Abstract
Very little is known regarding the function, origin, and turnover of airway smooth muscle (ASM). In this article, we discuss the embryological development of ASM, and provide information regarding candidate mesenchymal ASM progenitor cell populations specifically in relation to airway remodeling. This review also highlights the current limitations in studying ASM biology, and underscores the need for novel molecular tools and markers that will refine our understanding of this cell type in lung homeostasis and disease.
Keywords: stem cells, smooth muscle, progenitor cells, fibrocyte, myofibroblast
OVERVIEW
Very little is known regarding the role of stem cells in airway smooth muscle (ASM). While there has been considerable research on the contributions of local proliferation and circulating mesenchymal stem cells to hyperplasia of vascular smooth muscle (VSM) in both pulmonary hypertension and arthrosclerosis, there is no comparable body of work for ASM in diseases such as asthma. One of the greatest difficulties in understanding the role, if any, of stem cells in ASM is the role of ASM itself. To date there is no known “beneficial” or physiologic function to ASM (1).
There are several theories as to the possible ontology of ASM muscle. (1) The rhythmic peristalsis of ASM, in the developing fetal lung generates distending pressures, which promote development and maturation of airways and alveoli (2). (2) ASM may change the resistance of airways in parallel circuits to improve  /
/ matching and or decrease dead space (3). (3) ASM may increase the velocity of gas movement during cough to promote expellation of foreign bodies (4). (4) ASM may serve to balance hysteresis between small airways and alveolar units (5). (5) ASM may simply be an “evolutionary oversight,” that is, a vestige of the lung's origin from an organ, the foregut, already programmed to develop smooth muscle, with no true physiologic purpose.
matching and or decrease dead space (3). (3) ASM may increase the velocity of gas movement during cough to promote expellation of foreign bodies (4). (4) ASM may serve to balance hysteresis between small airways and alveolar units (5). (5) ASM may simply be an “evolutionary oversight,” that is, a vestige of the lung's origin from an organ, the foregut, already programmed to develop smooth muscle, with no true physiologic purpose.
When one attempts to study such a system, one usually begins teleologically, by working backward from its function, to understand the source; in this case, however, this approach is limited. This is further confounded by the lack of specific markers to distinguish ASM from VSM in the lung. While there has been considerable research into the role of stem cells in VSM, when one looks for similar progenitors in ASM, it becomes difficult to tease out whether or not what you are looking at is truly an airway or vascular cell. With these caveats in mind, we can proceed with some basic principles. (1) There is now an emerging understanding of the source and maturation of both local and circulating mesenchymal stem/progenitors cells with the capacity and or proclivity to mature into smooth muscle in vitro (6–10). (2) Asthma is a disease of increased ASM mass, due to both (in varying degrees) hypertrophy and hyperplasia (11–16). (3) In disease states, circulating and local mesenchymal/progenitor cells play a role in VSM accumulation, but related data in the case of asthma is not as forthcoming (10, 17–19).
EMBRYOLOGY
The precise origin of ASM in the developing lung is not known; accumulated data suggest, however, that these cells originate from the primitive embryonic lung mesenchyme. In the mouse, smooth muscle actin–positive cells associated circumferentially with the early trachea and mainstem bronchi can be observed at the earliest stages of lung development (20). This nascent smooth muscle cell compartment is likely already enervated, consistent with observations demonstrating the presence of pulsatile airway contractions in the early fetus (2, 20, 21).
The lack of distinguishing markers has hampered efforts to absolutely clarify the ontological relationship between VSM and ASM, and to elucidate whether these cell types have distinct differentiation programs. Interestingly, the transcription factor GATA-5 is selectively expressed in ASM during late gestation; the significance of this observation has not been further explored (22). Distinct cell differentiation programs and/or cell origins are inferred by the phenotypes of mice that carry select mutations in growth factor signaling molecules. In this regard, hypomorphic FGF-10 mice display selective loss of ASM mass. On the other hand, deletion of Wnt-7b appears to selectively disrupt development of VSM in pulmonary arteries (23, 24).
Since ASM accumulation is ongoing and coupled to branching morphogenesis, it has been suggested that signals originating in the airway epithelium regulate smooth muscle cell recruitment, differentiation, and organization. Consistent with this speculation, deletion of epithelial derived sonic hedgehog is associated with reduced ASM mass (25). Several studies have shown that components of the epithelial basement membrane, particularly laminin-2, may play a role in controlling ASM differentiation by facilitating attachment and spreading of primitive mesenchymal cells (22, 26). These changes in cell shape induce translocation of serum-response factor (SRF) from the cytoplasm to the nucleus (27). Changes in SRF localization have important functional consequences, since SRF is one of the key transcription factors involved in up-regulating expression of smooth muscle–related genes (27). Stretch has also been shown to promote bronchial smooth muscle differentiation by activating an SRF-dependent pathway (27, 28).
ORIGIN OF SMOOTH MUSCLE DURING AIRWAY REMODELING
Asthma is a disease estimated to affect 10% of people in the United States, causing considerable morbidity and mortality. Although inflammation is undoubtedly a cornerstone of the disease, it is clear that structural changes referred to cumulatively as “airway remodeling” contribute to the asthmatic diathesis. This airway remodeling consists of thickening of the airway wall, subepithelial fibrosis, smooth muscle myocyte hyperplasia and hypertrophy, and myofibroblast hyperplasia (29). These processes contribute to airway hyperresponsiveness and are associated with disease severity and decline of lung function (FEV1) (29). The origin of smooth muscle and fibrogenic cells responsible for this pathology is not currently known. In 2001 Johnson and coworkers showed that ASM obtained from endobronchial biopsies in individuals with asthma proliferated much more quickly (∼300%) than did those from healthy control subjects; these findings could be accounted for by expansion of local ASM progenitors or hyperplasia of already differentiated cells (16).
One potential progenitor for ASM is a circulating mesenchymal stem cell (MSC), first identified in outgrowths of adult murine bone marrow in 1976 (30). These cells have historically been isolated by in vitro culturing and expansion on plastic. They are characterized by expression of select surface markers such as Sca-1 (stem cell antigen 1) and by the lack of hematopoietic markers. When placed in appropriate media MSCs can differentiate into cartilage, bone, fat, or smooth muscle (6, 8, 31). In models of skeletal and cardiac injury, such marrow-derived cells have been found to contribute to the reconstitution of damaged muscle (32–34). Myofibroblasts, a cell type that actively produces connective tissue, have also been shown to be marrow derived, in part, in organ injury (35–37). Further, a circulating cell may contribute to smooth muscle cells during the healing phase of vascular wall injury (38).
To date, the study of circulating mesenchymal progenitors has been hampered by the lack of an effective surface marker for their isolation and characterization in the blood and marrow directly, forcing investigators to rely on less reliable techniques such as staining for internal markers and selection of cell types after culturing. A recent publication by Gang and colleagues has shown that the stage-specific early embryonic antigen 4 (SSEA-4) may serve as an effective and easily applied surface marker for MSCs obtained from both human and mouse bone marrow (8). Clonal analysis of CD45− SSEA-4+ cells showed that the majority (∼70%) of these cells had the capacity to differentiate into bone, fat, and cartilage cell types (8). While the authors did not specifically look at the ability of these cells to differentiate into smooth muscle, or characterize them in the peripheral circulation, this begs the question of their potential contribution to ASM accumulation in asthma.
Another potential source of ASM is the circulating fibrocyte, first described in 1994 in an article by Bucala and coworkers wherein they identified a circulating mesenchymal cell that could be cultured from the blood of mice and humans (39). These cells are isolated by plastic-adherent culture of peripheral blood mononuclear cells, express the panhematopoietic marker CD45 and the hematopoietic stem cell antigen CD34, and synthesize collagen-I (39–44). Fibrocytes enter sites of injury localizing to areas of matrix formation in vivo (44). A progenitor phenotype is suggested by their ability to proliferate in culture and to differentiate after injection, or under specific culturing conditions, into α-smooth muscle actin+ (SMA) cells that secrete matrix proteins (40, 45, 46). Whether or not the fibrocyte is the definitive circulating progenitor cell or is merely a subtype of a broader category of mesenchymal progenitor cells is not yet clear. It is important to note, however, that classical mesenchymal progenitor cells do not express hematopoeitic markers.
The fibrogenic potential of fibrocytes and the mechanisms responsible for their recruitment to lung tissue are of interest. One study showed that circulating fibrocytes from humans and mice express the chemokine receptor CXCR4 and migrated in response to its cognate ligand, CXCL12 (47). Notably, CXCR4+ fibrocytes traffic into murine lungs during bleomycin challenge; maximal recruitment directly correlated with increased collagen deposition. Furthermore, a CXCL12-neutralizing antibody inhibited recruitment of fibrocytes and attenuated pulmonary fibrosis, suggesting a pivotal role in the fibrotic response. Further, in an ovalbumin murine model of asthma, infused fibrocytes differentiated into lung myofibroblasts and smooth muscle cells beneath the bronchial epithelium (45). These cells were rapidly recruited to bronchial tissue after allergen exposure and could be re-isolated. Although freshly purified circulating fibrocytes do not express SMA, expression of SMA was induced in cells that engrafted in the bronchial wall. Interestingly, SMA is induced in human fibrocytes when cultured with endothelin-1 (ET-1) and transforming growth factor–β (TGF-B)—two fibrogenic cytokines up-regulated in the airways of patients with asthma (45).
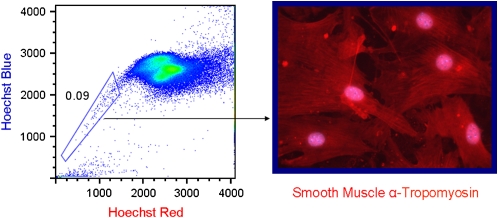
In our own lab we have made use of the vital dye Hoechst 33342, which is preferentially effluxed out of stem cells by the action of the ABC transporter BCRP-1 (breast cancer resistance protein 1) (6, 48–50). When performing flow cytometry on cells stained with Hoechst, one finds a population with little or no dye that has a characteristic fluorescence pattern off to the side (so called side population or SP cells). We found that the lung SP is composed of a heterogeneous population that can be distinguished by the presence or absence of the panhematopoietic marker CD45. The CD45+ lung SP population behaves like hematopoetic stem cells (HSCs), possessing the ability to reconstitute the bone marrow of radio-ablated hosts (49). The CD45− SP has at least two subpopulations: a CD31-positive and -negative population (49). Like marrow MSCs, the lung CD45− CD31− SP cells can give rise to a variety of differentiated mesenchymal cells types in vitro, including smooth muscle (Figure 1) (6). The rarity of these cells, along with the lack of specific markers, has hampered efforts to specifically identify their site(s) of localization in the adult lung. Our data indicate that these cells are resident in the embryonic and adult lung and not likely derived from the blood. Whether these cells contribute to airway remodeling is uncertain.
Figure 1.
Lung side population (SP) cells give rise to smooth muscle. (Left) Density dot plot of Hoechst stained lung digests. The boxed area represents cells (0.09%) that efflux dye (SP cells). (Right) The CD45− CD31− subtype differentiates into α-tropomyosin–positive cells after collection and culture in smooth muscle media. The CD45+ subgroup and the CD45− CD31+ subgroup do not display this capacity.
CONCLUSIONS AND FUTURE DIRECTIONS
Overall, there is a fundamental lack of knowledge regarding the function of ASM in homeostasis, and the source and mechanism of increased ASM mass in disease states. This state of affairs relates directly to the lack of definitive markers that distinguish ASM from VSM in the lung. The identification of such markers would facilitate our understanding of the basic biology and ontogeny of ASM, and the development of meaningful genetic models. Clarifying whether ASM cells originate from a defined progenitor or through expansion of differentiated cells may also become possible if specific markers are available; armed with this understanding, this knowledge would inform and guide new treatment strategies for airway remodeling.
This study was supported by K08 HL077138 (to R.S.) and by R21 HL08277 (to A.F.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Otis AB. A perspective of respiratory mechanics. J Appl Physiol 1983;54:1183–1187. [DOI] [PubMed] [Google Scholar]
- 2.Schittny JC, Miserocchi G, Sparrow MP. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol 2000;23:11–18. [DOI] [PubMed] [Google Scholar]
- 3.Crawford AB, Makowska M, Engel LA. Effect of bronchomotor tone on static mechanical properties of lung and ventilation distribution. J Appl Physiol 1987;63:2278–2285. [DOI] [PubMed] [Google Scholar]
- 4.Seow CY, Fredberg JJ. Historical perspective on airway smooth muscle: the saga of a frustrated cell. J Appl Physiol 2001;91:938–952. [DOI] [PubMed] [Google Scholar]
- 5.Widdicombe, JG. Regulation of tracheobronchial smooth muscle. Physiol Rev 1963;43:1–37. [DOI] [PubMed] [Google Scholar]
- 6.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol 2007;37:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minasi MG, Riminucci M, De AL, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De MR, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 2002;129:2773–2783. [DOI] [PubMed] [Google Scholar]
- 8.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 2007;109:1743–1751. [DOI] [PubMed] [Google Scholar]
- 9.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation 2002;106:1199–1204. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, Saito M, Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol 2004;32:1238–1245. [DOI] [PubMed] [Google Scholar]
- 11.Hossain S, Heard BE. Hyperplasia of bronchial muscle in chronic bronchitis. J Pathol 1970;101:171–184. [DOI] [PubMed] [Google Scholar]
- 12.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis 1993;148:720–726. [DOI] [PubMed] [Google Scholar]
- 13.Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C III, Flavell RA, Elias JA. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest 1996;98:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1990;3:507–511. [DOI] [PubMed] [Google Scholar]
- 15.Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol 1997;16:664–673. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 2001;164:474–477. [DOI] [PubMed] [Google Scholar]
- 17.Forte A, Cipollaro M, Cascino A, Galderisi U. Pathophysiology of stem cells in restenosis. Histol Histopathol 2007;22:547–557. [DOI] [PubMed] [Google Scholar]
- 18.Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med 2001;7:382–383. [DOI] [PubMed] [Google Scholar]
- 19.Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev 2003;13:537–542. [DOI] [PubMed] [Google Scholar]
- 20.Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn 2001;221:48–60. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow MP, Weichselbaum M, McCray PB. Development of the innervation and airway smooth muscle in human fetal lung. Am J Respir Cell Mol Biol 1999;20:550–560. [DOI] [PubMed] [Google Scholar]
- 22.Morrisey EE, Ip HS, Tang Z, Lu MM, Parmacek MS. GATA-5: a transcriptional activator expressed in a novel temporally and spatially-restricted pattern during embryonic development. Dev Biol 1997;183:21–36. [DOI] [PubMed] [Google Scholar]
- 23.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 2005;132:2157–2166. [DOI] [PubMed] [Google Scholar]
- 24.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002;129:4831–4842. [DOI] [PubMed] [Google Scholar]
- 25.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn 2004;231:57–71. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Palmer KC, Relan N, Diglio C, Schuger L. Role of laminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development 1998;125:2621–2629. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest 2000;106:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beqaj S, Jakkaraju S, Mattingly RR, Pan D, Schuger L. High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J Cell Biol 2002;156:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999;104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267–274. [PubMed] [Google Scholar]
- 31.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 2003;89:1235–1249. [DOI] [PubMed] [Google Scholar]
- 32.Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 2003;9:1520–1527. [DOI] [PubMed] [Google Scholar]
- 33.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999;401:390–394. [DOI] [PubMed] [Google Scholar]
- 34.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol 2006;34:219–229. [DOI] [PubMed] [Google Scholar]
- 36.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006;168:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol 2005;167:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med 2001;7:738–741. [DOI] [PubMed] [Google Scholar]
- 39.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 40.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 1998;160:419–425. [PubMed] [Google Scholar]
- 41.Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep 2000;2:501–505. [DOI] [PubMed] [Google Scholar]
- 42.Chesney J, Bucala R. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem Soc Trans 1997;25:520–524. [DOI] [PubMed] [Google Scholar]
- 43.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004;36:598–606. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Howarth PH, Roche WR. Cytokine production by cell cultures from bronchial subepithelial myofibroblasts. J Pathol 1996;180:95–101. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 46.Kimura A, Katoh O, Hyodo H, Kuramoto A. Transforming growth factor-beta regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br J Haematol 1989;72:486–491. [DOI] [PubMed] [Google Scholar]
- 47.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summer R, Kotton DN, Sun X, Fitzsimmons K, Fine A. Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L477–L483. [DOI] [PubMed] [Google Scholar]
- 49.Summer R, Kotton DN, Liang S, Fitzsimmons K, Sun X, Fine A. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol 2005;33:32–40. [DOI] [PubMed] [Google Scholar]
- 50.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 2003;285:L97–L104. [DOI] [PubMed] [Google Scholar]