Abstract
Proliferation, migration, and differentiation of smooth muscle (SM)–like lymphangioleiomyomatosis (LAM) cells in the lungs are pathologic manifestations of pulmonary LAM, a rare lung disease predominantly afflicting women and exacerbated by pregnancy. LAM cells form nodules throughout the lung without any predominant localization, but can also form small cell clusters dispersed within lung parenchyma. LAM cells have the appearance of “immature” SM-like cells, irregularly distributed within the nodule in contrast to organized SM cell layers in airways and vasculature. Progressive growth of LAM cells leads to the cystic destruction of the lung parenchyma, obstruction of airways and lymphatics, and loss of pulmonary function. Pathogenetically, LAM occurs from somatic or genetic mutations of tumor suppressor genes tuberous sclerosis complex 1 (TSC1) or TSC2. The TSC1/TSC2 protein complex is an integrator of signaling networks regulated by growth factors, insulin, nutrients, and energy. The observation that the TSC1/TSC2 functions as a negative regulator of the mammalian target of rapamycin (mTOR)/p70 S6 kinase (S6K1) signaling pathway yielded the first rapamycin clinical trial for LAM. Although LAM is a rare lung disease, the elucidation of disease-relevant mechanisms of LAM will provide a better understanding of not only SM-like cell growth, migration, and differentiation in LAM but may also offer insights into other metabolic diseases such as cardiovascular diseases, diabetes, and cancer. In this article, we will summarize the progress made in our understanding of LAM, and we will focus on how dysregulation of TSC1/TSC2 signaling results in abnormal proliferation and migration of SM-like LAM cells.
Keywords: lung cancer, TSC1, TSC2, signal transduction, mesenchymal cells
Smooth muscle (SM)–like lymphangioleiomyomatosis (LAM) cells represent a unique type of mesenchymal cell, expressing SM-specific markers, continuously proliferating, and invading the lung parenchyma. Although the origin of LAM cells remains unknown, some progress has been made in our understanding of the genetics and signal transduction mechanisms regulating SM-like LAM cell proliferation and migration. Over the last decade, linking somatic TSC2 (tuberous sclerosis complex 2) gene mutations to LAM pathology (1, 2), and discovering that TSC2 loss-of-function mutations led to the constitutive activation of p70 S6 kinase (S6K1) and abnormal LAM cell proliferation (3, 4), provided the foundation for the development of new therapeutic strategies for LAM (5–7). However, the inherent complexity of LAM pathology, LAM cells, and the TSC1/TSC2 signaling pathway are such that despite intensive efforts, the etiology, pathobiology, and signaling of LAM disease remain poorly understood. In this article, we will focus on current progress in understanding how abnormal growth, proliferation, and migration of SM-like LAM cells occur due to dysregulation of the TSC1/TSC2 signaling pathway. Some of the challenges in defining the regulatory mechanisms of LAM cell proliferation and migration, and most of the important observations highlighting TSC1/TSC2 signaling in LAM, have been previously reviewed (8).
WHAT ARE SM-LIKE LAM CELLS?
The abnormal and potentially metastatic growth of atypical SM-like LAM cells within the lungs and axial lymphatics are pathologic manifestations of LAM, a rare progressive cystic lung disease affecting primarily women of childbearing age (5–7). LAM can worsen during pregnancy and exogenous estrogen also exacerbates LAM (5–7), suggesting the role of estrogen in the etiology and pathology of LAM. The cystic destruction of the lung interstitium, obstruction of airways and pulmonary lymphatics, and formation of fluid-filled cystic structures (lymphangioleiomyomas) are caused by the abnormal growth of LAM cells; these destructive changes of the lungs ultimately lead to the loss of pulmonary function. Other rare diseases, such as Birt-Hogg-Dubé syndrome, Langerhans cell histiocytosis, and Sjögren's syndrome, also present with lung cysts (6); however, LAM disease, in addition to the lung cysts, is also characterized by lymphatic abnormalities and renal or abdominal angiomyolipomas (AMLs). AMLs are perivascular tumors consisting of a mixture of SM-like cells, fat cells, and blood vessels (6, 7). SM-like cells in AMLs often appears to merge with the walls of medium-sized blood vessels (9, 10). Interestingly, the blood vessels in AMLs lack the normal inner and outer layer of elastic tissue seen in normal small arteries (11). LAM is predominantly sporadic, but may also be manifested in association with tuberous sclerosis complex (TSC), an autosomal dominant inherited disorder affecting 1 in 5,800 individuals (12). Patients with TSC develop hamartomas and benign tumors in the brain, heart, and kidneys, and also manifest cognitive defects, epilepsy, and autism; the prevalence of LAM among women with TSC is 26 to 39%. Importantly, malignant tumors of the kidney, which develop either as malignant AMLs or renal cell carcinomas, occur in about 63% of sporadic LAM (13) and in most patients with TSC (14).
LAM cells, which infiltrate lungs, consist of two types of cell subpopulations: myofibroblast-like spindle-shaped cells and epithelioid-like polygonal cells. LAM cells predominantly form nodules (Figure 1) (3), but can also form small cell clusters dispersed within lung parenchyma (5, 6, 15). Spindle-shaped cells expressing SM-specific proteins, such as SM α-actin, desmin, and vimentin, form the core of the nodule surrounded by epithelioid-like cells, which have immunoreactivity for HMB45 (human melanoma black 45) antibody, which binds glycoprotein gp100, a marker of melanoma cells and immature melanocytes (5, 6). SM-positive LAM cells also express estrogen and progesterone receptors, suggesting that hormonal factors may modulate SM-like LAM cell proliferation (16, 17). Within the nodule, the spindle-shaped SM-like cells are haphazardly distributed (Figure 1), in contrast to SM cells in airways and vasculature where SM cells form well-organized SM layers (6). Interestingly, the dispersed LAM cells, which do not form larger nodules also express HMB45, suggesting that some SM-positive LAM cells have melanocytic differentiation (15). Importantly, SM-like cells in AMLs are also HMB45 positive (11). LAM cells also express another two melanocyte-specific proteins: CD63, a melanoma-associated protein, and PNL2, an uncharacterized melanocytic protein. Interestingly, the PNL2-positive LAM cells had decreased DNA synthesis detected by Ki67 immunostaining compared with PNL2-negative LAM cells (15). Similarly, SM-like LAM cells show high immunoreactivity for proliferating cell nuclear antigen (PCNA), a marker of DNA synthesis and cell proliferation, compared with the epithelioid-like HMB45-positive cells (6), suggesting the existence of two cell subpopulations with two different proliferative characteristics, and that SM-positive LAM cells represent the proliferative component of the LAM nodules. Currently, the role of melanocyte-specific markers as they relate to LAM cell functions is not established; however, it may be indicative of dysregulation of fundamental underlying cellular or molecular mechanisms, the pursuit of which may lead to a better understanding of LAM pathobiology.
Figure 1.
Pulmonary pathology in lymphangioleiomyomatosis (LAM) nodules (3). Tissue specimens from normal lung (upper panels) and LAM lung (lower panels) were stained with hematoxylin and eosin (H&E) or immunostained with anti–smooth muscle α-actin (SM α-actin)–fluorescein isothiocyanate (FITC)–conjugated mouse monoclonal antibody followed by analysis using a Nikon Eclipse E400 microscope (Nikon, Kawasaki, Japan) at an original magnification of ×200. Arrows indicate LAM nodules; V indicates blood vessels. Reprinted by permission from Reference 3.
SM-LIKE LAM CELL ORIGIN
The origin of LAM cells is controversial. Although initially LAM cells were believed to be derived either from airway or vascular smooth muscle cells, it is now known that LAM cells are found throughout the lungs without any predominant localization in proximity to airways, bronchus, or vasculature. LAM cells have the appearance of “immature” SM-like cells, irregularly distributed within the nodule without forming well-organized layers of SM cells, whereas SM cells in airways and vasculature form organized layers. Although the origin of LAM cells remains to be determined, clinical, genetic, and cell culture studies show that LAM cells have neoplastic potential, increased motility, and invasiveness. Thus, LAM cells were found in the blood, urine, and chylous fluids of patients with LAM (18), indicating that LAM cells can leave primary lesions, disseminate through blood or lymph vessels, and implant into secondary sites. Indeed, a LAM cell nodule recurrence after single-lung transplantation occurred in a patient with LAM (19). Formation of secondary tumors with identical mutations of tumor suppressor TSC2 in the lymph nodes of patients with LAM has also been reported (19, 20). Primary LAM-derived cell culture also shows increased motility in the absence of any stimuli and invasiveness into collagen matrix (21). Identical TSC2 gene mutations were also detected in pulmonary LAM cells and AML cells from renal tumors of TSC patients with LAM (22). Collectively, these data indicate that LAM cells are an unusual cell type with some non–SM cell features, such as an ability to disseminate in the body, suggesting that LAM cells may have inherent neoplastic potential. These features are indicative of similarities between migrating LAM cells and either mesenchymal stem cells (23, 24) or migrating cancer stem cells (25); however, the origin of LAM cells remains to be determined.
TUMOR SUPPRESSOR GENES TSC1 AND TSC2 ARE MUTATED IN SM-LIKE LAM CELLS
The somatic or germline mutations of the tumor suppressor gene TSC2 were found in SM-like LAM cells microdissected from LAM lung tissue sections (1, 2). The mutations in another tumor suppressor gene, TSC1, were also linked to the LAM disease (20). Importantly, mutations in the TSC2 gene arise more frequently (the majority of LAM, and about 60% of TSC cases) than TSC1 mutations (6, 7, 14, 18). The prevailing model for LAM is that the disease develops through a two-hit mechanism: a mutation in either TSC1 or TSC2 is followed by a second hit referred to as loss of heterozygosity (LOH), leading to the loss of function of either TSC1 or TSC2 proteins. Thus, somatic LAM develops due to two acquired mutations (predominantly in TSC2), and patients with LAM and TSC have one germline mutation (again predominantly in TSC2) and one acquired mutation (5). Until recently, the prevailing opinion suggested that mutational inactivation of either TSC1 or TSC2 genes results in a similar phenotype of the disease. However, genetic analysis (26, 27) and some animal studies (28) challenge this paradigm. Thus, patients with TSC with TSC1 mutations exhibited a milder disease phenotype, on average, compared with patients with TSC with TSC2 mutations in similar age groups (26, 27). Mutational analysis of familial and sporadic cases of TSC found a similar predominance of TSC2 mutations and TSC2-associated disease severity compared with TSC1 mutations and a milder disease phenotype (27). Another challenge in LAM relates to linking the distribution and type of TSC1 or TSC2 mutations to a specific region of the gene (14, 29), and linking these specific mutations to the disease phenotype and severity. The prognostic significance of mutations in different structural and functional regions of TSC1 or TSC2 is critically important for predicting disease outcome, and, if identified, may be decisive in determining the therapeutic approaches employed to treat the disease.
ABNORMAL GROWTH OF SM-LIKE LAM CELLS IS DUE TO TSC1/TSC2 PROTEIN COMPLEX DYSFUNCTION
Although TSC2 mutations were identified in SM-like LAM cells, the function of the gene and how its dysregulation results in abnormal LAM cell proliferation were unknown. Because the mammalian target of rapamycin (mTOR)/S6K1 signaling cascade is a critical regulator of cell growth and proliferation, we examined activation of this pathway in SM α–positive cells in the LAM nodules using immunostaining for phosphoribosomal protein S6, a molecular signature of mTOR/S6K1 activation (Figure 2) (3). Ribosomal protein S6 is phosphorylated by an activated S6K1, an effector of mTOR and a component of the PI3K signaling cascade (30, 31). As seen in Figure 2, SM-like LAM cells showed high phosphorylation levels of phospho-S6, suggesting activation of the mTOR/S6K1 signaling pathway, in contrast to normal SM cells, for example, in a blood vessel. However, a cell culture model was required to perform critical and fundamental experiments that can elucidate the intricate molecular components leading to activation of the mTOR/S6K1 signaling pathway. Consequently, we established primary cultures of SM α–positive LAM-derived cells from different patients with sporadic LAM without TSC and identified the mutations in the TSC2 gene in LAM-derived cells (Figure 3) (3, 4). Phosphorylation of ribosomal protein S6 was detected in LAM cells (Figure 3), which confirmed our in vivo observations. Furthermore, S6K1, an upstream activator of S6, was also constitutively activated. To establish that the constitutively activated mTOR/S6K1 signaling occurred due to TSC2 dysfunction, we demonstrated that reexpression of TSC2 in SM-positive LAM cell cultures inhibited mTOR/S6K1 signaling. Indeed, TSC2 reexpression inhibited the constitutive activation of S6K1, thus establishing TSC2 as an upstream regulator of mTOR/S6K1 signaling (3). LAM cells also showed increased proliferation under serum-free conditions, which was inhibited by TSC2 reexpression or treatment with rapamycin (3, 4). Rapamycin (i.e., sirolimus, an antifungal macrolide antibiotic approved by the U.S. Food and Drug Administration for immunosuppression) acts as a specific inhibitor of mTOR, a serine-threonine kinase, an obligatory upstream regulator of S6K1. The identification of the function of TSC2 as a negative regulator of the mTOR/S6K1 signaling pathway had a profound impact on our understanding of LAM. Thus, the linkage of the mutational inactivation of TSC2 to the constitutive activation of S6K1 in LAM cells (3, 4) identified a potential molecular target to treat LAM that led to the first rapamycin clinical trial. While the results of the first pilot studies using rapamycin in clinic at the University of Cincinnati are awaited, the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) randomized controlled trial has begun. Some of the challenges in using rapamycin analogs to inhibit mTOR/S6K1 activation in LAM have been previously reviewed (8).
Figure 2.
Activation of the mTOR/S6K1 signaling pathway in smooth muscle (SM) α-actin–positive lymphangioleiomyomatosis (LAM) cells (3). Confocal microscopy images taken at an original magnification of ×1,000 of an LAM nodule (upper panels) and a blood vessel (lower panels) were probed with anti–SM α-actin–FITC-conjugated (green) and anti-phosphoribosomal protein S6 (P-S6) (red) antibodies; yellow fluorescence results from the colocalization of P-S6–positive and SM α-actin–positive LAM cells. Reprinted by permission from Reference 3.
Figure 3.
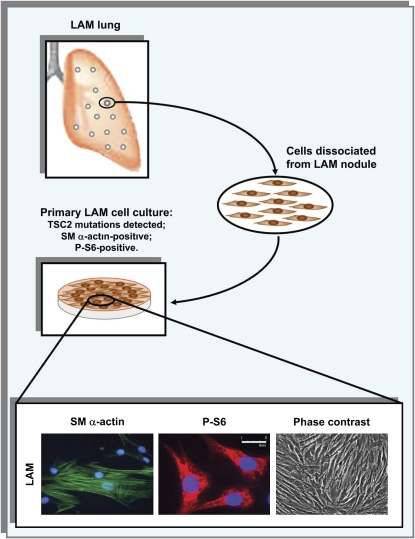
Smooth muscle (SM)–like lymphangioleiomyomatosis (LAM) cells in culture (3, 4, 21, 34, 68, 71). LAM cells, dissociated by enzymatic digestion from LAM nodules dissected from the lungs of patients with LAM who had undergone lung transplant, were plated on tissue culture plates coated with collagen and were passaged twice a week. LAM cells in culture maintain the filamentous SM α-actin expression pattern (lower left panel), increased ribosomal protein S6 phosphorylation (P-S6) (lower middle panel), and show SM-like cell morphology (lower right panel) during the 1st through 10th cell passages. The representative images of LAM cells immunostained with anti–SM α-actin (green), anti–P-S6 (red) antibodies, and 4,6-diamidino-2-phenylindole to detect nuclei (blue) were taken using a Nikon Eclipse TE2000-E microscope under appropriate filters at and original magnification of ×400. The representative phase contrast image is taken on an Olympus IX microscope (Olympus, Tokyo, Japan) under ×200 original magnification.
SIGNALING BY TSC1/TSC2 TUMOR SUPPRESSOR PROTEIN COMPLEX
Tumor suppressor genes TSC1 and TSC2 encode TSC1 and TSC2 proteins known as hamartin and tuberin, respectively (8, 31). TSC1, a 130-kD protein, and TSC2, an approximately 200-kD protein, form a physical and functional complex, in which TSC1 apparently functions as the regulatory component stabilizing TSC2, and facilitating TSC2 catalytic function as a GTPase activating protein (GAP) for the small GTPase Rheb (Figure 4). A recent study suggests that, in the absence of growth factor or insulin stimulation, TSC1 localizes TSC2 at the intracellular membrane, a requirement for the stabilization of TSC2 and the efficiency of TSC2 function as a RhebGAP (8, 32). In response to growth factor stimulation, TSC2 is inactivated by Akt-dependent phosphorylation that disrupts TSC2 interaction with TSC1. The dissociation of the TSC1/TSC2 complex promotes relocalization of the phosphorylated TSC2 into the cytosol, where it binds different isoforms of 14-3-3 proteins and, apparently, becomes highly ubiquitinated (32, 33). Dissociation of TSC2 from TSC1 makes TSC2 also available for binding with HERC1, a protein with an E3 ubiquitin ligase HECT (homology to the E6AP carboxyl terminal) domain (33). Importantly, both HERC1 and TSC1 bind to the N-terminal region of TSC2 (21, 31, 33, 34) (Figure 4), and TSC1 prevents the interaction of TSC2 and HERC1. Thus, a possible mechanism by which TSC1 binding stabilizes TSC2 is that TSC1 inhibits the interaction between TSC2 and HERC1, thereby inhibiting TSC2 ubiquitination and degradation (33). Indeed, in TSC1−/− mouse embryonic fibroblasts, TSC2 levels are markedly decreased (35); in contrast, when TSC2 is knocked-out in TSC2−/− mouse embryonic fibroblasts, TSC1 levels are unchanged (36). Ubiquitination and proteasomal degradation of TSC2 are potentially critical factors in the regulation of TSC2 cell functions. It is a possibility that TSC2 mutations that weaken its interaction with TSC1 destabilize TSC2 and target it for degradation, thus promoting activation of small GTPase Rheb and mTOR/S6K1 signaling, stimulating cell growth and the development of disease-relevant pathologies.
Figure 4.
Schematic representation of TSC1/TSC2 signaling (8). The TSC1/TSC2 complex controls cell growth depending on inputs from growth factors, nutrients, cellular energy levels, oxygen levels, and cell adhesion. Under metabolic stress or hypoxic conditions, TSC1/TSC2 is positively regulated by the phosphorylation of RTP801 (REDD1) and RTP801L (REDD2) or AMPK, respectively, which augment TSC1/TSC2 complex stability. This leads to inhibition of mTOR-dependent phosphorylation of S6K1 and 4EBP1 and abrogation of protein translation, cell growth, and proliferation. TSC1/TSC2 regulates the activities of Rac1 and RhoA small GTPases, actin cytoskeleton, and cell adhesion. Under growth factor stimulation, TSC2 is subjected to inhibitory phosphorylation by Akt, ERK1/2, and ERK1/2-dependent RSK1, which results in the potential dissociation of the TSC1/TSC2 complex and the binding of TSC2 with 14-3-3 protein or an HERC1 ubiquitin ligase. This, in turn, promotes activation of Rheb GTPase, which activates mTORC1 by directly binding to the Raptor. mTORC1 positively regulates protein translation by phosphorylation translation control proteins S6K1 and 4E-BP1, which promote cell growth and proliferation. TSC1/TSC2 down-regulation also leads to reduction of p27(kip1) levels, increasing CDK2 activity and cell proliferation. FAK and FIP200 phosphorylate TSC2, which leads to inhibition of S6K1 activity. Wnt-dependent GSK3 inhibition results in suppression of TSC1/TSC2 activity and stimulation of protein translation and cell growth in an mTORC1-dependent manner. In addition to mTORC1, mTOR is a member of the functionally distinct complex mTORC2 (mTOR/rictor/mLST8/Sin1); upstream regulators of mTORC2 are currently unknown. mTORC2 apparently controls microtubule organization and actin dynamics through CLIP-170 and PKCα, respectively. In addition, mTORC2 phosphorylates Akt at Ser473, which promotes Akt-dependent phosphorylation of FoxO1, 3A, and 4 transcription factors. Red or blue coloring indicates signaling molecules and regulatory events which are either positively or negatively, respectively, involved in TSC1/TSC2 signaling. Arrows indicate functional enhancement; flat bars indicate functional suppression.
Compelling evidence demonstrates that the TSC1/TSC2 protein complex integrates growth factor, insulin, and energy signaling transduction pathways in coordinated regulation of critical cellular processes, including cell cycle, cell size, cell proliferation, actin dynamics, cell motility, microtubule organization, and adhesion (Figure 4). Because of constraints, we will cite only the most recent original studies; references to previously published original studies can be found in several reviews (8, 30, 37–45).
The majority of inhibitory TSC2 phosphorylation sites are regulated by Akt activation (Figure 4); they inactivate TSC2 RhebGAP activity leading to mTOR/S6K1 activation. In addition to PI3K-Akt–dependent inactivating phosphorylation, the activity of the TSC1/TSC2 tumor suppressor complex is also regulated by other upstream kinases, which relay the signals from growth factors, nutrients, and energy levels (Figure 4), including extracellular signal-regulated kinase (ERK) 1/2 (46), and ERK-activated p90 ribosomal S6 kinase 1 (RSK1) (41). Importantly, phosphorylation by ERK suppresses TSC2 tumor suppressor function not only in vitro but also in vivo as demonstrated using a xenographic animal model (46). Furthermore, immunohistochemical analysis of subependymal giant cell astrocytomas, a common TSC-associated brain lesion, shows activation of ERK and Akt in parallel to inactivating phosphorylation of TSC2 on Ser1462 and activation of S6K1 (47). Thus, in addition to classical LOH of TSC2 and the consequent loss of tumor suppressor function of TSC2, the inhibitory phosphorylation of TSC2 may serve as another critical mechanism for TSC2 inactivation in vivo, which may contribute to the development of pathologic conditions and cancer.
Another pathway of TSC2-dependent regulation of S6K1 activity involves focal adhesion kinase (FAK) and cell adhesion (48). FAK directly associates with TSC2 without apparent interaction with TSC1 and promotes tyrosine phosphorylation of TSC2, which is required for FAK-dependent S6K1 activation. Importantly, FAK-dependent activation of S6K1 is critical for cell adhesion–induced S6K1 phosphorylation; in contrast, in cell suspension, S6K1 activity is inhibited by TSC2. This evidence indicates that TSC2 tumor suppressor activity can negatively regulate not only growth factor effects but also cell adhesion, which plays a critical role in regulating cell growth, cell cycle progression, and proliferation. A recent study demonstrates that TSC1 binds to FIP200 (a FAK family interacting protein of 200 kD), a newly identified protein that binds to the kinase domain of FAK and inhibits its kinase activity (49). The interaction of TSC1 with FIP200 prevents TSC1/TSC2 complex formation, leading to increased S6K1 activity and cell growth (49), which further supports the notion that TSC2 activity as a tumor suppressor is regulated by TSC1 binding. Others identify FIP200 as a putative inhibitor of TSC1/TSC2 tumor suppressor activity (50).
Growth factor– or insulin-induced inactivating phosphorylation of TSC2 inhibits RhebGAP activity and leads to activation of small GTPase Rheb, which directly binds to and activates mTOR (40, 44). Rheb activity is also positively regulated by the recently discovered guanine nucleotide exchange factor activity of translationally controlled tumor protein (TCTP) (51); whether translationally controlled tumor protein acts downstream or parallel to TSC1/TSC2 remains to be elucidated. The role of TSC2 in regulating the mTOR/S6K1 signaling pathway has been intensively studied and the TSC1/TSC2 complex clearly modulates mTOR/S6K1 signaling. Phosphorylation-induced inhibition of TSC2 RhebGAP activity releases Rheb GTPase activity, which directly activates mTOR in the mTOR/Raptor complex (TORC1). Serine-threonine kinase mTOR, an upstream activator of S6K1, is part of both complexes: the rapamycin-sensitive mTOR/Raptor (mTORC1) phosphorylating S6K1, and the rapamycin-insensitive mTOR/Rictor (mTORC2). Comprehensive reviews of the manner in which dysregulation of TSC1/TSC2–mTORC1 modulates intracellular signaling (e.g., negative feedback inhibition of insulin signaling and Akt activation) are published in Inoki and colleagues (43) and Sabatini (52).
The recent discovery that mTORC2 phosphorylates Akt at Ser473 suggested the possibility that mTORC2 may regulate the activation of TSC2 and mTORC1 (52). Although Akt phosphorylation on Ser473 indicates activation, the physiologic function of this phosphorylation has remained illusive (8). Knock-out of Rictor in mice resolved a puzzle about the role of mTORC2 in the regulation of Akt (53, 54). Rictor is necessary for normal growth and development of the mouse embryo and for Akt phosphorylation at Ser473 during embryogenesis (55–57). Importantly, knock-down of mTORC2 proteins had little effect on TSC2, mTOR, S6K1, and GSK3, which are known effectors of Akt (58). In contrast, Rictor knock-down affected phosphorylation levels of the Forkhead transcription factors FoxO3 and FoxO4A, which are negatively regulated by Akt and are required for cell survival (55, 56, 58–60) (Figure 4). Collectively, these studies demonstrate that Akt–Ser473 is not essential for Akt-dependent regulation of TSC1/TSC2 and that mTORC2 does not modulate TSC1/TSC2–mTORC1 activity.
In addition to growth factors and insulin, TSC1/TSC2 activity is regulated by cellular energy (i.e., the availability of glucose and ATP) (Figure 4). Energy depletion decreases ATP levels and increases AMP levels leading to activation of AMP-activated protein kinase (AMPK) (61). In mammalian cells, AMPK is activated by glucose deprivation and other stresses, which lead to ATP depletion, such as attenuation of ATP synthesis during hypoxia or increased ATP consumption during physical exertion. Activated AMPK initiates two major signaling cascades: one to switch on catabolic pathways to generate ATP and another to switch off ATP-consuming processes, which are not essential for short-term survival (i.e., protects against apoptosis during glucose starvation). Thus, decreased cellular ATP levels activate AMPK, which directly phosphorylates TSC2; this is followed by inhibition of mTOR/S6K1, leading to inhibition of translation and attenuation of protein synthesis (30). AMPK phosphorylation primes TSC2 for its subsequent phosphorylation by GSK3 (62). GSK3 activity is negatively regulated by the Wnt signaling pathway, which plays a critical role in cellular proliferation, especially during development (63). Wnt-dependent activation of S6K1 requires inhibition of GSK3 and TSC1/TSC2 activity (62). Inhibition of GSK3 activity by Wnt is specific and distinct from its inhibition by Akt and RSK1 (64). Thus, TSC2 integrates both energy and Wnt signaling in coordinated inhibition of mTOR/S6K1 signaling, protein translation, and cell growth.
Prolonged stressors (e.g., hypoxia) also regulate TSC1/TSC2 by up-regulating the TSC1/TSC2 complex activity through two stress-induced proteins, RTP801/REDD1 and RTP801L/REDD2 (65, 66). These two proteins have approximately 50% homology to each other but have little homology to other known proteins. RTP801/REDD1 and RTP801L/REDD2 are mammalian orthologs of Scylla and Charybdis, which were identified as negative regulators of the TOR pathway in Drosophila (67). Activated Scylla and Charybdis inhibited S6K with concomitant inhibition of cell size and growth, and acted upstream of TSC1/TSC2. The mechanism of RTP801-dependent regulation of TSC1/TSC2 complex activity remains unknown.
Thus, the role of TSC1/TSC2 tumor suppressor complex in integrating signaling networks from growth factors, nutrients, and energy has been clearly demonstrated. However, most studies were based on data obtained using biochemical and molecular in vitro models; the relative contribution of potentially differential regulation of TSC1/TSC2 by growth factors, nutrients, and energy in LAM remains to be determined. Ultimately, in vitro studies remain to be bridged to LAM disease. A LAM cell model is urgently needed not only to validate TSC1/TSC2 signaling in physiologically relevant cells but also to serve as the preclinical LAM cell model for validation of therapeutic approaches to treat LAM disease.
MODULATION OF THE ACTIVITY OF Rho GTPases AND LAM CELL MOTILITY DUE TO TSC1/TSC2 DYSFUNCTION OPENS A POSSIBILITY FOR COMBINATIONAL THERAPY IN LAM
Although the link between the loss of TSC2 function and abnormal LAM cell growth is well established, little information is available about the precise role of TSC1 and TSC2 in regulating migration and metastatic LAM cell dissemination. Thus, primary LAM cell cultures show increased migration and invasiveness, which are abolished by the reexpression of TSC2 (21, 68). Increased RhoA activity was also found in serum-deprived primary LAM cells cultures; and reexpression of TSC2 inhibited RhoA activity (21). It is well established that the Rho family of small GTPases plays a critical role in regulating cell motility, transformation, invasion, and metastasis. Pharmacologic inhibition of RhoA GTPase or its downstream effector RhoA kinase (ROCK) abolished the increased migratory activity of LAM cells, indicating that RhoA activation is critical for the up-regulation of LAM cell migration (21). These data suggest that LAM cells have the potential to migrate abnormally and metastasize in vitro. Furthermore, RhoA GTPase may serve as a novel target for a therapeutic approach in LAM.
The findings that RhoA is activated in primary cultures of LAM cells (21) suggest a potential link between TSC1/TSC2 signaling and Rho GTPases. Indeed, the role of TSC1 in regulating RhoA activity and cell adhesion was demonstrated (69) before the discovery that the interaction between TSC1 and TSC2 stabilizes both proteins in a functional complex, in which TSC2 has the catalytic GAP activity toward Rheb GTPase. Overexpression of TSC1 in NIH 3T3 fibroblasts activated RhoA and promoted stress fiber formation (69). Membrane binding of TSC1 through its C-terminus with the ezrin–radixin–moesin (ERM) family of actin-binding proteins is a prerequisite for TSC1-dependent RhoA activation. However, the mechanism of RhoA activation by TSC1 remains unknown.
It is important to note that the RhoA-activating domain of TSC1 (amino acids 145–510) overlaps with the domain that binds to TSC2: the amino acids 302–430 of TSC1 associate with amino acids 1–418 of TSC2 and are required for TSC1/TSC2 complex formation. These data suggested that the interaction between TSC1 and TSC2 is critical for TSC1-dependent RhoA activation and focal adhesion formation (34). Reexpression of TSC2 in TSC2-null cells inhibited RhoA activation and stress fiber and focal adhesion formation. Importantly, only the TSC1-binding domain of TSC2 or small interfering RNA (siRNA)-induced TSC1 depletion generated similar effects, indicating that increased RhoA activity and stress fiber and focal adhesion formation require TSC1 and are regulated by the formation of the TSC1/TSC2 complex. Our studies also showed that TSC1-induced RhoA activation is regulated by the TSC1-dependent reciprocal inhibition of Rac1 by an as yet unidentified mechanism. Collectively, these studies suggest that TSC1 and TSC2 modulate the activities of small Rho GTPases and may also regulate cell adhesion and motility. Findings that loss of TSC2 results in RhoA activation in increased LAM cell migration open potential novel approaches for the treatment of LAM disease (8). There is evidence that simvastatin inhibits Rho activity and the migration of primary human LAM cell cultures (70). Importantly, simvastatin also inhibits LAM cell proliferation; furthermore, combined treatment of LAM cells with simvastatin and rapamycin abrogated cell proliferation compared with the partially inhibitory effects of each agent alone (70). Thus, simultaneous inhibition of mTOR/S6K1 and RhoA GTPases may have potential as a therapeutic strategy for combinational treatment in LAM.
CONCLUSIONS
In the last few years, our understanding of the SM-like cells in LAM has greatly improved. Abnormal growth, proliferation, and migration of atypical SM-like LAM cells in the lungs lead to cystic destruction of the lungs. TSC1/TSC2 tumor suppressor complex mutations had been linked to the abnormal growth (3, 4, 71) and migration (21, 68) of LAM cells. TSC2 dysfunction in LAM cells results in the constitutive activation of S6K1, which identifies S6K1 as a molecular target for treatment of LAM disease.
In summary, it is apparent that LAM cell behavior, including growth, proliferation, adhesion, and motility, plays a critical role in LAM disease. However, whether only TSC1 or TSC2 dysfunction accounts for the multisystem pathologic changes seen in pulmonary LAM needs to be established. LAM pathology provides clues to the enigmatic complexity of LAM disease, the solving of which may lead not only to elucidating cellular and molecular mechanisms but may also pave the way toward finding the cure for LAM. The importance of further investigations into a role of SM-like cells in the etiology and pathology of LAM cannot be overestimated, not only because they may uncover mechanisms of LAM pathology but may also have the potential to develop novel therapeutic strategies for LAM treatment.
Acknowledgments
The author thanks Dr. Elena A. Goncharova for help with figures, Mr. Andrew Eszterhas for helpful discussions and critical reading of the manuscript, and Ms. Beatrice Delamerced for help in preparation of the manuscript.
Supported by grants from NIH/NHLBI 2RO1HL071106, American Lung Association CI-9813, and The LAM Foundation LAM059P07-06.
Conflict of Interest Statement: V.P.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC.2 mutations: chromosome 16p13 loss of heterozygosity in angiolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet 1998;62:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem 2002;277:30958–30967. [DOI] [PubMed] [Google Scholar]
- 4.Goncharova EA, Goncharov DA, Spaits M, Noonan D, Talovskaya E, Eszterhas A, Krymskaya VP. Abnormal smooth muscle cell growth in lymphangioleiomyomatosis (LAM): role for tumor suppressor TSC2. Am J Respir Cell Mol Biol 2006;34:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol 2006;38:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taveira-DaSilva AM, Moss J. Lymphangioleiomyomatosis. Cancer Control 2006;13:276–285. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J 2006;27:1056–1065. [DOI] [PubMed] [Google Scholar]
- 8.Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): progress and current challenges. J Cell Biochem [E-pub ahead of print 31 May 2007]. [DOI] [PubMed]
- 9.Matsui K, Beasley MB, Nelson WK, Barnes PJ, Bechtle J, Falk R, Ferrans VJ, Moss J, Travis WD. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol 2003;25:479–484. [DOI] [PubMed] [Google Scholar]
- 10.Moss J, editor. LAM and other diseases characterized by smooth muscle proliferation. New York: Marcel Dekker; 1999. 663pp.
- 11.Travis WD, Usuki J, Horiba K, Ferrans VJ. Histopathological studies on lymphangioleiomyomatosis. In: Moss J, editor. LAM and other diseases characterized by smooth muscle proliferation. New York: Marcel Dekker; 1999. pp. 171–217.
- 12.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–1356. [DOI] [PubMed] [Google Scholar]
- 13.Astrinidis A, Khare L, Carsillo T, Smolareck T, Au KS, Northrup H, Henske EP. Mutational analysis of the tuberous sclerosis gene TSC2 in patients with pulmonary lymphangioleiomyomatosis. J Med Genet 2000;37:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet 2003;67:87–96. [DOI] [PubMed] [Google Scholar]
- 15.Zhe X, Schuger L. Combined smooth muscle and melanocytic differentiation in lymphangioleiomyomatosis. J Histochem Cytochem 2004;52:1537–1542. [DOI] [PubMed] [Google Scholar]
- 16.Logginidou H, Ao X, Russo I, Henske EP. Frequent estrogen and progesterone receptor immunoreactivity in renal angiomyolipomas from women with pulmonary lymphangioleiomyomatosis. Chest 2000;117:25–30. [DOI] [PubMed] [Google Scholar]
- 17.Brentani MM, Carvalho CR, Saldiva PH, Pacheco MM, Oshima CT. Steroid receptors in pulmonary lymphangiomyomatosis. Chest 1984;85:96–99. [DOI] [PubMed] [Google Scholar]
- 18.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP Jr, Wang J-a, Kumaki F, Darling T, Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2004;101:17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med 2003;167:976–982. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Seyama K, Fujii H, Maruyama H, Setoguchi Y, Iwakami S, Fukuchi Y, Hino O. Mutational analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangileiomyomatosis. J Hum Genet 2002;47:20–28. [DOI] [PubMed] [Google Scholar]
- 21.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 2006;34:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Astrinidis A, Henske EP. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med 2001;164:1537–1540. [DOI] [PubMed] [Google Scholar]
- 23.Herzog EL, Krause DS. Engraftment of marrow-derived epithelial cells: the role of fusion. Proc Am Thorac Soc 2006;3:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med 2007;175:547–553. [DOI] [PubMed] [Google Scholar]
- 25.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells: an integrated concept of malignant tumor progression. Nat Rev Cancer 2005;5:744–749. [DOI] [PubMed] [Google Scholar]
- 26.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001;68:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S, Halley D, van den Ouweland A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype-phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet 2005;13:731–741. [DOI] [PubMed] [Google Scholar]
- 28.Wilson C, Bonnet C, Guy C, Idziaszczyk S, Colley J, Humphreys V, Maynard J, Sampson JR, Cheadle JP. Tsc1 haploinsufficiency without mammalian target of rapamycin activation is sufficient for renal cyst formation in tsc1+/− mice. Cancer Res 2006;66:7934–7938. [DOI] [PubMed] [Google Scholar]
- 29.Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum Genet 2000;107:97–114. [DOI] [PubMed] [Google Scholar]
- 30.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 2006;3:393–402. [DOI] [PubMed] [Google Scholar]
- 31.Krymskaya VP. Tumor suppressors hamartin and tuberin: intracellular signaling. Cell Signal 2003;15:729–739. [DOI] [PubMed] [Google Scholar]
- 32.Cai S-L, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by Akt-mediated phosphorylation and membrane partitioning. J Cell Biol 2006;173:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Rosa JL, Guan K-L. Tsc1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem 2006;281:8313–8316. [DOI] [PubMed] [Google Scholar]
- 34.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the RAC1 GTPase. J Cell Biol 2004;167:1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of tsc1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6K kinase activity in tsc1 null cells. Hum Mol Genet 2002;11:525–534. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of TSC1/TSC2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest 2003;112:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krymskaya VP, Shipley JM. Lymphangioleiomyomatosis: a complex tale of serum response factor-mediated tissue inhibitor of metalloproteinase-3 regulation. Am J Respir Cell Mol Biol 2003;28:546–550. [DOI] [PubMed] [Google Scholar]
- 38.Findlay GM, Harrington LS, Lamb RF. TSC1-2 tumor suppressor and regulation of mTOR signalling: linking cell growth and proliferation? Curr Opin Genet Dev 2005;15:69–76. [DOI] [PubMed] [Google Scholar]
- 39.Lee C-H, Inoki K, Guan K-L. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 2007;47:443–467. [DOI] [PubMed] [Google Scholar]
- 40.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 2006;25:6361–6372. [DOI] [PubMed] [Google Scholar]
- 41.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441:424–430. [DOI] [PubMed] [Google Scholar]
- 42.Inoki K, Ouyang H, Li Y, Guan K-L. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 2005;69:79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoki K, Corradetti MN, Guan K-L. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 2005;37:19–24. [DOI] [PubMed] [Google Scholar]
- 44.Nobukini T, Thomas G. The mTOR/S6K signaling pathway: the role of the TSC1/2 tumor suppressor complex and the proto-oncogene Rheb. Novartis Found Symp 2005;262:148–159. [PubMed] [Google Scholar]
- 45.Vivekanand P, Rebay I. Intersection of signal transduction pathways and development. Annu Rev Genet 2006;40:139–157. [DOI] [PubMed] [Google Scholar]
- 46.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by ERK: implications for tuberous sclerosis and cancer pathogenesis. Cell 2005;121:179–193. [DOI] [PubMed] [Google Scholar]
- 47.Han S, Santos TM, Puga A, Roy J, Thiele EA, McCollin M, Stemmer-Rachamimov A, Ramesh V. Phosphorylation of tuberin as a novel mechanism for somatic inactivation of the tuberous sclerosis complex proteins in brain lesions. Cancer Res 2004;64:812–816. [DOI] [PubMed] [Google Scholar]
- 48.Gan B, Yoo Y, Guan J-L. Association of focal adhesion kinase with tuberous sclerosis complex 2 in the regulation of S6 kinase activation and cell growth. J Biol Chem 2006;281:37321–37329. [DOI] [PubMed] [Google Scholar]
- 49.Gan B, Melkoumian ZK, Wu X, Guan K-L, Guan J-L. Identification of FIP200 interaction with the TSC1-TSC2 complex and its role in regulation of cell size control. J Cell Biol 2005;170:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gan B, Peng X, Nagy T, Alcaraz A, Gu H, Guan J-L. Role of fip200 in cardiac and liver development and its regulation of TNFα and TSC-mTOR signaling pathways. J Cell Biol 2006;175:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu Y-C, Chern JJ, Cai Y, Liu M, Choi K-W. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 2007;445:785–788. [DOI] [PubMed] [Google Scholar]
- 52.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 2006;6:729–734. [DOI] [PubMed] [Google Scholar]
- 53.Polak P, Hall MN. mTORC2 caught in a sinful Akt. Dev Cell 2006;11:433–434. [DOI] [PubMed] [Google Scholar]
- 54.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 2007;12:487–502. [DOI] [PubMed] [Google Scholar]
- 55.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or MLST8 reveals that mTORC2 is required for signaling to Akt-Foxo and PKCα, but not S6K1. Dev Cell 2006;11:859–871. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q, Inoki K, Ikenoue T, Guan K-L. Identification of SIN1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev 2006;20:2820–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiota C, Woo J-T, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell 2006;11:583–589. [DOI] [PubMed] [Google Scholar]
- 58.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006;127:125–137. [DOI] [PubMed] [Google Scholar]
- 59.Guertin DA, Guntur KVP, Bell GW, Thoreen CC, Sabatini DM. Functional genomics identifies TOR-regulated genes that control growth and division. Curr Biol 2006;16:958–970. [DOI] [PubMed] [Google Scholar]
- 60.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307:1098–1101. [DOI] [PubMed] [Google Scholar]
- 61.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 2007;47:185–210. [DOI] [PubMed] [Google Scholar]
- 62.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006;126:955–968. [DOI] [PubMed] [Google Scholar]
- 63.Clevers H. Wnt/β-catenin signaling in development and disease. Cell 2006;127:469–480. [DOI] [PubMed] [Google Scholar]
- 64.Choo AY, Roux PP, Blenis J. Mind the gap: Wnt steps onto the mTORC1 train. Cell 2006;126:834–836. [DOI] [PubMed] [Google Scholar]
- 65.Ellisen LW. Growth control under stress. Cell Cycle 2005;4:e58–e60. [DOI] [PubMed] [Google Scholar]
- 66.Corradetti MN, Inoki K, Guan K-L. The stress-inducted proteins RTP801 and RTP801l are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 2005;280:9769–9772. [DOI] [PubMed] [Google Scholar]
- 67.Reiling JH, Hafen E. The hypoxia-induced paralogs scylla and charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev 2004;18:2879–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nat Protocols 2006;1:2933–2939. [DOI] [PubMed] [Google Scholar]
- 69.Lamb RF, Roy C, Diefenbach TJ, Vinters HV, Johnson MW, Jay DG, Hall A. The TSC1 tumor suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase rho. Nat Cell Biol 2000;2:281–287. [DOI] [PubMed] [Google Scholar]
- 70.Goncharova EA, Goncharov DA, Lim PN, Krymskaya VP. Simvastatin modulates rhoa activity, proliferation and migration of LAM-derived cells. Am J Respir Crit Care Med 2007;175:A345. [Google Scholar]
- 71.Goncharova EA, Lin P, Goncharov DA, Eszterhas A, Panettieri RA Jr, Krymskaya VP. Assays for in vitro monitoring proliferation of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cells. Nat Protocols 2006;1:2905–2908. [DOI] [PubMed] [Google Scholar]