Abstract
Airway smooth muscle abnormalities are central to the pathophysiology of asthma. These airway smooth muscle cell abnormalities may include changes in cell number, size, phenotype, or function. Gene expression studies performed using asthmatic airway smooth muscle cells represent one approach to identifying the abnormalities of airway smooth muscle that occur in asthma in vivo. However, due to the technical challenges involved, only two studies have been performed to date using freshly obtained tissue from subjects with asthma. The first of these studies suggested increased expression of myosin light-chain kinase in airway smooth muscle from patients with asthma, whereas the second study found no difference in myosin light-chain kinase expression, nor any difference in other markers of smooth muscle phenotype in asthma. Studies performed in cell culture through the application of gene expression microarrays to profile airway smooth muscle cells exposed to potential mediators of asthma yield more consistent results, including induction by IL-13 of tenascin, the H1 histamine receptor, and IL-13 receptor subunits. However, the significance of these microarray findings for smooth muscle function is uncertain. Furthermore, gene expression studies have a fundamental limitation in that many functional properties of airway smooth muscle are regulated at other levels (e.g., protein phosphorylation). Thus, gene expression studies ultimately must be integrated with other methodological approaches to adequately study airway smooth muscle in asthma in vivo.
Keywords: airway smooth muscle; gene expression, microarray; polymerase chain reaction
Asthma is characterized by an exaggerated response of airway smooth muscle to constrictor agonists, which is manifest both in the dose required to initiate bronchial constriction and in the degree of bronchial constriction that results from any given dose of agonist. These exaggerated responses are a central mechanism of airway obstruction in asthma and are thought to be due to some combination of increases in the amount of smooth muscle in the airway (due to increases in cell number and/or cell size) and functional abnormalities of those smooth muscle cells (increased contractility and/or decreased relaxation). In addition, airway smooth muscle may contribute to asthma through the production of mediators that amplify the inflammatory response. One goal of studying gene expression in asthmatic airway smooth muscle cells is to distinguish which of these pathophysiological processes actually occurs in asthma to guide therapeutic approaches to reversing smooth muscle dysfunction in asthma. This review focuses on gene expression studies performed using airway smooth muscle cells that were designed to identify the pathophysiological processes that occur in asthma, either by testing preexisting hypotheses regarding airway smooth muscle dysfunction in asthma, or through genome-wide analysis of gene expression using microarrays.
HYPOTHESES REGARDING AIRWAY SMOOTH MUSCLE DYSFUNCTION IN ASTHMA THAT MAY BE TESTED IN GENE EXPRESSION STUDIES
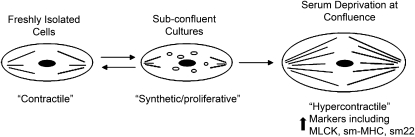
One hypothesis regarding airway smooth muscle dysfunction in asthma is that these cells have an altered phenotype, which contributes to either increased proliferative capacity or to hypercontractility. Smooth muscle cells are not terminally differentiated and, in culture, demonstrate remarkable plasticity of phenotype. In subconfluent cell culture, freshly isolated cells will “dedifferentiate” and acquire characteristics of a synthetic cell while proliferating (a “synthetic/proliferative” phenotype) (1). Even when confluence is achieved, smooth muscle cells in culture do not fully revert to the contractile state observed in fresh samples. However, if deprived of serum, these cells may “redifferentiate” and acquire hypercontractile characteristics (2) (Figure 1). A hypercontractile phenotype may also be produced in culture through transduction of temperature-sensitive simian virus 40 large tumor antigen and growth arrest at 39°C (3). Phenotypic plasticity of smooth muscle cells has been demonstrated in the vascular wall in atherosclerosis (4, 5). Whether similar phenotypic plasticity of smooth muscle occurs in the airway or is associated with smooth muscle changes in asthma is unknown, but advances in vascular biology have spurred interest in this possibility (6). Because there is no single discriminating marker that is indicative of synthetic or hypercontractile smooth muscle cell phenotypes, classification is based on ultrastructural changes or the pattern of expression of a repertoire of structural and contractile proteins (1). In freshly isolated contractile airway smooth muscle cells, smooth muscle–specific myosin heavy chain (MHC) isoforms (slow myosin heavy chain-1 [SM-1] and SM-2) predominate over nonmuscle MHC isoforms and h-caldesmon predominates over l-caldesmon. With acquisition of the synthetic phenotype in vitro, nonmuscle MHC isoforms and l-caldesmon are preferentially expressed. Other changes that accompany acquisition of the synthetic phenotype include decreased expression of α-smooth muscle actin (α-SMA), M-calponin, SM-22, and preferential expression of the intermediate filament, vimentin, rather than desmin. With serum deprivation, differentiation to the hypercontractile phenotype is associated with increased expression of smooth muscle–specific MHC isoforms (2, 7), α-sm actin (2), and SM-22 (7), as well as increased expression of the regulatory protein, myosin light chain (MLC) kinase (MLCK) (2). Whether airway smooth muscle cells in asthma acquire either a synthetic–proliferative phenotype (accompanied by increased smooth muscle cell proliferation) or a hypercontractile phenotype (accompanied by an increased capacity for force generation or increased velocity of shortening) is uncertain, and has been the subject of gene expression studies performed using freshly isolated airway smooth muscle cells, as described subsequently here.
Figure 1.
Airway smooth muscle phenotypic plasticity may contribute to either increased proliferative capacity or to hypercontractility. Whether phenotypic plasticity occurs in the airway in asthma is unknown, but expression of phenotypic markers, such as myosin light-chain kinase, smooth muscle–specific myosin heavy-chain isoforms, and SM-22 (smooth muscle 22-kD protein), has been measured in gene expression studies. MLCK = myosin light-chain kinase; SM = smooth muscle; SM-MHC = SM–specific myosin heavy-chain.
A second hypothesis regarding airway smooth muscle dysfunction in asthma is that these cells may express a smooth muscle MHC transcript variant that is thought to increase the velocity of shortening of these cells. There are four known isoforms of smooth muscle MHC formed through two independent, alternative splicing events of a single gene (8). In the 5′ region of the gene (exon 5b), differential splicing either results in the presence (+ insert) or absence (− insert) of a seven–amino acid sequence (9). Isoforms omitting the insert are denoted by “A,” and isoforms containing the insert are denoted by “B.” Near the 3′ terminus of the gene, inclusion of exon 41, encoding a stop codon, generates a foreshortened isoform, denoted SM-2 (10) to distinguish it from SM-1. Thus, the four known isoforms of smooth muscle MHC are the four possible transcripts produced by these independent splicing events, and are denoted SM-1A, SM-1B, SM-2A, and SM-2B. The exon 5b insert involves the ATP pocket of the smooth muscle MHC head region, and is thought to affect mechanical performance (11), increasing the velocity of contraction. The SM-B isoform expression is more prominent in smooth muscle with higher rates of shortening in animals (9), and SM-B is also expressed in human trachea (12). Whether expression of SM-B isoforms is increased in human asthma is uncertain.
A third hypothesis regarding airway smooth muscle dysfunction in asthma is that these cells contribute to the inflammatory environment through the production of proinflammatory mediators (13, 14). According to this hypothesis, airway smooth muscle cells do not simply regulate airway tone, but contribute to the regulation and perpetuation of asthmatic airway inflammation.
REGULATION OF GENE EXPRESSION IN AIRWAY SMOOTH MUSCLE
In general, gene expression is only one of many levels at which protein expression may be regulated in the cell to influence cell phenotype or function. Because many of the functional properties of airway smooth muscle are regulated at the level of protein phosphorylation, gene expression analyses have fundamental limitations in the study of airway smooth muscle dysfunction in asthma. Nonetheless, specific transcription factors have been found to play seminal roles in the expression of smooth muscle contractile proteins (15), and may play a role in regulating phenotypic modulation. One such transcription factor is serum response factor (SRF). The majority of smooth muscle–specific genes contain at least one CArG (CC(AT)6GG) box in their promoter regions that binds SRF (15, 16). Although SRF is a ubiquitously expressed MADS box transcription factor, smooth muscle cell–type restricted function is thought to be regulated by the binding of specific cofactors, such as myocardin family members (17), and forced expression of myocardin can direct the expression of many (but not all) smooth muscle–specific genes (18). Another transcription factor family relevant to smooth muscle changes in asthma is the transforming growth factor-β (TGF-β) family. TGF-β activation is known to occur in asthma, and is thought to contribute to airway remodeling (19, 20). Many smooth muscle cell–specific genes, including α-sm actin and SM-22α, contain a TGF-β control element and are induced by TGF-β. TGF-β also influences the expression of smooth muscle cell–specific genes through direct and indirect effects on SRF (21). Thus, both SRF and TGF-β are transcription factors potentially important in smooth muscle cell differentiation and in the accumulation of structural and contractile proteins in smooth muscle cells. Other families of transcription factors are also involved in the embryological development of airway smooth muscle (22) and in the expression of contractile proteins (15), but SRF and TGF-β are likely to be central to airway smooth muscle cell differentiation in the adult and in disease states. Finally, CCAAT/enhancer binding protein α (C/EBPα) is a transcription factor that can regulate rate of smooth muscle proliferation through induction of the cell cycle inhibitor, p21waf1/cip1. Investigations using airway smooth muscle obtained from patients with asthma and healthy control subjects have demonstrated decreased expression of C/EBPα in asthma and decreased responsiveness to the antiproliferative effects of glucocorticoids on these cells (23). Interestingly, C/EBPα could also play a role in proinflammatory cytokine production and in MLCK expression (23, 24). However, C/EBPα is thought to be regulated at the level of mRNA translation (24) and, thus, represents another potential mechanism of airway smooth muscle cell dysfunction that would be difficult to examine directly using gene expression profiling.
OVERVIEW OF GENE EXPRESSION STUDIES
To date, gene expression studies performed using airway smooth muscle freshly isolated from human subjects with asthma have been fairly limited in number and in scope, examining a small number of genes that are thought to be markers of phenotypic modulation (25, 26), genes that are known to regulate smooth muscle contraction (MLCK), or the smooth muscle MHC transcript variant, which is thought to increase the velocity of smooth muscle shortening (25). Other studies have taken the approach of modeling the changes that may occur in asthma by using cultured smooth muscle cells exposed to potential mediators of asthma (27–30). These studies have not definitively established the pathophysiologic changes in airway smooth muscle in asthma, but have provided some insights that are reviewed here. Indeed, it is possible that gene expression studies, per se, cannot definitively establish the pathophysiologic changes that occur in asthma if those changes are due to post-transcriptional regulatory events (3, 21, 31, 32) or to physiological processes that are regulated by intracellular signaling pathways, Ca2+ handling, or enzymatic activity. These potential limitations of the use of gene expression to study the phenotype of asthmatic airway smooth muscle must be borne in mind.
GENE EXPRESSION STUDIES USING FRESHLY ISOLATED ASTHMATIC SMOOTH MUSCLE
At least two studies have examined gene expression in airway smooth muscle cells isolated from bronchial biopsies obtained from subjects with asthma and healthy control subjects (Table 1). Ma and colleagues (25) studied the contractility of airway smooth cells isolated from bronchial biopsies by enzymatic digestion, and the expression of smooth muscle MLCK and smooth muscle MHC isoforms by reverse transcription–polymerase chain reaction (RT-PCR) in either isolated smooth muscle cells or bronchial biopsies. Single cells isolated from five subjects with asthma showed increased maximal shortening capacity and velocity to electrical field stimulation as compared with cells from five healthy control subjects. In accompanying experiments, mRNA was isolated from either dispersed airway smooth muscle cells or from whole bronchial biopsies, and RT-PCR was performed. The authors found increased expression of smooth muscle MLCK in 7 subjects with asthma as compared with 11 healthy control subjects when normalized to glyceraldehyde-3-phosphate dehydrogenase (a housekeeping gene expressed by all cells), but no differences in smooth muscle MHC expression. In these analyses, only the SM-A isoform of smooth muscle MHC was detected. SM-B, the isoform associated with increased velocity of smooth muscle contraction, was not detected, either in patients with asthma or in healthy control subjects. To account for the possibility of increased numbers of smooth muscle cells in asthmatic bronchial biopsies (because their analyses used biopsy homogenates with a mixed cell population, in some instances), the authors also normalized gel loading to smooth muscle MHC expression, and again found increased expression of smooth muscle MLCK in asthma. Overall, their findings are reminiscent of a previous study performed using sensitized canine airway smooth muscle strips, which showed increased contractility and increased protein expression of smooth muscle MLCK (33), and provide one possible mechanism for increased airway smooth muscle contractility in asthma: increased expression of smooth muscle MLCK. Conceivably, increased smooth muscle MLCK expression could also be consistent with a hypercontractile airway smooth muscle phenotype. The absence of SM-B expression in their sample argues against this transcript variant as a cause of hypercontractility in asthma; however, other investigators have presented preliminary data in abstract form in support of this alternative mechanism (34).
TABLE 1.
GENE EXPRESSION STUDIES USING FRESHLY ISOLATED ASTHMATIC SMOOTH MUSCLE
| Author (Reference No.) | Subjects Studied | Method of Isolation | Method of Gene Expression Profiling (Reference No.) | Findings |
|---|---|---|---|---|
| Ma and coworkers (25) | 7 Patients with asthma | Enzymatic dispersion (or whole bronchial biopsies) | RT-PCR | Increased expression of smooth muscle MLCK |
| 11 Healthy control subjects | SM-A was present but no SM-B was detected | |||
| Woodruff and coworkers (26) | 11 Patients with asthma | Laser capture microdissection from bronchial biopsies | Two-step real-time PCR (35) | No differences in the expression of MLCK, SM-MHC, SM22, calponin, caldesmon |
| 8 Healthy control subjects |
Definition of abbreviations: MHC = myosin heavy chain; MLCK = myosin light-chain kinase; RT-PCR = reverse transcription–polymerase chain reaction; SM = smooth muscle.
Our laboratory has studied the morphological characteristics of airway smooth muscle in bronchial biopsies using design-based stereology and the expression of a panel of genes related to the phenotypic modulation of airway smooth muscle using laser microdissection (26) and two-step, real-time PCR (35). We found that airway smooth muscle cell size was similar in both groups, but cell number was nearly twofold higher in subjects with asthma (P = 0.03), and the amount of smooth muscle in the submucosa was increased 50–83% (P < 0.005). Gene expression profiling in smooth muscle cells showed no difference in the expression of six genes coding for phenotypic markers in cells (including smooth muscle MLCK and the SM-1 isoform of smooth muscle MHC) from 11 subjects with asthma and 8 healthy control subjects. These data suggested that submucosal airway smooth muscle content in asthma is increased due to an accumulation of airway smooth muscle cells, but that these cells do not show evidence of phenotypic modulation in vivo. One important caveat of this study is that enrolled subjects had mild to moderate asthma, and these findings may not apply to patients with more severe disease. Our findings differed from those of Ma and colleagues (25) in that we did not find increased expression of smooth muscle MLCK. The factors that may account for this disparity are not immediately apparent, although many of the methods applied were different (e.g., the use of dispersed cells or biopsy homogenates vs. laser capture–microdissected tissue, and the use of RT-PCR vs. real-time PCR with preamplification). Increased smooth muscle MLCK expression in asthmatic airway smooth muscle remains an attractive possible explanation for airway hyperresponsiveness in asthma, in part because airway smooth muscle from ragweed pollen–sensitized dogs shows increased smooth muscle MLCK content accompanied by increased phosphorylation of the regulatory MLC20 (33).
GENE EXPRESSION STUDIES USING CULTURED AIRWAY SMOOTH MUSCLE CELLS
A significant body of literature in the past 10 years indicates that asthma is associated with a T-helper type 2–skewed pattern of airway inflammation (36–38) in which IL-4, IL-5, and IL-13 (and, more recently, IL-25) play a pivotal role. This inflammation is accompanied by structural changes in the airway wall (known as airway remodeling), which are thought to be the result of stimulation by locally produced growth factors, including TGF-β (39), epidermal growth factor receptor ligands (40), connective tissue growth factor (41), and many others. Based on these data, several investigators have applied relevant cytokines and growth factors to cultured airway smooth muscle cells in an effort to model the smooth muscle changes that one may observe in the asthmatic airway and perform genome-wide analyses of smooth muscle cell gene expression using microarrays (27, 28, 30) (Table 2). More recently, microarrays have been applied to study asthma therapy by investigating the effects of β-agonists on gene expression in cultured airway smooth muscle cells (29).
TABLE 2.
GENE EXPRESSION STUDIES USING CULTURED AIRWAY SMOOTH MUSCLE CELLS
| Author (Reference No.) | Source of Cells | Exposures | Method of Gene Expression Profiling | Findings |
|---|---|---|---|---|
| Lee and coworkers (28) | Commercially available human airway smooth muscle cells | IL-13 (100 ng/ml) | Affymetrix Genechip HuGeneFL arrays coding for ∼6,500 human genes | 204 Genes induced two-fold, including smooth muscle MHC, phospholipase A2, diacyglycerol kinase δ, and IL-13Rα1 |
| Syed and coworkers (30) | 3 Lung transplant donors | IL-13 (10–100 ng/ml) | Research Genetics and Incyte Genomics cDNA microarrays coding for 8,159 genes | A large number of genes induced 1.5-fold or by FDR analyses (total no. not given), including VCAM1, IL-13Rα2, Tenascin C, and histamine receptor H1 |
| IL-13R130Q* | ||||
| Jarai and coworkers (27) | 2 Patients undergoing lung resection | IL-13† | Affymetrix HG-U95Av2 microarrays coding for ∼12,500 genes | H1 receptor and tenascin were highly induced by IL-13 |
| TGF-β† | ||||
| IL-1β† | ||||
| McGraw and coworkers (29) | Wild-type and transgenic mice overexpressing β2-adrenergic receptor | None | University of Cincinnati Genomic and Microarray Laboratory array coding for 8,734 cDNAs | 319 Genes induced |
| 164 Repressed (including phospholamban) |
Definition of abbreviations: FDR = false discovery rate; IL-13Rα1 = IL-13 receptor α1; MHC = myosin heavy chain; RT-PCR = reverse transcription–polymerase chain reaction; TGF = transforming growth factor; VCAM = vascular cell adhesion molecule.
A naturally occurring isoform of IL-13.
All at a dose of 10 ng/ml.
The first of these studies was performed by Lee and colleagues (28) and examined the effect of exposure of commercially available human airway smooth muscle cells, airway epithelial cells, and lung fibroblasts (Clonetics, San Diego, CA) to recombinant IL-13 (100 ng/ml) for 6 hours using Affymetrix gene expression microarrays (Genechip HuGene FL array; Santa Clara, CA). Using twofold induction in at least three of four culture dishes as criteria, a greater number of genes were induced in airway smooth muscle cells as compared with the other lung cell types, including smooth muscle MHC (3.6-fold), phospholipase A2, diacyglycerol kinase δ, components of the mitogen-activated protein kinase signaling pathways, and IL-13 receptor α (IL-13Rα) 1 (which could represent a positive feedback loop for IL-13 signaling in these cells). There was little overlap in the genes induced across the three cell types. This study established that airway smooth muscle cells have the capacity to respond to direct stimulation by IL-13, and that IL-13 can influence the expression of a wide variety of genes in a cell-type–dependent manner.
Syed and colleagues (30) used human airway smooth muscle cells enzymatically dissociated from the trachea of three human lung transplant donors and exposed them to IL-13 (10–100 ng/ml) for 6 and 18 hours at passage 2 through 5 (passages at which these cells retained native contractile protein expression). Total cellular mRNA was isolated and analyzed using single color cDNA microarrays coding for 8,159 genes from Research Genetics (IMAGE consortium, Huntsville, AL) and Incyte Genomics (Santa Clara, CA). Duplicate chips were used for each RNA sample. Analyses were performed using analyis of variance and Benjamini–Hochberg false discovery rate methods to assign statistical significance while adjusting for multiple comparisons and, alternatively, using 1.5-fold induction as a cut off (as in Lee and colleagues [28]). Syed and colleagues also exposed cells to a naturally occurring isoform of IL-13 (IL-13R130Q), and found that IL-13 and IL-13R130Q induced the same set of genes in smooth muscle cells, and combined these data in their analyses. Induced genes included genes potentially important in airway inflammation, airway remodeling, and bronchial hyperresponsiveness. The authors highlighted increased expression of vascular cell adhesion molecule-1 (twofold), IL-13Rα2 (1.6-fold), tenascin C (twofold), and the histamine H1 receptor (1.3-fold), which were validated by TaqMan PCR (for all four genes) and flow cytometry (for vascular cell adhesion molecule-1 and IL-13Rα2). This study again demonstrated that airway smooth muscle cells have the capacity to respond to direct stimulation by IL-13, and suggested some potentially interesting possible mediators of asthma. In addition, both Lee and colleagues (28) and Syed and colleagues (30) identified induction of IL-13 receptor subunits (albeit different subunits), suggesting intact feedback mechanisms for IL-13 signaling in smooth muscle cells.
In a third study, Jarai and colleagues (27) used airway smooth muscle cells enzymatically digested from surgical specimens from two patients undergoing lung resection, and exposed them to IL-13, TGF-β, or IL-1β at a dose of 10 ng/ml each for 4 and 24 hours at passage 5. Total cellular RNA was isolated and analyzed using HG-U95Av2 microarrays from Affymetrix. Duplicate chips were used for each RNA sample. Analyses were performed using fold-induction values (> twofold for induction and > threefold for repression). IL-1β had the strongest overall effect on gene expression, and increased the expression of chemokines and cytokines, which may contribute to the inflammatory milieu of asthma. TGF-1β had the next-strongest effect, and stimulated the expression of genes coding for growth factors, structural and extracellular matrix proteins, and enzymes. IL-13 had the weakest effect, and many of the genes induced are poorly characterized. However, among the genes most highly induced by IL-13 were two relevant chemokines (eotaxin and monocyte chemotactic protein-1 [MCP-1]). In addition, the histamine H1 receptor and tenascin were also highly induced by IL-13 in this study (as they were in the study by Syed and colleagues [30]). Indeed, the histamine H1 receptor is known to mediate smooth muscle contraction using the same pathways mediated by muscarnic receptors (although antihistamines have long since fallen out of favor in asthma therapy). Taken together, a comparison of the data presented by these three cell culture articles yields some interesting overlap with regard to the induction of two genes potentially important in asthma: histamine H1 receptor and tenascin. These studies also provide additional evidence that airway smooth muscle cells are capable of producing proinflammatory cytokines and chemokines in cell culture. However, whether the production of proinflammatory mediators by airway smooth muscle contributes significantly to the asthmatic inflammatory milieu in vivo remains uncertain.
Finally, one recent article used airway smooth muscle cells in culture to examine the effects of β2-adrenergic receptor agonism using gene expression microarrays (29). Airway smooth muscle cells were derived from wild-type and transgenic mice overexpressing β2-adrenergic receptor, total cellular mRNA was isolated and analyzed using microarray slides manufactured by the Genomic and Microarray Laboratory (Center for Environmental Genetics, University of Cincinnati, Cincinnati, OH), which code for 8,734 cDNAs. A large number of genes were differentially expressed (319 genes were increased and 164 were decreased), including phospholamban (an intracellular Ca2+ handling protein), which showed a 60% decrease in β2-adrenergic receptor overexpressing mice (P = 0.008). This finding was confirmed at the protein level, and the physiological effect of decreased phospholamban expression in airway smooth muscle contraction and relaxation responses was studied in phospholamban−/− mice. These mice had a markedly reduced constrictive response to methacholine without a reduction in the bronchodilatory effect of β2 agonists. These results suggest that inhibition of phospholamban may act synergistically with the bronchodilating action of β2 agonists, and represent an additional target for asthma therapy.
CONCLUSIONS
Thus far, only a small number of studies have examined gene expression in airway smooth muscle in vivo, primarily because of the technical obstacles to obtaining adequate tissue, isolating airway smooth muscle from these samples, and measuring gene expression using the small RNA samples that result. Furthermore, results of the studies that have been performed are somewhat conflicting, although increased expression of smooth muscle MLCK in asthma remains an intriguing possibility. Other studies have sought to model the asthmatic milieu in vitro by exposing airway smooth muscle cells in culture to relevant mediators and performed non–hypothesis-directed studies using gene expression microarrays. These studies manifest some modest overlap in the expression of IL-13–induced genes, including the histamine H1 receptor, tenascin, proinflammatory mediators, and receptors involved in IL-13 signaling. However, these studies share the limitation that airway smooth muscle cells in culture may not adequately model airway smooth muscle in vivo due to important effects of the local environment on smooth muscle cell differentiation and function. Furthermore, all studies that use gene expression to test hypotheses regarding the nature of smooth muscle dysfunction in asthma share the important limitation that the relevant aspects of smooth muscle function may be regulated at the level of translation, or by physiological processes, such as protein phosphorylation, intracellular signaling pathways, Ca2+ handling, or enzymatic activity. Thus, gene expression studies comprise only one of several approaches that must be integrated in the study of airway smooth muscle in asthma. The daunting challenge in all of these approaches remains establishing which pathophysiological process actually occur in the human lung.
Supported by National Center for Research Resources, National Institutes of Health, grant K23 RR17002.
Conflict of Interest Statement: P.G.W. has received a grant from Genentech Inc. in the amount of $350,000/year (for the years 2007–2011) for asthma-related research.
References
- 1.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol 1996;270:L1040–L1051. [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Wang Y, Stephens NL. Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am J Physiol 1998;274:C1206–C1214. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, Li J, Goldsmith AM, Newcomb DC, Giannola DM, Vosk RG, Eves EM, Rosner MR, Solway J, Hershenson MB. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med 2004;169:703–711. [DOI] [PubMed] [Google Scholar]
- 4.Babaev VR, Bobryshev YV, Stenina OV, Tararak EM, Gabbiani G. Heterogeneity of smooth muscle cells in atheromatous plaque of human aorta. Am J Pathol 1990;136:1031–1042. [PMC free article] [PubMed] [Google Scholar]
- 5.Frid MG, Dempsey EC, Durmowicz AG, Stenmark KR. Smooth muscle cell heterogeneity in pulmonary and systemic vessels: importance in vascular disease. Arterioscler Thromb Vasc Biol 1997;17:1203–1209. [DOI] [PubMed] [Google Scholar]
- 6.Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol 2001;90:358–368. [DOI] [PubMed] [Google Scholar]
- 7.Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, Hershenson MB, Solway J. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol 1999;276:L197–L206. [DOI] [PubMed] [Google Scholar]
- 8.Low R, Leguillette R, Lauzon AM. (+)Insert smooth muscle myosin heavy chain (SM-B): from single molecule to human. Int J Biochem Cell Biol 2006;38:1862–1874. [DOI] [PubMed] [Google Scholar]
- 9.White S, Martin AF, Periasamy M. Identification of a novel smooth muscle myosin heavy chain cDNA: isoform diversity in the S1 head region. Am J Physiol 1993;264:C1252–C1258. [DOI] [PubMed] [Google Scholar]
- 10.Babij P, Periasamy M. Myosin heavy chain isoform diversity in smooth muscle is produced by differential RNA processing. J Mol Biol 1989;210:673–679. [DOI] [PubMed] [Google Scholar]
- 11.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 1993;268:12848–12854. [PubMed] [Google Scholar]
- 12.Leguillette R, Gil FR, Zitouni N, Lajoie-Kadoch S, Sobieszek A, Lauzon AM. (+)Insert smooth muscle myosin heavy chain (SM-B) isoform expression in human tissues. Am J Physiol Cell Physiol 2005;289:C1277–C1285. [DOI] [PubMed] [Google Scholar]
- 13.Lazaar AL, Panettieri RA Jr. Airway smooth muscle as a regulator of immune responses and bronchomotor tone. Clin Chest Med 2006;27:53–69. [DOI] [PubMed] [Google Scholar]
- 14.McKay S, Sharma HS. Autocrine regulation of asthmatic airway inflammation: role of airway smooth muscle. Respir Res 2002;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solway J, Forsythe SM, Halayko AJ, Vieira JE, Hershenson MB, Camoretti-Mercado B. Transcriptional regulation of smooth muscle contractile apparatus expression. Am J Respir Crit Care Med 1998;158:S100–S108. [DOI] [PubMed] [Google Scholar]
- 16.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 2003;35:577–593. [DOI] [PubMed] [Google Scholar]
- 17.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2006;116:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, Kawai-Kowase K, Owens GK. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol 2004;24:1596–1601. [DOI] [PubMed] [Google Scholar]
- 19.Postma DS, Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:434–439. [DOI] [PubMed] [Google Scholar]
- 20.Howell JE, McAnulty RJ. TGF-β: its role in asthma and therapeutic potential. Curr Drug Targets 2006;7:547–565. [DOI] [PubMed] [Google Scholar]
- 21.Camoretti-Mercado B, Fernandes DJ, Dewundara S, Churchill J, Ma L, Kogut PC, McConville JF, Parmacek MS, Solway J. Inhibition of transforming growth factor β-enhanced serum response factor–dependent transcription by SMAD7. J Biol Chem 2006;281:20383–20392. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes DJ, McConville JF, Stewart AG, Kalinichenko V, Solway J. Can we differentiate between airway and vascular smooth muscle? Clin Exp Pharmacol Physiol 2004;31:805–810. [DOI] [PubMed] [Google Scholar]
- 23.Roth M, Johnson PR, Borger P, Bihl MP, Rudiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, et al. Dysfunctional interaction of C/EBPα and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med 2004;351:560–574. [DOI] [PubMed] [Google Scholar]
- 24.Borger P, Tamm M, Black JL, Roth M. Asthma: is it due to an abnormal airway smooth muscle cell? Am J Respir Crit Care Med 2006;174:367–372. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol 2002;283:L1181–L1189. [DOI] [PubMed] [Google Scholar]
- 26.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med 2004;169:1001–1006. [DOI] [PubMed] [Google Scholar]
- 27.Jarai G, Sukkar M, Garrett S, Duroudier N, Westwick J, Adcock I, Chung KF. Effects of interleukin-1β, interleukin-13 and transforming growth factor-β on gene expression in human airway smooth muscle using gene microarrays. Eur J Pharmacol 2004;497:255–265. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol 2001;25:474–485. [DOI] [PubMed] [Google Scholar]
- 29.McGraw DW, Fogel KM, Kong S, Litonjua AA, Kranias EG, Aronow BJ, Liggett SB. Transcriptional response to persistent β2-adrenergic receptor signaling reveals regulation of phospholamban, which alters airway contractility. Physiol Genomics 2006;27:171–177. [DOI] [PubMed] [Google Scholar]
- 30.Syed F, Panettieri RA Jr, Tliba O, Huang C, Li K, Bracht M, Amegadzie B, Griswold D, Li L, Amrani Y. The effect of IL-13 and IL-13R130Q, a naturally occurring IL-13 polymorphism, on the gene expression of human airway smooth muscle cells. Respir Res 2005;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camoretti-Mercado B, Liu HW, Halayko AJ, Forsythe SM, Kyle JW, Li B, Fu Y, McConville J, Kogut P, Vieira JE, et al. Physiological control of smooth muscle–specific gene expression through regulated nuclear translocation of serum response factor. J Biol Chem 2000;275:30387–30393. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith AM, Hershenson MB, Wolbert MP, Bentley JK. Regulation of airway smooth muscle α-actin expression by glucocorticoids. Am J Physiol Lung Cell Mol Physiol 2007;292:L99–L106. [DOI] [PubMed] [Google Scholar]
- 33.Kong SK, Halayko AJ, Stephens NL. Increased myosin phosphorylation in sensitized canine tracheal smooth muscle. Am J Physiol 1990;259:L53–L56. [DOI] [PubMed] [Google Scholar]
- 34.Leguillette R, Laviolette M, Lauzon AM. Quantitative smooth muscle RNA analysis of human bronchial biopsies. Proc Am Thorac Soc 2005;2:A338. (abstract). [Google Scholar]
- 35.Dolganov GM, Woodruff PG, Novikov AA, Zhang Y, Ferrando RE, Szubin R, Fahy JV. A novel method of gene transcript profiling in airway biopsy homogenates reveals increased expression of a Na+ K+ Cl− cotransporter (NKCC1) in asthmatic subjects. Genome Res 2001;11:1473–1483. [DOI] [PubMed] [Google Scholar]
- 36.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humbert M, Durham SR, Kimmitt P, Powell N, Assoufi B, Pfister R, Menz G, Kay AB, Corrigan CJ. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J Allergy Clin Immunol 1997;99:657–665. [DOI] [PubMed] [Google Scholar]
- 38.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 39.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-β expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 1997;156:591–599. [DOI] [PubMed] [Google Scholar]
- 40.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000;14:1362–1374. [DOI] [PubMed] [Google Scholar]
- 41.Burgess JK, Johnson PR, Ge Q, Au WW, Poniris MH, McParland BE, King G, Roth M, Black JL. Expression of connective tissue growth factor in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med 2003;167:71–77. [DOI] [PubMed] [Google Scholar]