Abstract
Gold nanoparticles functionalized with water-soluble zwitterionic ligands form kinetically stable complexes with hydrophobic drugs and dyes. These drugs and dyes are efficiently released into cells, as demonstrated through fluorescence microscopy and cytotoxicity assays. Significantly, there is little or no cellular uptake of particle, making these low toxicity particles promising for delivery applications.
Drug delivery systems (DDSs) provide an important tool for increasing efficacy of pharmaceuticals through improved pharmacokinetics and biodistribution.1 A wide variety of nanoscale materials such as liposomes, polymeric micelles, and dendrimers, have been employed as drug carriers. 2 Both covalent and non-covalent approaches can be applied to the conjugation of drugs into/onto these DDSs.3 Non-covalent approaches have the capability of employing active drugs, whereas covalent attachment generally requires chemical modification which can cause reduced efficiency of drug release or incomplete intracellular processing of a prodrug.4
Recently, gold nanoparticle (AuNP) based drug/gene delivery systems have attracted attention due to their functional versatility,5 biocompatibility,6 and low toxicity,7 Recent studies have demonstrated controlled release of payload by intracellular thiols.8 However, controlled dissociation of drugs in active form from covalent AuNP-drug conjugates remains a challenge for clinical applications.2
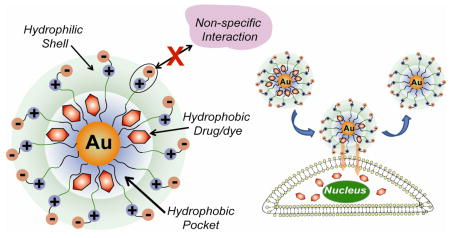
Noncovalent incorporation of drugs into AuNP monolayers provides an alternative delivery strategy with the potential for avoiding drug release and prodrug processing issues. The structure of commonly used water-soluble AuNPs is similar to that of unimolecular micelles such as dendrimers, featuring a hydrophobic interior and a hydrophilic exterior.9 The alkanethiol monolayer of the nanoparticle coupled with the radial nature of the ligands10 creates “hydrophobic pockets” inside monolayer of AuNP where organic solutes can be partitioned, as demonstrated by Lucarini and Pasquato.11 We report here the use of these pockets to encapsulate drugs and deliver them with high efficiency to cells.
The biocompatible AuNPs used in this study features two functional domains: a hydrophobic alkanethiol interior and a hydrophilic shell composed of a tetraethylene glycol (TEG) unit terminated with a zwitterionic headgroup. Particles with this general structure have been shown to minimize non-specific binding with biomacromolecules.12
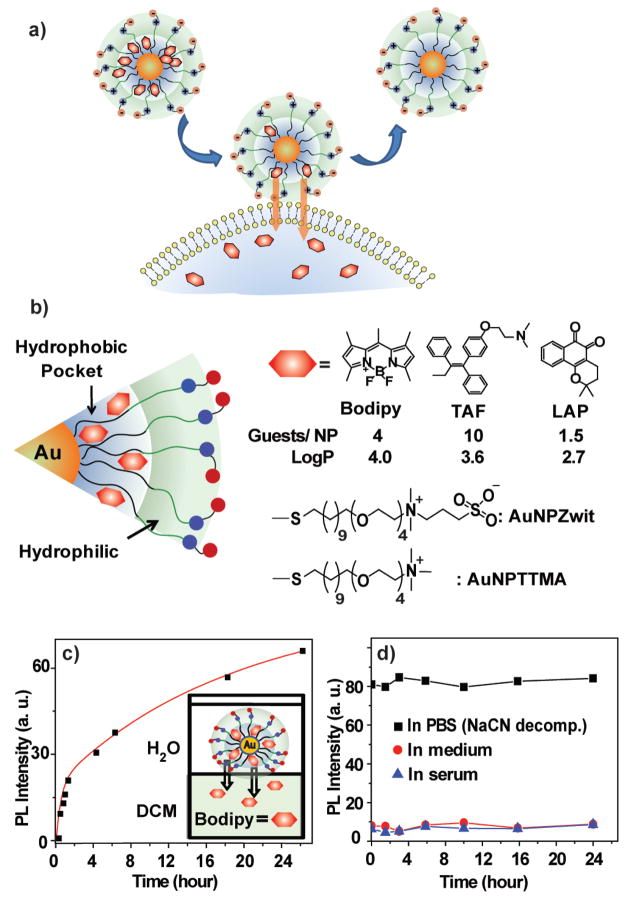
We chose three different hydrophobic guest compounds: 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (Bodipy) as a fluorescent probe,13 and the highly hydrophobic therapeutics tamoxifen (TAF) and β-lapachone (LAP) as drugs (Figure 1). The nanoparticle-payload conjugates (AuNPZwit-Bodipy, TAF, and LAP) were prepared by solvent displacement method.14 First, AuNPZwit (Au core: 2.5 ± 0.4 nm) and guest were dissolved in an acetone/water and the solvent slowly evaporated. The bulk of the excess guest precipitated out and was removed by filtration; the particles were further purified by multiple filtrations through a molecular weight cutoff filter until no free guest was observed, followed by dialysis against buffer. The number of entrapped guest molecules per particle was determined from 1H NMR spectrum and NaCN-induced decomposition experiments (see Supporting Information) and varied depending on size, hydrophobicity (logP), and molecular structure of hydrophobic molecules (Figure 1). The particle/guest complexes are stable in buffer for >1 month and to extended dialysis, a level of kinetic entrapment greater than that observed with dendrimers.4
Figure 1.
a) Delivery of payload to cell through monolayer-membrane interactions. b) Structure of particles and guest compounds: Bodipy, TAF, and LAP, the number of encapsulated guests per particle, and logP of the guests. c) Release of Bodipy from AuNPZwit-Bodipy in DCM-aqueous solution two-phase systems (λex = 499 nm, λem = 517 nm) d) PL intensity AuNPZwit-Bodipy in cell culture medium and 100 % serum, indicating little or no release relative to AuNPZwit-Bodipy in PBS after NaCN-induced release of guest molecules ((λex = 499 nm, λem = 510 nm).
The ability of the delivery systems to release their payload was first explored in vitro using AuNPZwit-Bodipy in a two-phase dichloromethane (DCM)-water system, 15 where the dye is quenched by the AuNP, and photoluminescence (PL) only observed upon dye release. In these studies a rapid increase in PL intensity is observed along with transfer of Bodipy into the DCM layer (Figure 1c). Significantly, since no release is observed in monophasic aqueous conditions and no particle was observed in the DCM layer, payload release occurs interfacially.
Payload delivery to cells using AuNPZwit-Bodipy was determined by confocal laser scanning microscopy (CLSM) using human breast cancer (MCF-7) cells. Efficient delivery of the dye to the cytosol is observed after 2 h incubation with AuNPZwit-Bodipy (Figure 2a–c). Cellular uptake of nanoparticle was studied using transmission electron microscopy (TEM), and inductively coupled plasma mass spectrometry (ICP-MS), using the analogous cationic particle/dye conjugate AuNPTTMA-Bodipy as a positive control. Little cellular uptake of AuNPZwit was observed by either TEM (Figure 2 d,e) or ICP-MS for AuNPZwit-Bodipy (31 ng/well at 4h (Figures 2), 71 ng/well at 24h Figure S3, corresponding to uptake of 0.06% and 0.14% of available particle, respectively), whereas substantial particle uptake was observed with AuNPTTMA-Bodipy (1750 ng/well (4 h), 2150 ng/well (24 h)) Since no free dye was observed during the 24 h incubation of AuNPZwit-Bodipy in medium or serum solution at 37 °C (Figure 1 d), Bodipy delivery presumably occurs via a monolayer-membrane transfer process, consistent with our in vitro studies16
Figure 2.
CLSM images of MCF-7 cell treated with AuNPZwit-Bodipy for 2h: a) green channel b) bright field, and c) overlap. TEM images of fixed cell treated with d) AuNPZwit-Bodipy and e) AuNPTTMA as a positive control, Endosomally trapped AuNPs are marked by arrow. (f) ICP-MS measurement. (200,000 cells/well), indicating low cellular uptake of AuNPZwit (31 ng/well after 4 h)
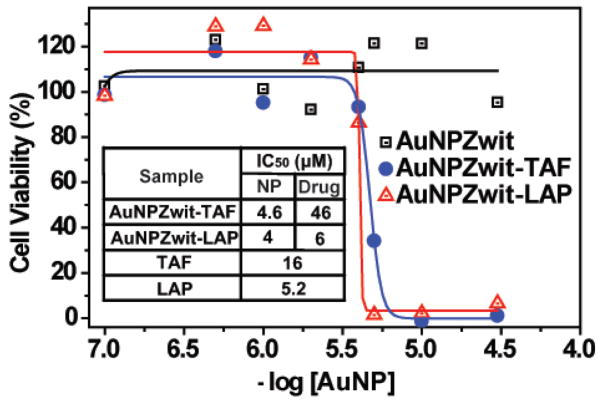
Demonstration of drug delivery to MCF-7 cells through presumably the same mechanism was determined through cytotoxicity studies of free and encapsulated drugs using an Alamar blue assay (Figure 3). Notably, AuNPZwit itself was non-toxic at 30 μM. In contrast, IC50 values of 4 μM and 4.6 μM were observed using AuNPZwit-LAP and AuNPZwit-TAF, respectively. The delivery process was quite efficient, with the per drug molecule IC50 of AuNPZwit-TAF (46 μM) only three-fold higher than that of TAF (16 μM), and that of AuNPZwit-LAP (6.0 μM) essentially identical to that of LAP (5.2 μM).
Figure 3.
Cytotoxicity of AuNPZwit complexes measured by Alamar blue assay after 24h incubation with MCF-7 cells. IC50 of AuNP (NP), equivalent drugs (Drug), and free drugs are shown in table.
In conclusion, we have demonstrated that hydrophobic dyes/drugs can be stably entrapped in hydrophobic pocket of AuNPs and released into cell by membrane-mediated diffusion without uptake of the carrier nanoparticle. Importantly, the small size of these nanocarriers coupled with their biocompatible surface functionality should provide long circulation lifetimes and preferential accumulation in tumor tissues by the enhanced permeability and retention (EPR) effect.17 Additionally, the noninteracting nature of their monolayer should make these systems highly amenable to targeting strategies. We are currently exploring these applications as well as the role of monolayer and guest structure in the encapsulation process.
Supplementary Material
Experimental procedures, synthesis of gold nanoparticles and complexes, and TEM of gold nanoparticles. This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was support by the NIH (GM077173)
References
- 1.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 3.(a) Torchilin VP. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]; (b) Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW. Cancer Res. 2006;66:11913–11921. doi: 10.1158/0008-5472.CAN-06-2066. [DOI] [PubMed] [Google Scholar]
- 5.Templeton AC, Wuelfing MP, Murray RW. Acc Chem Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 6.De M, Ghosh PS, Rotello VM. Adv Mater. 2008;20:4225–4241. [Google Scholar]
- 7.Bhattacharya R, Mukherjee P. Adv Drug Deliver Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 8.(a) Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. J Am Chem Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]; (b) Paciotti GF, Kingston DGI, Tamarkin L. Drug Develop Res. 2006;67:47–54. [Google Scholar]; (c) Gibson JD, Khanal BP, Zubarev ER. J Am Chem Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]; (d) Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv Drug Deliver Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]; (e) Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C. J Am Chem Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crampton HL, Simanek EE. Polymer International. 2007;56:489–496. doi: 10.1002/pi.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hostetler MJ, Stokes JJ, Murray RW. Langmuir. 1996;12:3604–3612. [Google Scholar]
- 11.Lucarini M, Franchi P, Pedulli GF, Gentilini C, Polizzi S, Pengo P, Scrimin P, Pasquato L. J Am Chem Soc. 2005;127:16384–16385. doi: 10.1021/ja0560534. [DOI] [PubMed] [Google Scholar]
- 12.(a) Rouhana LL, Jaber JA, Schlenoff JB. Langmuir. 2007;23:12799–12801. doi: 10.1021/la702151q. [DOI] [PubMed] [Google Scholar]; (b) Jin Q, Xu JP, Ji J, Shen JC. Chem Commun. 2008:3058–3060. doi: 10.1039/b801959b. [DOI] [PubMed] [Google Scholar]
- 13.Loudet A, Burgess K. Chem Rev. 2007;107:4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 14.Jones MC, Leroux JC. Eur J Pharm Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C. J Am Chem Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HT, Kim SW, Li L, Wang SY, Park K, Cheng JX. Proc Natl Acad Sci USA. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Emanuele A, Attwood D. Adv Drug Deliver Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, synthesis of gold nanoparticles and complexes, and TEM of gold nanoparticles. This information is available free of charge via the Internet at http://pubs.acs.org.