Abstract
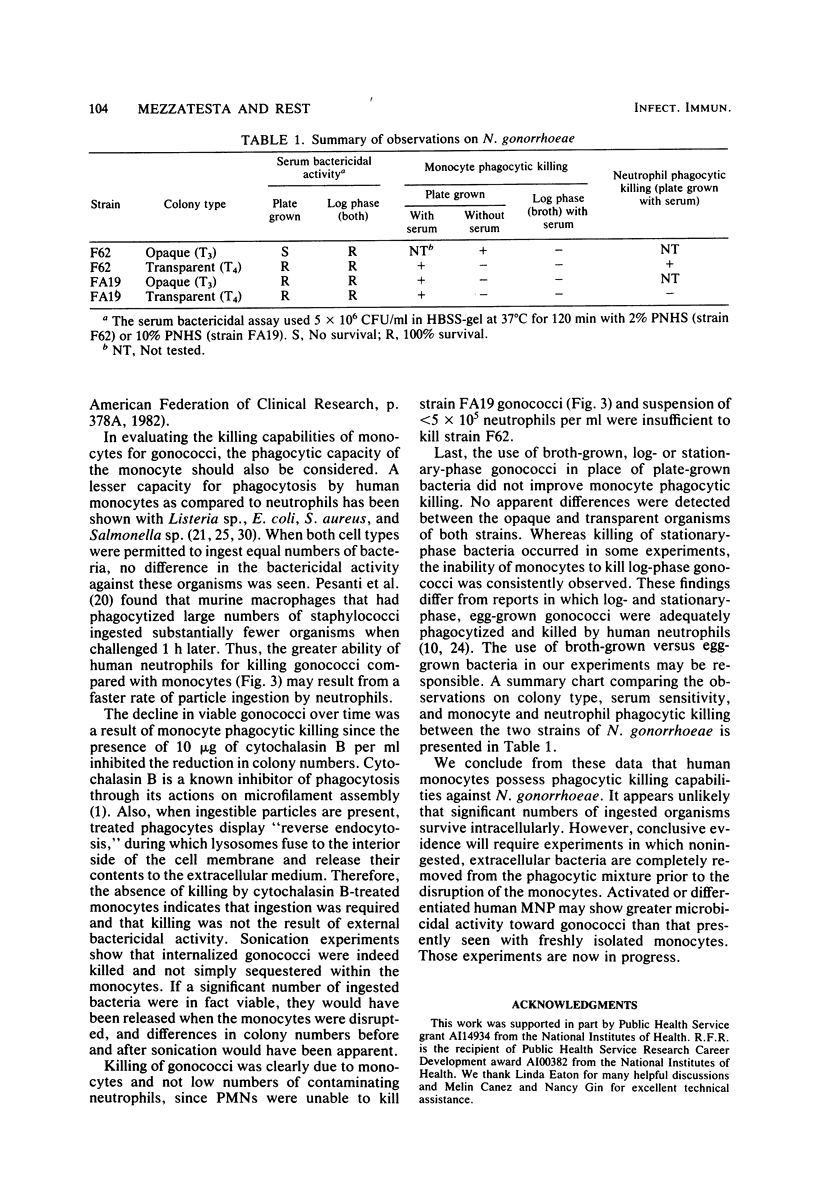
The ability of human monocytes to phagocytize and kill nonpiliated opaque (T3) and transparent (T4) gonococci was investigated in a tumbling tube suspension assay. A serum-sensitive strain, F62, and a serum-resistant strain, FA19, were studied. CFU remaining after incubation with monocytes were used to assess the extent of killing. The data show that 50% of T3 and T4 gonococci of both strains were killed by monocytes over a 2-h period. Serum was necessary for the killing of transparent gonococci of both strains as well as for FA19 T3. Concentrations of serum ranging from 0.5 to 10% were equally effective, and heat-labile components were required. Killing of F62 T3, however, occurred in the absence of serum. An increased ratio of bacteria to monocytes decreased the rate of killing. A 30-min preopsonization of gonococci in 10% serum resulted in an enhanced rate of killing. Monocytes were able to kill plate-grown, but not log-phase, organisms. Disruption of the monocytes by sonication to release internalized bacteria did not increase the number of viable organisms. The addition of 10 micrograms of cytochalasin B per ml completely inhibited the reduction in colony numbers over time. These data indicate that freshly isolated human monocytes are capable of phagocytizing and killing nonpiliated gonococci.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M., Swanson J. Studies on Gonococcus infection. IX. In vitro decreased assocation of pilated gonococci with mouse peritoneal macrophages. Infect Immun. 1975 Jun;11(6):1402–1404. doi: 10.1128/iai.11.6.1402-1404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey S. G., Veale D. R., Smith H. Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J Gen Microbiol. 1979 Aug;113(2):395–398. doi: 10.1099/00221287-113-2-395. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Floyd S. A. In vitro kinetics of phagocytosis and intracellular killing of gonococci by peritoneal macrophages from mice deficient in complement component 5. Infect Immun. 1982 Apr;36(1):363–370. doi: 10.1128/iai.36.1.363-370.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Buchanan T. M. Quantitative measurement of phagocytosis of Neisseria gonorrhoeae by mouse peritoneal macrophages. Infect Immun. 1978 Jun;20(3):732–738. doi: 10.1128/iai.20.3.732-738.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Newland J. C., Olsen D. A., Buchanan T. M. Immune-enhanced phagocytosis of Neisseria gonorrhoeae by macrophages: characterization of the major antigens to which opsonins are directed. J Gen Microbiol. 1980 Dec;121(2):365–372. doi: 10.1099/00221287-121-2-365. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ota F., Morita J., Yoshida N., Ashton F., Diena B. Studies on gonococcal infection. I. Electron microscopic studies on phagocytosis of Neisseria gonorrhoeae by macrophages. Jpn J Microbiol. 1975 Apr;19(2):149–155. doi: 10.1111/j.1348-0421.1975.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Br J Vener Dis. 1971 Dec;47(6):419–439. doi: 10.1136/sti.47.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L., Nugent K. M. Inhibition of macrophage phagocytosis after contact with ingestible particles. J Reticuloendothel Soc. 1981 Sep;30(3):157–166. [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Friedman G. L., Roberts R. B. The role of natural IgG and complement in the phagocytosis of type 4 Neisseria gonorrhoeae by human polymorphonuclear leukocytes. J Infect Dis. 1979 Nov;140(5):698–707. doi: 10.1093/infdis/140.5.698. [DOI] [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale D. R., Finch H., Smith H. Penetration of penicillin into human phagocytes containing Neisseria gonorrhoeae: intracellular survival and growth at optimum concentrations of antibiotic. J Gen Microbiol. 1976 Aug;96(2):353–363. doi: 10.1099/00221287-95-2-353. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K., Veale D. R., Smith H. Resistance of Neisseria gonorrhoeae to ingestion and digestion by phagocytes of human buffy coat. J Med Microbiol. 1976 Feb;9(1):1–12. doi: 10.1099/00222615-9-1-1. [DOI] [PubMed] [Google Scholar]



