacrylamide gels in Tris-glycine buffer (pH 8.5) and the gels were stained with Coomassie blue.
Demonstration of tyrosine-sulfate in human trypsinogens by alkaline hydrolysis and thin-layer chromatography
To obtain conclusive evidence for the chemical nature of the modifying group, we set out to isolate the modified tyrosine from human trypsinogens and to identify the modifying group by thin-layer chromatography (TLC). In the design of the experimental protocol, we took advantage of the following published observations (6–9). Tyrosine-sulfate and usually tyrosine-phosphate are resistant to alkaline hydrolysis. Due to the presence of the strongly acidic group, tyrosine-sulfate and tyrosine-phosphate do not bind to a Dowex cation exchange column at pH 2.5, whereas under these conditions all other amino-acids remain bound to the resin. Tyrosine-sulfate and tyrosine-phosphate migrate differently on a silica thin-layer chromatography plate in 80 % ethanol solvent. This property allows the clear identification of the modified tyrosine species using appropriate standards.
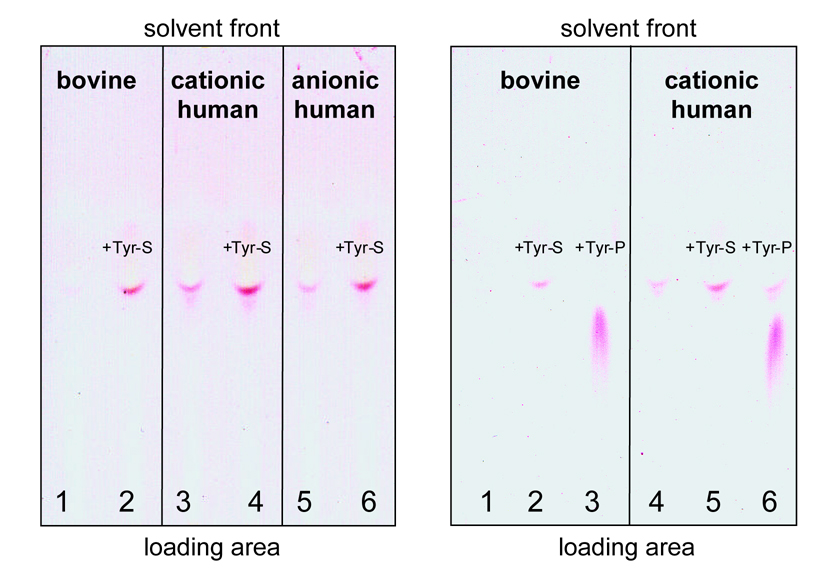
Human pancreatic trypsinogens were subjected to alkaline hydrolysis, and the modified tyrosines were isolated and analyzed by thin-layer chromatography, as described in Experimental Procedures. As a negative control, we used commercial bovine trypsinogen, which is devoid of any phosphate or sulfate modifying groups. Ninhydrin staining of thin-layer silica plates revealed that no tyrosine-sulfate or tyrosine-phosphate amino acids were isolated from bovine trypsinogen hydrolysates (see lanes 1 in Fig 2 left and right panels). In contrast, samples isolated from human cationic trypsinogen or anionic trypsinogen clearly showed a ninhydrin-positive spot (lanes 3 and 5, Fig 2 left panel), which co-migrated with the tyrosine-sulfate standard (lanes 2, 4, 6; Fig 2, left panel). No tyrosine-phosphate was detected in any of the samples analyzed. As shown in Fig 2 (right panel), the tyrosine-phosphate was clearly distinguished from tyrosine-sulfate by its slower migration. Taken together, these experiments unambiguously proved that human trypsinogens contain tyrosine-sulfate, not tyrosine-phosphate.
Figure 2.
Thin-layer chromatography of tyrosine-sulfate isolated from human trypsinogens after alkaline hydrolysis. The silica plate was developed using 80 % ethanol as solvent. The plate was then air-dried, sprayed with ninhydrin solution and heated with a hair-dryer. See text for experimental details. Tyrosine sulfate (Tyr-S) and (Tyr-P) standards were co-loaded with the trypsinogen-derived samples where indicated.
Human cationic trypsinogen expressed in HEK 293T cells incorporates [35S] from inorganic [35S]Na2SO4 into Tyr-154
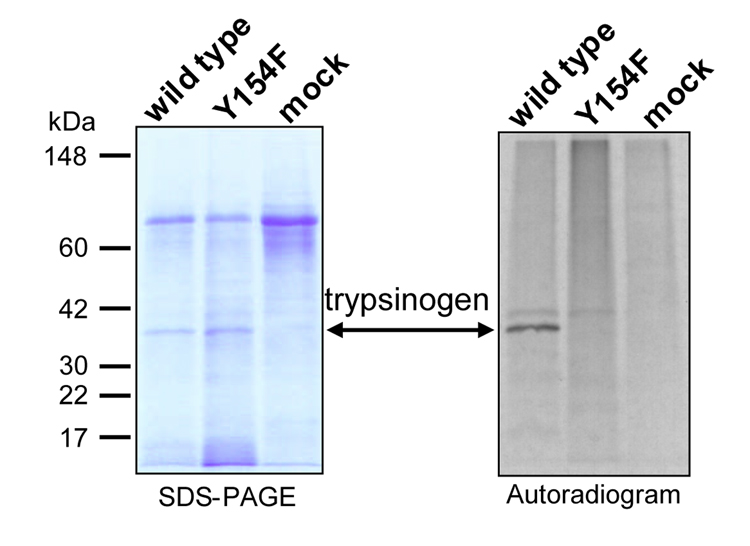
HEK 293T cells transiently transfected with the cDNA for human cationic trypsinogen secrete trypsinogen into the growth medium. Addition of [35S]Na2SO4 to the medium resulted in the incorporation of [35S] into the secreted trypsinogen (Fig 3). Importantly, no [35S] incorporation was observed into the proteins of conditioned media of mock-transfected HEK 293T cells. Mutation of Tyr-154 to Phe (Y154F) abolished radioactive sulfate incorporation into the major trypsinogen band, confirming that the site of sulfation is Tyr-154 in cationic trypsinogen.
Figure 3.
Incorporation of [35S]SO4 into Tyr-154 of human cationic trypsinogen expressed in human embryonic kidney (HEK) 293T cells. Transiently transfected HEK 293T cells expressing wild-type or Y154F mutant trypsinogens were incubated with [35S]Na2SO4 in F-12 medium, as described in Experimental Procedures. Conditioned media were harvested 48 h after transfection and 200 µL (wild-type) or 400 µL (Y154F and mock) medium was precipitated with 10 % trichloroacetic acid (final concentration). The precipitate was recovered by centrifugation, resuspended in Laemmli sample buffer with 100 mM dithiothreitol, heat-denatured and on run on a 13 % gel. The gel was stained with Coomassie blue, dried between two cellophanes, and exposed to film for 48 h. The markers used were MultiMark pre-stained standards from Invitrogen. The faint bands above the main trypsinogen band on the autoradiogram are probably aberrantly glycosylated trypsinogens, which become visible due to [35S]SO4 incorporation into their sugar moiety.
Tyrosine-sulfation increases autoactivation of human cationic trypsinogen
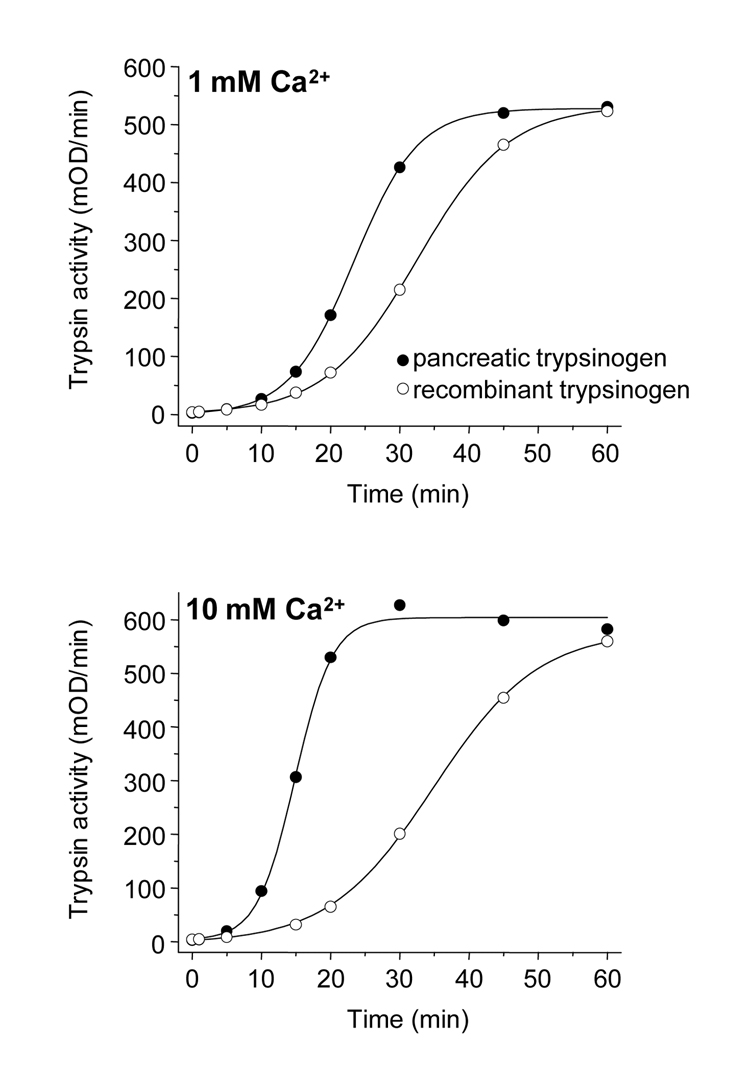
Catalytic activity of sulfated pancreatic and non-sulfated recombinant cationic trypsins were essentially identical on the chromogenic peptide substrate N-CZB-Gly-Pro-Arg-p-nitroanilide (not shown). On the other hand, a clear difference was observed in autoactivation experiments. Autoactivation of trypsinogens is a self-amplifying proteolytic reaction, in which trypsin activates trypsinogen to trypsin. As shown in Fig 4, sulfated pancreatic cationic trypsinogen autoactivated faster than its recombinant non-sulfated form, particularly at higher calcium concentrations. Interestingly, autoactivation of human anionic trypsinogen seemed unaffected by tyrosine sulfation, as both native and recombinant preparations autoactivated at comparable rates (not shown).
Figure 4.
Autoactivation of pancreatic (solid circles) and recombinant (empty circles) cationic trypsinogens. Trypsin-mediated trypsinogen activation was measured at 37 °C, using 2 µM trypsinogen and 10 nM trypsin initial concentrations in 0.1 M Tris-HCl (pH 8.0), 2 mg/mL bovine serum albumin and the indicated CaCl2 concentrations. At given times, 2 µL aliquots were removed from the activation reactions and trypsin activity was measured with the synthetic chromogenic substrate, N-CBZ-Gly-Pro-Arg-p-nitroanilide at 0.14 mM final concentration. One-minute time-courses of p-nitroaniline release were followed at 405 nm in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2, at room temperature using a Spectramax Plus 384 microplate reader (Molecular Devices). The slope of the linear portion of the absorbance increase was expressed in mOD/min units. The maximal trypsin activity was 600 mOD/min. The calculated rates of autoactivation of recombinant and pancreatic trypsinogens were 1.5 nM/min and 2.1 nM/min in 1 mM CaCl2 and 1.5 nM/min and 3.6 nM/min in 10 mM CaCl2, respectively.
DISCUSSION
The present study offers conclusive evidence that human trypsinogens undergo post-translational tyrosine-sulfation in the pancreas. Post-translational O-sulfation of tyrosine residues was first described in bovine fibrinopeptide B in 1954 (6), and its common occurrence in proteins of various mammalian cells was demonstrated by Huttner in 1982 (7). In contrast to phosphorylation, tyrosine sulfation is not part of dynamic regulatory mechanisms, as mammalian tissues have no sulfatase activity for the removal of the sulfate moiety (10). Tyrosine-sulfation is catalyzed by membrane-bound tyrosylprotein sulfotransferases (TPST-1 and TPST-2) in the trans-Golgi network (11–13). Genetic deletion of TPST-1 in mice causes reduced body weight and increased postimplantation fetal death (14), whereas deletion of TPST-2 results in male infertility (15). The functional role of tyrosine-sulfation has been characterized only in a handful of proteins so far. The most prominent examples include the HIV co-receptor and chemokine receptor CCR5, which requires tyrosine-sulfation in its N-terminal extracellular domain for proper function; the leech-derived thrombin-inhibitor hirudin, which has 10-fold higher affinity in its sulfated form; and P-selectin glycoprotein ligand-1, the sulfation of which is required for high-affinity binding to P-selectin (10 and references therein). In general, tyrosine sulfation is believed to modify protein-protein interactions in various physiological processes (10).
Inspection of the human cationic or anionic trypsinogen sequence in the vicinity of Tyr-154 reveals features characteristic of known tyrosine sulfation sites (10). Thus, acidic residues have been shown to enhance sulfation, particularly on the N-terminal side (−1 position) of the Tyr residue. In addition, turn-inducing residues (Gly, Pro) are often found in the vicinity of sulfated Tyr residues, but basic residues (Arg, Lys) are generally excluded. In human trypsinogens, the Gly-Ala-Asp-Tyr-Pro-Asp-Glu sequence contains 3 acidic residues, one N-terminal (−1) and two C-terminal (+2 and +3) of the sulfated Tyr-154. Furthermore, the presence of two turn-inducing residues (Gly-151 and Pro-155) and the absence of any basic residues (Arg or Lys) are also readily apparent. Thus, Tyr-154 seems to conform to the general criteria of tyrosine sulfation sites. This sequence is fully conserved in all 3 human trypsinogens. Genomic sequences of the chimpanzee and the rhesus monkey predict that all potentially expressed trypsinogens contain the human sulfation motif (Table 1). A probably innocuous Gly→Thr change is noted in the chimpanzee mesotrypsinogen sequence at position −3. In stark contrast to the primate trypsinogens, the sulfation motif is absent in all other mammalian, avian, amphibian or fish trypinogens. Although Tyr-154 per se is conserved in the majority of the sequences examined, the potentially critical Asp-153 is uniformly absent. Furthermore, one or both of the two C-terminal acidic residues (Asp-156 and Glu-157 in human) are also missing in most trypsinogens. These structural differences, particularly the absence of the N-terminal (−1) acidic residue, suggest that all known animal trypsinogens are unmodified at Tyr-154 and tyrosine-sulfation of pancreatic trypsinogens is primate-specific.
Table 1.
Alignment of the human trypsinogen sulfation motif (from Gly-151 to Glu-157) with the homologous sequences of vertebrate trypsinogens.
| Human cationic | GADYPDE |
| Human anionic | GADYPDE |
| Human mesotrypsinogen | GADYPDE |
| Chimpanzee anionic | GADYPDE |
| Chimpanzee C | GADYPDE |
| Chimpanzee mesotrypsinogen | TADYPDE |
| Rhesus monkey (all isoforms) | GADYPDE |
| Bovine cationic | GTSYPDV |
| Bovine anionic | GVNYPDL |
| Pig cationic | GSSYPSL |
| Dog cationic | GQNYPDV |
| Dog anionic | GTNYPEL |
| Rat 1 | GVNNPDL |
| Rat 2 | GVNEPDL |
| Rat 3 | GTNYPSL |
| Rat 4 | GGKYPAL |
| Rat 5 | GFESPSV |
| Mouse 7 | GTNYPSL |
| Mouse 4, 5 | GGKYPAL |
| Mouse 8, 9, 20 | GVNNPDL |
| Guinea pig | GVKNPDL |
| Ostrich | GSSFPEI |
| Chicken I-P38 | GSLYPDV |
| Chicken II-P29 | GYNYPEL |
| Chicken I-P1 | GSLYPDV |
| Xenopus laevis 1 | GSNYPDL |
| Xenopus laevis 2 | GTNYPDL |
| Xenopus tropicalis 1 | IGEFPDR |
| Xenopus tropicalis 10 | GTNYPDL |
| Atlantic salmon 1 | STADSNK |
| Atlantic salmon 2 | STADKNK |
| Atlantic salmon 3 | SSNYPDT |
| Chum salmon anionic | STADKNK |
| Zebrafish | GSNYPSR |
| Atlantic cod 1 | SVADGDK |
| Atlantic cod Y | EVNLPTR |
| Pufferfish 1 | DVFNPFN |
| Pufferfish 2 | ADRNKLQ |
| Japanese flounder 1 | STANRDM |
| Japanese flounder 2 | SSTDSSR |
| Japanese flounder 3 | QVFNPFN |
It is interesting to note that in our experiments human pancreatic trypsinogens appeared fully sulfated, as judged by native gel electrophoresis or by MonoQ anion-exchange chromatography. Given the fact that trypsinogens are among the most abundant secretory proteins, the human pancreas seems to exhibit extraordinary tyrosine-sulfating capacity. In this respect, Ouyang and Moore (1998) reported that mRNA expression of the TPST-2 isoform was drastically higher in the pancreas than any other tissues examined (13). This observation also raises the possibility that trypsinogens expressed in extra-pancreatic tissues may be incompletely sulfated.
The functional significance of tyrosine sulfation in human trypsinogens remains obscure at this time. Tyr-154 forms part of the S2′ subsite on trypsin and thus might modify interactions with various substrates and inhibitors. In this respect, Szilágyi et al. (2001) measured binding of pancreatic secretory trypsin inhibitor to native (sulfated) and recombinant (non-sulfated) trypsins and found only a 2-fold difference in the equilibrium dissociation constants (4). In our preliminary experiments, native cationic trypsinogens exhibited faster autoactivation than recombinant cationic trypsinogen, particularly in the presence of higher calcium concentrations. In contrast, autoactivation of anionic trypsinogen was unaffected. Because cationic trypsinogen is the predominant trypsinogen isoform secreted, these observations suggest that tyrosine-sulfation might be important for efficient zymogen activation in the human digestive tract.
EXPERIMENTAL PROCEDURES
Materials
De-identified samples of pure human pancreatic juice were received in lyophilized form, as a kind gift from Dr. Niels Teich (University of Leipzig, Gemany). The juice was collected from patients who underwent pancreas transplantation with bladder drainage of exocrine pancreatic secretions. To prevent zymogen activation, Trasylol (aprotinin) was added at the time of collection. See ref (16) for details. Carrier-free [35S]Na2SO4 (1 mCi; 10 mCi/mL in water) was purchased from American Radiolabeled Chemicals, Inc (St. Louis, Missouri). Tyrosine-sulfated cholecystokinine fragment 26–33 (CCK-8) was obtained from Peptides International (Louisville, Kentucky). The CCK-8 peptide (0.52 mg) was hydrolyzed and the tyrosine-sulfate was isolated as described below. Dowex 50WX2-400 cation-exchange resin and tyrosine-phosphate was from Sigma (St. Louis, Missouri). Tyrosine-phosphate was dissolved in water at 1 mM concentration. F-12 nutrient mixture (Ham) containing 1 mM L-glutamine was purchased from Invitrogen (Carlsbad, California). Whatman flexible plates for TLC (AL SIL G; 20 × 20 cm; aluminum backing; silica gel coating, 250 µM layer) were obtained from Fisher Scientific. Ninhydrin, ozone friendly ready-to-use spray for TLC (in 2-propanol as solvent) was from Acros Organics (Geel, Belgium).
Purification of native trypsinogens from pancreatic juice
Lyophilized pancreatic juice powder (50–100 mg) was solubilized with 1 mL 10 mM HCl, and insoluble material was removed by centrifugation. The supernatant was diluted with 3 mL water and loaded onto a MonoQ anion-exchange column (Pharmacia) equilibrated with 20 mM Tris-HCl (pH 8.0). Proteins were eluted with a 0-0.5 M NaCl gradient at 1 mL/min flow rate and 1 mL fractions were collected. The two trypsinogen peaks were collected and further purified with ecotin-affinity chromatography. See ref (17) for more detail.
Expression of recombinant trypsinogens in Escherichia coli
Expression plasmids and strains, growth conditions; isolation of inclusion bodies, in vitro refolding and ecotin-affinity chromatography were described previously (18–20).
Isolation and identification of tyrosine-sulfate from native trypsinogens by thin-layer chromatography
Approximately 40 nmol (1 mg) of purified trypsinogens were dialyzed against 1 mM HCl and lyophilized. The protein was re-suspended in 480 µL of water and 20 µL 5 N NaOH was added to a final concentration of 0.2 N. Trypsinogens were then hydrolyzed at 100 °C for 16–18 hours in glass vials. The sample was cooled to room temperature and 500 µL 1 M HCOOH was added to acidify the solution. The 1 mL samples were loaded onto Dowex mini-columns (0.8 mL resin equilibrated with 0.1 M HCOOH), and the columns were washed with 0.1 M HCOOH. Fractions (1 mL) were collected, lyophilized, re-suspended in 100 µL water and dried again. Finally, the dried material was re-suspended in 10 µL of water and 0.5 µL samples were analyzed by thin-layer chromatography on silica plates in 80 % ethanol as solvent. Amino-acids were visualized by spraying with ninhydrin and heating with a blow dryer. The developed plates were then scanned for documentation.
Transfection of HEK 293T cells and metabolic labeling with [35S]Na2SO4
The pcDNA3.1 expression plasmid containing the gene for human cationic trypsinogen was described previously (17). Mutation Y154F was introduced by oligonucleotide-directed site-specific mutagenesis using the overlap extension PCR method. HEK 293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) with 10 % fetal bovine serum (FBS), and 4 mM L-glutamine to 95 % confluence in 60 mm Petri-dishes. Transfections were carried out in Opti-MEM I reduced-serum medium with 2 mM L-glutamine (Invitrogen) using 8 µg plasmid DNA and 20 µL Lipofectamine 2000™ reagent (Invitrogen). After 5 hours, the medium was supplemented with DMEM and FBS to a 10 % final concentration. After 24 hours, cells were washed with sulfate-free F-12 medium (Ham); covered with 2.5 mL of the same medium and supplemented with 10 µL [35S]Na2SO4 (10 mCi/mL). Conditioned medium was harvested after 48 h incubation.
ACKNOWLEDGMENTS
This work was supported by NIH grants AA014544 and DK058088 to M. S.-T. Special thanks to Niels Teich (University of Leipzig, Germany) for providing pancreatic juice samples for this study. Suchismita Das and John Samuelson are acknowledged for their help with the radioactive experiments.
REFERENCES
- 1.Teich N, Rosendahl J, Tóth M, Mössner J, Sahin-Tóth M. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum Mutat. 2006;27:721–730. doi: 10.1002/humu.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witt H, Sahin-Tóth M, Landt O, Chen JM, Kahne T, Drenth JP, Kukor Z, Szepessy E, Halangk W, Dahm S, Rohde K, Schulz HU, Le Marechal C, Akar N, Ammann RW, Truninger K, Bargetzi M, Bhatia E, Castellani C, Cavestro GM, Cerny M, Destro-Bisol G, Spedini G, Eiberg H, Jansen JB, Koudova M, Rausova E, Macek M, Jr, Malats N, Real FX, Menzel HJ, Moral P, Galavotti R, Pignatti PF, Rickards O, Spicak J, Zarnescu NO, Bock W, Gress TM, Friess H, Ockenga J, Schmidt H, Pfützer R, Löhr M, Simon P, Weiss FU, Lerch MM, Teich N, Keim V, Berg T, Wiedenmann B, Luck W, Groneberg DA, Becker M, Keil T, Kage A, Bernardova J, Braun M, Guldner C, Halangk J, Rosendahl J, Witt U, Treiber M, Nickel R, Ferec C. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–673. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaboriaud C, Serre L, Guy-Crotte O, Forest E, Fontecilla-Camps JC. Crystal structure of human trypsin 1: unexpected phosphorylation of Tyr151. J Mol Biol. 1996;259:995–1010. doi: 10.1006/jmbi.1996.0376. [DOI] [PubMed] [Google Scholar]
- 4.Szilágyi L, Kénesi E, Katona G, Kaslik G, Juhász G, Gráf L. Comparative in vitro studies on native and recombinant human cationic trypsins. Cathepsin B is a possible pathological activator of trypsinogen in pancreatitis. J Biol Chem. 2001;276:24574–24580. doi: 10.1074/jbc.M011374200. [DOI] [PubMed] [Google Scholar]
- 5.Scheele G, Bartelt D, Bieger W. Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology. 1981;80:461–473. [PubMed] [Google Scholar]
- 6.Bettelheim FR. Tyrosine-O-sulfate in a peptide from fibrinogen. J Am Chem Soc. 1954;76:2838–2839. [Google Scholar]
- 7.Huttner WB. Sulphation of tyrosine residues - a widespread modification of proteins. Nature. 1982;299:273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- 8.Huttner WB. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- 9.Blode H, Heinrich T, Diringer H. A quantitative assay for tyrosine sulfation and tyrosine phosphorylation in peptides. Biol Chem Hoppe Seyler. 1990;371:145–151. [PubMed] [Google Scholar]
- 10.Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J Biol Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang Y, Lane WS, Moore KL. Tyrosylprotein sulfotransferase: purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci USA. 1998;95:2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beisswanger R, Corbeil D, Vannier C, Thiele C, Dohrmann U, Kellner R, Ashman K, Niehrs C, Huttner WB. Existence of distinct tyrosylprotein sulfotransferase genes: molecular characterization of tyrosylprotein sulfotransferase-2. Proc Natl Acad Sci USA. 1998;95:11134–11139. doi: 10.1073/pnas.95.19.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang YB, Moore KL. Molecular cloning and expression of human and mouse tyrosylprotein sulfotransferase-2 and a tyrosylprotein sulfotransferase homologue in Caenorhabditis elegans. J Biol Chem. 1998;273:24770–24774. doi: 10.1074/jbc.273.38.24770. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang YB, Crawley JT, Aston CE, Moore KL. Reduced body weight and increased postimplantation fetal death in tyrosylprotein sulfotransferase-1-deficient mice. J Biol Chem. 2002;277:23781–23787. doi: 10.1074/jbc.M202420200. [DOI] [PubMed] [Google Scholar]
- 15.Borghei A, Ouyang YB, Westmuckett AD, Marcello MR, Landel CP, Evans JP, Moore KL. Targeted disruption of tyrosylprotein sulfotransferase-2, an enzyme that catalyzes post-translational protein tyrosine O-sulfation, causes male infertility. J Biol Chem. 2006;281:9423–9431. doi: 10.1074/jbc.M513768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keim V, Iovanna JL, Orelle B, Verdier JM, Busing M, Hopt U, Dagorn JC. A novel exocrine protein associated with pancreas transplantation in humans. Gastroenterology. 1992;103:248–254. doi: 10.1016/0016-5085(92)91120-s. [DOI] [PubMed] [Google Scholar]
- 17.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–11886. doi: 10.1074/jbc.M600124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin-Tóth M. Human cationic trypsinogen. Role of Asn-21 in zymogen activation and implications in hereditary pancreatitis. J Biol Chem. 2000;275:22750–22755. doi: 10.1074/jbc.M002943200. [DOI] [PubMed] [Google Scholar]
- 19.Sahin-Tóth M, Tóthx M. Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem Biophys Res Commun. 2000;278:286–289. doi: 10.1006/bbrc.2000.3797. [DOI] [PubMed] [Google Scholar]
- 20.Kukor Z, Tóth M, Sahin-Tóth M. Human anionic trypsinogen. Properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur J Biochem. 2003;270:2047–2058. doi: 10.1046/j.1432-1033.2003.03581.x. [DOI] [PubMed] [Google Scholar]