Abstract
Contractility of airway smooth muscle requires elevation of intracellular calcium concentration. Under resting conditions, airway smooth muscle cells maintain a relatively low intracellular calcium concentration, and activation of the surface receptors by contractile agonists results in an elevation of intracellular calcium, culminating in contraction of the cell. The pattern of elevation of intracellular calcium brought about by agonists is a dynamic process and involves the coordinated activities of ion channels located in the plasma membrane and the sarcoplasmic reticulum. Among the signaling molecules involved in this dynamic calcium regulation in airway smooth muscle cells are inositol 1,4,5-trisphosphate and cyclic ADP-ribose, which mobilize calcium from the sarcoplasmic reticulum by acting via the inositol 1,4,5-trisphosphate and ryanodine receptors, respectively. In addition, calcium influx from the extracellular space is critical for the repletion of the intracellular calcium stores during activation of the cells by agonists. Calcium influx can occur via voltage- and receptor-gated channels in the plasma membrane, as well as by influx that is triggered by depletion of the intracellular stores (i.e., store-operated calcium entry mechanism). Transient receptor potential proteins appear to mediate the calcium influx via receptor- and store-operated channels. Recent studies have shown that proinflammatory cytokines regulate the expression and activity of the pathways involved in intracellular calcium regulation, thereby contributing to airway smooth muscle cell hyperresponsiveness. In this review, we will discuss the specific roles of cyclic ADP-ribose/ryanodine receptor channels and transient receptor potential channels in the regulation of intracellular calcium in airway smooth muscle cells.
DYNAMIC INTRACELLULAR CALCIUM REGULATION IN AIRWAY SMOOTH MUSCLE CELLS
Exposure of airway smooth muscle (ASM) cells to contractile agonists results in a biphasic elevation of intracellular calcium concentration ([Ca2+]i) that is characterized by an initial rapid and transient rise in calcium, followed by a decline to a lower, sustained steady-state concentration above the basal level (1–3). This biphasic [Ca2+]i response results from calcium influx from the extracellular space and release of calcium from the intracellular stores (i.e., the sarcoplasmic reticulum [SR]). Earlier studies have attributed the initial rapid and transient phase of the [Ca2+]i response to release from the SR, while the sustained phase of the response was thought to be due to influx from the extracellular space (2–6). Recent investigations using the improved temporal and spatial resolution features of real-time confocal microscopy have shed light on the dynamics of the [Ca2+]i response in ASM cells. These studies have shown that the biphasic [Ca2+]i response of ASM cells elicited by contractile agonists in reality consists of propagating regenerative calcium oscillations that originate at a location within a cell and propagate to other regions of the cell (7–9). As the basal [Ca2+]i concentration increases after activation of the cell surface receptor by the agonist, the absolute amplitude of the calcium oscillations decreases (9). The steady-state phase of the response consists of calcium oscillations of similar amplitude. Another feature of these calcium oscillations is that the peak amplitude of the oscillations is similar, but the frequency of the oscillations, while higher during the initial phase of the response, reaches a lower steady-state level as the basal calcium level decreases. Furthermore, the rise time of the calcium oscillations also correlates directly with the basal [Ca2+]i (9). Calcium oscillations can be initiated on exposure to the agonist in the absence of extracellular calcium, but cannot be sustained. The frequency and propagation velocity of the calcium oscillations are directly correlated to the basal [Ca2+]i (9). These observations indicate that the basal intracellular calcium level influences the kinetics of the calcium oscillations. In smooth muscle cells, the basal [Ca2+]i under unstimulated conditions is approximately 200 nM, and exposure to contractile agonists increases this level in a concentration-dependent fashion (10, 11). Therefore, we predicted that there would be agonist concentration–dependent changes in the frequency and propagation velocity of the calcium oscillations.
The dynamic nature of the calcium oscillations has been investigated in ASM cells after exposure to the muscarinic agonist acetylcholine (Ach). Within a small region of the cell, exposure to Ach caused calcium oscillations that were biphasic (12). Real-time confocal imaging also revealed that the calcium oscillations originate from one end of the cell and propagate toward the other end. However, in a few cells, the calcium wave appeared to originate in the middle part of the cell and spread to both ends of the cell. With higher concentrations of Ach, the peak of regional intracellular calcium oscillations appeared similar, while the frequency and propagation velocity of the oscillations both increased (12). When the [Ca2+]i was measured from the whole cell, the spatial and temporal integration of the regional responses consisted of an Ach concentration–dependent increase in peak and mean cellular calcium concentrations (12). Thus, in ASM cells Ach concentration–dependent changes in mean [Ca2+]i are achieved via modulation of oscillation frequency and propagation velocity, and increases in either or both would elevate the mean [Ca2+]i.
Liu and Farley, in their initial studies of ASM cells, showed that calcium oscillations arise from intracellular stores and are sustained by calcium influx from the extracellular space (7). Other studies have examined the underlying mechanisms of calcium oscillations in ASM cells after activation of the muscarinic receptors. The results of these investigations reveal that calcium oscillations do not arise from repetitive changes in membrane potential, but rather from repetitive calcium release and reuptake from the SR. During the steady-state phase of calcium oscillations elicited by Ach, exposure to caffeine or ryanodine inhibited the oscillations (9). Exposure to thapsigargin, an inhibitor of the sarcoplasmic-endoplasmic reticulum calcium ATPase (SERCA), resulted in an increase in the basal [Ca2+]i and eventual inhibition of the calcium oscillations (9). The inhibition of the calcium oscillations after exposure to thapsigargin was due to depletion of the SR calcium stores. These results collectively indicate that calcium oscillations arise from activation of caffeine-sensitive ryanodine receptors in the SR through a repetitive process that is often referred to as calcium-induced calcium release (CICR) and that calcium reuptake into the SR is important to sustain these oscillations. By varying the kinetics of the release and reuptake process from a limited pool of SR calcium, the cell achieves an agonist concentration–dependent change in the mean [Ca2+]i. Later studies from our laboratory also revealed that activation of the inositol 1,4,5-trisphosphate (IP3) receptors in the SR is crucial for the initiation of the calcium oscillations by Ach (13). Once initiated, the calcium oscillations are maintained through CICR involving activation of the ryanodine receptors.
SPONTANEOUS CALCIUM RELEASE EVENTS IN AIRWAY SMOOTH MUSCLE CELLS
Recent investigations have shown that localized and spontaneous intracellular calcium release events (i.e., calcium sparks) also arise from calcium release through ryanodine receptor channels in skeletal, cardiac, and smooth muscle cells (14–20). In porcine ASM cells, earlier studies revealed occurrence of calcium sparks that were grouped and coupled across adjacent regions of the cell (16). Often, individual calcium sparks fused to give rise to a large local elevation of [Ca2+]i, which resulted in a propagating wave of calcium. Exposure of the cells to low concentrations of ryanodine or caffeine increased calcium spark incidence, but the calcium sparks were preserved in the absence of extracellular calcium (16). There were regions of high spark incidence in a cell and exposure to Ach resulted in propagating calcium oscillations that often originated in these regions (16). These studies revealed that unitary calcium release events in ASM cells also arise from calcium release through ryanodine receptor channels in the SR. Furthermore, agonist-induced calcium oscillations most likely reflect the spatial and temporal integration of calcium sparks due to activation of the ryanodine receptor channels. However, the mechanisms by which ryanodine receptor channels are activated and the role of second messengers in this process are not clearly understood.
The functional role of calcium sparks in ASM cells is not clearly known. Unlike the case in vascular smooth muscle cells, where calcium sparks appear to play a role in the control of membrane potential by virtue of their occurrence in close proximity to the plasma membrane, calcium sparks in ASM cells, by providing trigger sites for the initiation of calcium oscillations, may contribute to contractility of the muscle (17). Recent investigations have examined the role of specific ryanodine receptor isoforms in the generation of calcium sparks in smooth muscle cells. In the smooth muscle cells obtained from the bladder of RyR3 null mice, Ji and coworkers showed that calcium sparks are unaltered relative to the wild-type controls (21). In addition, the frequency, amplitude, and kinetics of the transient outward currents, which reflect the local membrane response to calcium sparks, were also similar in the myocytes obtained from the RyR3-null and the wild-type mice (21). These results demonstrate that deletion of RyR3 in bladder myocytes does not result in any significant alterations in the properties of the calcium sparks. However, calcium release through the RyR2 channels was shown to have a major contribution to the generation of spontaneous and evoked calcium sparks and that RyR-associated proteins (i.e., FK506-binding proteins [FKBP]) are involved in this process (see below). It remains to be seen whether this is also true for ASM cells. The facts that RyR2 is the predominant RyR isoform expressed in ASM cells, and that the characteristics of the calcium sparks in these cells are modulated by activation of the RyR by ryanodine and caffeine, strongly point to the role of RyR2 in the generation of calcium sparks (22). In this context, a recent report in equine ASM cells by Wang and colleagues provided evidence for RyR2 in spontaneous calcium release and that FKBP12.6 and the NAD metabolite cyclic ADP-ribose (cADPR) (23) (see subsequent section) are involved in the regulation of calcium release through activation of RyR2 in the tracheal myocytes.
CYCLIC ADP-RIBOSE AS AN ENDOGENOUS LIGAND FOR THE RYANODINE RECEPTOR CHANNELS IN AIRWAY SMOOTH MUSCLE CELLS
The role of ryanodine receptor channels in intracellular calcium regulation and the mechanisms underlying their activation in ASM cells have been the subject of recent investigations. Airway smooth muscle cells from different species express all three isoforms of the ryanodine receptors, although RyR2 and RyR3 appear to be the predominant isoforms (22, 24). Calcium release from the SR through RyR is an important component of the intracellular calcium response after activation of G protein–coupled receptors (GPCRs) in ASM cells. Prior studies demonstrated that during ongoing calcium oscillations elicited by Ach, exposure to caffeine resulted in a large calcium transient and eventual, but transient, abolition of the calcium oscillations (25). On the other hand, exposure to the inhibitors of RyR channels (i.e., high concentrations of ryanodine or ruthenium red) resulted in inhibition of the ongoing calcium oscillations. These observations suggest that Ach-induced calcium oscillations arise from activation of caffeine-sensitive RyR channels in ASM cells. During ongoing calcium oscillations elicited by Ach in ASM cells, exposure to a zero-calcium containing solution resulted in disappearance of the oscillations, which reappeared on readmission of normal calcium in the perfusate. In ASM cells permeabilized with β-escin and maintained in a defined calcium environment, exposure to Ach caused calcium oscillations, which were inhibited by ruthenium red (25). Furthermore, in the presence of 8-NH2-cADPR, a competitive antagonist of cADPR, the ongoing Ach-induced calcium oscillations were blocked (25). In intact ASM cells pretreated with 8-Br-cADPR, a membrane-permeant cADPR antagonist, intracellular calcium responses to Ach and endothelin-1 were attenuated compared with responses in control cells (13). In the presence of ryanodine, there was no further attenuation of the calcium responses to these contractile agents by 8-Br-cADPR (13). These results collectively demonstrate that contractile agonists mobilize the release of SR calcium through RyR channels and that cADPR mediates this response. In addition, calcium influx is critical for the repletion of the intracellular calcium stores and the sustenance of the intracellular calcium response to the agonist. How extracellular calcium enters ASM cells to refill SR calcium stores after their depletion by IP3- or ryanodine receptor channel-stimulated release is important to understand in terms of the overall calcium homeostasis. It is probable that localized SR calcium depletion could limit agonist-elicited SR quantal release. Calcium influx can occur through a variety of mechanisms, including voltage- and receptor-operated membrane channels, and store-operated calcium entry (SOCE) (1, 6, 26–29). Calcium influx by SOCE is triggered by depletion of the intracellular stores and may be influenced by the second messenger systems eliciting calcium release (30). In this context, transient receptor potential (TRP) channels appear to have a major role in calcium influx and repletion of SR calcium stores. TRP channels are members of a superfamily of nonselective cation channels responsive to agonists, as well as physical and other stimuli (30). Recent studies have also shown that TRP channels are responsive to phospholipase C–dependent pathways. It is known that TRPC3 can interact with calcium regulatory proteins that are associated with GPCR signaling, which includes receptors for IP3 and Ryanodine (31). Thus, TRP channel activity may share cross-talk with second messenger systems such as the CD38/cADPR signaling pathway. A study by White and coworkers provided evidence for the expression of multiple TRPC isoforms in human bronchial smooth muscle cells, although TRPC3 appears to be the predominant isoform involved in receptor- and store-operated calcium influx (32). It is worth noting that calcium release through the IP3 receptor channels is critical for activation of the RyR channels in ASM cells, since blocking IP3 generation resulted in complete abolition of the calcium response to Ach (13). The mechanisms involved in the activation of RyR in ASM cells are not clearly understood.
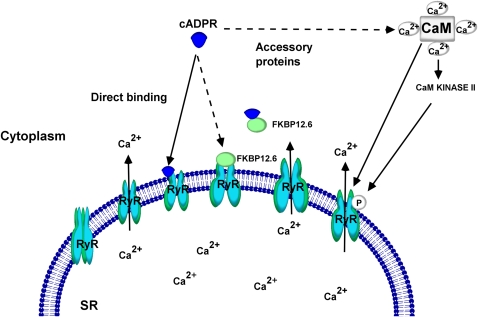
In this context, Wang and colleagues have provided evidence relevant to the mechanisms by which calcium release through RyR channels is brought forth in airway myocytes (23). The open state of the RyR channels in cardiac muscle cells is regulated by FK506-binding proteins (33). Specifically, FKBP12.6 is known to associate with the RyR2 isoform in smooth muscle cells to regulate its function (23, 34). In ASM cells, Wang and coworkers showed that, although both FKBP12 and FKBP12.6 mRNAs are expressed, the FKBP12.6 fusion proteins only associate with the RyR2 (23). In the same study, the authors demonstrated that cADPR, in a concentration-dependent fashion, produced macroscopic calcium release and increased spontaneous calcium release events. The effects of cADPR were blocked by excess FKBP12.6 or after exposure to FK506 (23). These results, while providing the molecular basis for the actions of cADPR that were described in previous studies, show that RyR channel gating in ASM cells by cADPR requires the interaction of FKBP12.6 and RyR2 to form a complex. In the pancreatic β-cells, studies by Noguchi and colleagues showed that prior exposure to FK506 prevented calcium release induced by cADPR (35). In the absence of FKBP12.6, such as the case in the myocytes from FKBP12.6 null mice, sensitivity to cADPR-induced calcium release was lost. These observations suggest that FKBP12.6 is the binding site for cADPR and dissociation of FKBP12.6 from the RyR2 is required for the gating of the RyR channels. It should be emphasized that other mechanisms of RyR channel activation by cADPR have been advanced in some cell types. For example, direct activation of RyR channels by cADPR has been suggested in studies involving coronary artery smooth muscle (36). The role of calmodulin in cADPR-mediated calcium release in sea urchin eggs and pancreatic β-cells has also been demonstrated (37–39). In the sea urchin eggs, calmodulin is known to sensitize and enhance cADPR-mediated calcium release by orders of magnitude (38). However, in the pancreatic β-cells, the calmodulin effects appear to be mediated via phosphorylation of the RyR channels by a calmodulin kinase II (39). In permeabilized ASM cells maintained in a calcium-containing solution, cADPR caused a concentration-dependent calcium response that was transient in nature (25). When cADPR was added to the cells during ongoing Ach-induced calcium oscillations, there was an increase in basal calcium and in the frequency of the oscillations (25). While these results indicate that cADPR can release intracellular calcium and alter the characteristics of the calcium oscillations, it is not clear how these actions are brought forth in ASM cells. It is likely that binding to FKBP12.6 to cause its dissociation from RyR2, resulting in the activation of the RyR channels is the underlying mechanism. No studies to date have described whether calmodulin has a role in this process in ASM cells. A model describing the mechanisms by which cADPR causes calcium release through RyR channels is outlined in Figure 1.
Figure 1.
Mechanisms of cyclic-ADP-ribose (cADPR)-mediated Ca2+ mobilization through ryanodine receptor. cADPR interacts directly or indirectly with the ryanodine receptor (RyR) to increase the open probability of the ion channel. FK506-binding protein 12.6 (FKBP12.6) is physically associated with the subunits of RyR and keeps it in a closed state. Interaction of cADPR with the RyR causes dissociation of FKBP12.6 and an increase in the open probability of RyR to release sarcoplasmic reticular (SR) Ca2+. Calmodulin (CaM), a Ca2+-binding protein, is known to play a critical role in cADPR-mediated Ca2+ mobilization in sea urchin egg microsomes. In pancreatic β-cells, the CaM is known to recruit CaM kinase II to phosphorylate the RyR. The phosphorylation increases the open probability of the RyR. cADPR can also act directly on RyR to open the channel.
ADP-RIBOSYL CYCLASE, CD38, AND CYCLIC ADP-RIBOSE IN AIRWAY SMOOTH MUSCLE CELLS
Cyclic ADP-ribose is a potent calcium ion mobilizing molecule initially discovered in sea urchin egg microsomes (40). A family of enzymes known as ADP-ribosyl cyclases generates cADPR from β-NAD (41, 42). The presence of ADP-ribosyl cyclase in a wide range of mammalian tissues, including ASM, has been reported by several investigators (42–45). A broad range of mammalian cells also were shown to possess cADPR as the endogenous calcium ion–mobilizing molecule (46). The role of cADPR as an intracellular calcium–mobilizing agent has been well characterized in ASM cells isolated from different mammalian species (13, 25, 47, 48).
In ASM cells, cADPR and ADPR are generated by the multifunctional enzyme activity localized in a trans-membrane protein called CD38 (45). CD38, considered a cell surface receptor in hematopoietic cells, is also a member of a broader superfamily of enzymes called ADP-ribosyl cyclases (49). The superfamily of ADP-ribosyl cyclases includes two mammalian members (CD38 and CD157) and a cyclase from the invertebrate Aplysia (sea slug) species (42, 50). The primary amino acid sequence and the partial crystal structure of these three major ADP-ribosyl cyclases have been resolved (51–53). Analysis of the sequence and structural data indicates that the mammalian and invertebrate ADP-ribosyl cyclases have 25 to 30% identity in their primary structure (54, 55). CD38, the typical mammalian ADP-ribosyl cyclase, is a 45-kD type II transmembrane protein expressed in a wide variety of tissues (44). CD38 possesses multiple enzymatic activities (41). The ADP-ribosyl cyclase activity of CD38 cyclizes β-NAD to generate cADPR (41). The NADase (NAD glycohydrolase) activity generates ADPR from β-NAD. Unlike the Aplysia cyclase, which predominantly generates cADPR from β-NAD, CD38 converts the bulk of the β-NAD to ADPR (∼95%); only a minor fraction (∼5%) of the products represents cADPR (41, 56). These enzymes also possess a transglycosidation (base-exchange) activity capable of exchanging the nicotinamide moiety of NAD(P) with nicotinic acid or other nucleophiles. In the presence of nicotinic acid and NADP, the base-exhange reaction of the ADP-ribosyl cyclase family is responsible for generating nicotinic acid adenine dinucleotide phosphate (NAADP), another compound capable of regulating calcium release from intracellular stores in a variety of cell types, including those of some smooth muscles (57–62).
CD38 possesses a relatively long extracellular domain (carboxyl terminal) and shorter cytoplasmic and transmembrane domains (55). The single catalytic domain responsible for the multiple enzymatic activities also is located at the extracellular portion of the protein (63). It is not well understood how the single catalytic domain acts on multiple substrates to generate different products. However, investigators have proposed models based on structural and kinetics data that explain the multifunctional enzyme activity of CD38 (53, 64, 65). According to previous investigations on the multiple enzymatic activities, critical residues necessary for the specific enzymatic functions line the catalytic pocket of the protein (63, 66). Another aspect of CD38 that received much attention in the recent past was the “topological paradox” (67). The paradox pertains to the question of how the substrate, β-NAD reaches the extracellular catalytic domain of the CD38 and how the product cADPR is transported back into the cytoplasm. De Flora and coworkers have proposed a model that explains the delivery of β-NAD to the CD38 and the transport of cADPR back into the cytoplasm (67). According to their model, connexin-43 hemi channels act as transporter for β-NAD and makes this substrate available for the extracellular catalytic domain of CD38. The cADPR generated by the ADP-ribosyl cyclase activity of CD38 is transported back into the cells by specialized nucleoside transporters as well as by the oligomerized CD38 itself. Structural studies on CD38 indicate that the full-length CD38 could naturally exist in oligomerized high-molecular-weight forms (53, 68). Investigators have reported ADP-ribosyl cyclase activity that is independent of CD38 (69). The non-CD38 ADP-ribosyl cyclase activity has been reported in brain homogenates obtained from CD38 knockout mice (69). However, CD38 is reported to be the only ADP-ribosyl cyclase present in the ASM (47). Lung homogenates from CD38 knockout mice did not show detectable levels of ADP-ribosyl cyclase activity (47). The Ach- and endothelin-induced cellular calcium transients were also significantly attenuated in tracheal myocytes isolated from CD38 knockout mice, indicating the significant role of CD38/cADPR system in mediating agonist-induced calcium transients in ASM cells (47).
ACTIVATION OF CD38/cADPR-MEDIATED CALCIUM RELEASE PATHWAYS BY AGONISTS
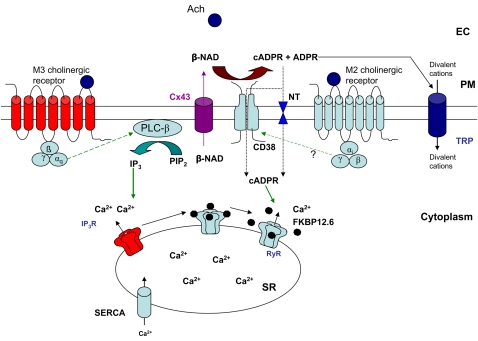
Contractile agonists acting on membrane receptors elicit cellular calcium transients in ASM cells (70). Many of these membrane-bound receptors for the agonists belong to the family of GPCRs. GPCRs transduce their signals downstream through heterotrimeric G proteins. Recruitment of the cADPR-mediated calcium release mechanism by contractile agonists has been investigated in our laboratory as well as by other investigators (13, 71–73). Experiments in freshly isolated porcine ASM cells demonstrated that the contractile agonist Ach elicits cellular calcium transients in a cADPR-dependent manner (13). However, the recruitment of cADPR-dependent calcium release mechanism was cholinergic receptor subtype-specific (13). Airway myocytes predominantly express M2 and M3 subtypes of cholinergic receptors (74). Application of acetylcholine causes the activation of both subtypes of the muscarinic receptors and the activation of multiple ion channels and calcium release (75). Kotlikoff and colleagues showed that M2 muscarinic receptor–mediated gating of nonselective cation channels requires a rise in cytosolic calcium mediated by M3 receptors in smooth muscle cells (75). Studies in our laboratory showed that selectively blocking M2 subtype of muscarinic receptors with methoctramine attenuated the Ach-mediated intracellular calcium transients (13). The possibility that methoctramine may have off-target effects on M3 muscarinic receptors cannot be ruled out. Addition of 8Br-cADPR to the myocytes failed to further suppress the methoctramine-insensitive calcium transients (13). This indicates that the intracellular calcium transients elicited by the activation of M2 subtype of cholinergic receptor is largely mediated through cADPR. It has previously been reported that the M3 subtype of cholinergic receptor elicits cellular calcium transients through the IP3-mediated calcium release mechanism (76). The M2 subtype of receptor is coupled to Gαi-type G proteins, whereas the M3 subtype is linked to Gαq-type G proteins (74). The observations suggest that in porcine ASM cells, there is receptor subtype specificity in recruiting the cADPR-mediated calcium release pathway possibly due to the difference in the types of G protein to which these receptors are coupled. Investigations in other types of smooth muscles have suggested a similar phenomenon. In bovine coronary arterial smooth muscles, the CD38/cADPR pathway of calcium release is recruited by activation of the M1 subtype of cholinergic receptor (77). Barone and coworkers demonstrated that, in peritubular smooth muscle cells isolated from rat testes, endothelin-1 recruited cADPR-mediated calcium release pathway in a receptor subtype-specific manner (71). It was found that in murine small mesenteric arteries activation of endothelin-1 subtype B receptors exclusively recruits cADPR-mediated calcium release, whereas the endothelin-1 subtype A receptors signal through both cADPR and IP3 (78). Therefore, recruitment of cADPR-mediated calcium mobilization by GPCRs and agonist- and receptor-subtype specificity of such recruitment are not uncommon in various types of smooth muscles. A model describing the mechanisms by which the CD38/cADPR signaling pathway is recruited by different GPCRs is presented in Figure 2.
Figure 2.
An integrative model of differential recruitment of cellular calcium mobilization mechanisms. Acetylcholine acts on M2 and M3 subtypes of muscarinic cholinergic receptors on the ASM cells to elicit a calcium response. M3 subtype of receptor, which is coupled to Gαq protein, recruits phospholipase C-β (PLC-β) and generates inositol 1,4,5-tris-phosphate (IP3). IP3 acts on IP3-receptor (IP3R) to increase its open probability to release Ca2+ from SR. Activation of the M2 subtype of receptor, which is coupled to Gαi protein, recruits CD38/cADPR signaling pathway. The β-NAD is transported out from the cytoplasm of the myocyte by connexin 43 hemichannel proteins (Cx43). cADPR generated by CD38 is transported back into the myocyte cytoplasm either through specialized nucleoside transporters (NT) or via the oligomerized CD38. ADPR, the predominant product of the CD38 enzymatic activity, acts on transient receptor potential (TRP) channels to elicit influx of divalent cations including Ca2+. Upon interaction of cADPR with RyR (direct or indirect), dissociation of FK506-binding protein 12.6 (FKBP12.6) from RyR results in open probability of the ion channel and releases SR Ca2+. SR Ca2+-ATPase 2 (SERCA2) pumps the cytosolic Ca2+ back into SR to replenish the SR Ca2+ stores. The TRP channels also play major role in replenishing the SR Ca2+ store through store-operated Ca2+ entry (SOCE). EC = extra cellular space; PM = plasma membrane.
INFLAMMATORY CYTOKINES AND CD38/cADPR-MEDIATED CELLULAR CALCIUM DYNAMICS
Inflammatory cytokines play critical roles in pathophysiologic conditions such as asthma (79). T helper type 2 (Th2) cytokines, such as IL-13 and IL-4, are known to play central roles in the pathophysiology of inflammatory airway diseases (80–82). Cytokines like these act both on the transient inflammatory cells and the resident cells, such as airway myocytes. Therefore, it is likely that the inflammatory cytokines also play a role in the altered spasmogen-induced smooth muscle contractility during inflammation of the airways. Since the CD38/cADPR pathway contributes to cellular calcium transients, the molecular and functional effects of the inflammatory cytokines on the CD38/cADPR cascade were investigated in our laboratory. When isolated human ASM cells (HASM) were incubated with IL-13, the expression of CD38 was significantly elevated compared to the vehicle-treated controls (83). The elevated expression of CD38 was also reflected in the ADP-ribosyl cyclase activity of the cellular homogenates (83). The HASM cells pretreated with IL-13 also showed a significantly augmented net increase in cellular calcium in response to a range of contractile agonists, and the agonist-mediated cellular calcium transients were attenuated upon pretreatment of the cells with 8-Br-cADPR (83). These findings suggest that IL-13 has a permissive effect on the CD38/cADPR-mediated cellular calcium mobilization. In order to determine if the in vitro effect of IL-13 on airway myocytes is representative of the physiologic effects of this cytokine, in vivo experiments were carried out in mouse models. When CD38 knockout and wild-type mice were intranasally exposed to IL-13, CD38 knockout mice displayed significantly attenuated airway hyperresponsiveness to inhaled methacholine compared with the wild-type controls (84). Isolated tracheal rings from CD38 knockout mice, when pretreated with IL-13, showed significant reduction in rate of force development to carbachol compared with that of the IL-13–treated tracheae from the wild-type mice (84). However, intranasal exposure to IL-13 elicited a comparable inflammatory response in the both groups of mice (84). The findings of the above study indicate that IL-13–induced airway hyperresponsiveness requires CD38 expression in the airways and airway inflammation per se is not sufficient in the development of airway hyperresponsiveness.
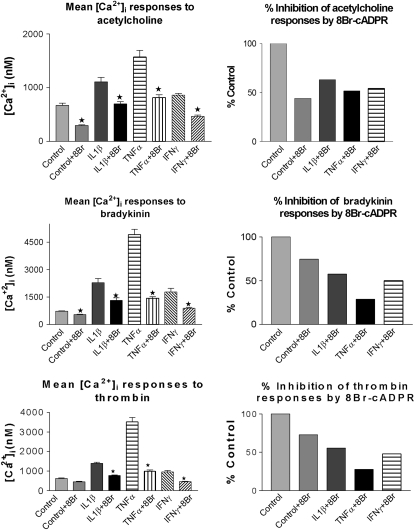
TNF-α, another important inflammatory cytokine, was previously demonstrated to induce expression of CD38 in airway and myometrial smooth muscles (48, 85). The pathophysiologic role of TNF-α is shown by the fact that it is found in high levels in bronchoalveolar lavage fluid and sputum of patients with asthma and chronic obstructive pulmonary disease (86, 87). Results of recent human clinical trials with anti–TNF-α-immunomodulators (etanercept) (88, 89) and anti–TNF-α monoclonal antibodies (infliximab) (90) have shown that TNF-α has a pathobiologic role in asthma, particularly in severe asthma that is refractory to corticosteroids. TNF-α appears to have a central role in the initiation of airway inflammation and AHR, hallmarks of asthma. Treatment of the HASM cells with human recombinant TNF-α, IL-1β, or IFN-γ resulted in increased expression of CD38, reflected in elevated ADP-ribosyl cyclase activity, and augmented intracellular calcium responses to contractile agonists (48). The augmented calcium responses to the agonists were inhibited by 8Br-cADPR, a membrane-permeant cADPR antagonist (Figure 3). Furthermore, the TNF-α–induced augmented calcium response was attenuated in the cells that were transiently transduced with adenoviral vectors carrying an anti-sense CD38 construct (91). These findings indicate that TNF-α augments the sensitivity of HASM cells to spasmogens by up-regulating the expression of CD38. Further studies demonstrated that the up-regulation of CD38 by TNF-α is mediated through the transcription factors NF-κB and AP-1 and involves the MAP kinases (92, 93). White and colleagues reported a significantly increased expression of TRPC3 after TNF-α treatment of ASM cells (32). Moreover, the increased TRPC3 expression was associated with augmented Ach-elicited calcium responses as well as a sixfold increase in SOCE (32). Transient transfection using small interfering RNA, which decreased TRPC3 expression, also resulted in attenuation of SOCE and agonist-induced [Ca2+]i responses (32). These studies provided an additional mechanism of intracellular calcium regulation in ASM cells by the inflammatory cytokine TNF-α involving TRPC3. It will be interesting to investigate the transcriptional mechanisms of TRPC3 expression by TNF-α.
Figure 3.
Treatment of cells with 8Br-cADPR results in attenuated [Ca2+]i responses to agonists in control and cytokine-treated human airway smooth muscle (HASM) cells. Fura-2/AM-loaded control and cytokine-treated HASM cells were incubated with 8Br-cADPR and [Ca2+]i responses to agonists were measured. The net [Ca2+]i values in the presence of 8Br-cADPR in control and cytokine-treated cells were compared with those of the corresponding controls (in the absence of 8Br-cADPR). Net [Ca2+]i responses to acetylcholine, bradykinin, and thrombin in control and cytokine-treated HASM cells, in the presence or absence of 8Br-cADPR, are shown. Panels on the right represent % inhibition of the [Ca2+]i responses by 8Br-cADPR. Note significant (P ≤ 0.05) attenuation of [Ca2+]i responses in the presence of 8Br-cADPR in control and cytokine-treated HASM cells. Data are from three different cell preparations. IL-1β = interleukin-1β; TNF-α = tumour necrosis factor-α; IFN-γ = interferon-γ. Reprinted by permission from Reference 48.
CONCLUSIONS
In this article we have presented evidence from recent investigations for the contribution of cADPR to intracellular calcium regulation in ASM cells. Since the discovery of cADPR as a calcium-mobilizing second messenger molecule in lower invertebrates in the late 1980s, there has been active interest in defining and identifying the pathways involved in the synthesis of cADPR, the mechanisms by which intracellular calcium release is brought forth by this agent, and how this pathway of calcium regulation is regulated in pathophysiologic states. The evidence from these studies reveals that cADPR-mediated calcium release results from activation of Gαi-type G proteins and involves ryanodine receptor channels in the SR. Activation of the ryanodine receptor channels by cADPR appears to result from dissociation of FKBP12.6, and smooth muscle cells obtained from FKBP12.6-null mice lose their sensitivity to cADPR-mediated calcium release. Airway myocytes obtained from CD38 knockout mice have attenuated calcium responses to contractile agonists, and CD38-knockout mice have reduced capacity to develop airway responsiveness to methacholine. Furthermore, CD38 knockout mice also develop attenuated airway hyperresponsiveness after intranasal challenge with the Th2 cytokine IL-13. The CD38/cADPR signaling pathway is up-regulated by TNF-α and Th2 cytokines, resulting in augmented intracellular calcium signaling attributable to cADPR. The enhanced calcium influx in bronchial smooth muscle cells after treatment with TNF-α is also attributable to activation of the TRPC3 channels in the plasma membrane. These results are important since TNF-α has an important role in asthma, and since TNF-α blockade in animal models of asthma as well as in human asthma reduces the airway inflammation and airway hyperresponsiveness. This can result from modulation of the TRPC3 and the CD38/cADPR signaling pathways of intracellular calcium regulation in airway smooth muscle. Future studies should test the role of the CD38/cADPR pathway and the therapeutic potential of CD38 inhibitors and/or cADPR antagonists initially in animal models of asthma and eventually in human asthma.
Acknowledgments
The authors thank Drs. White, Deshpande, Kang, and Tirumurugaan for their contributions. They thank Dr. Reynold A. Panettieri for providing human airway smooth muscle cells used in some of the studies reported in this review.
Some of the work presented in this review was supported by grants from the National Institutes of Health (HL057498 to M.S.K. and DA11806 to T.F.W.).
Conflict of Interest Statement: J.A.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.F.W. received $20,676 in 2006 from Genmab B.V. as a research grant. M.S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 1991;435:123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shieh CC, Petrini MF, Dwyer TM, Farley JM. Concentration-dependence of acetylcholine-induced changes in calcium and tension in swine trachealis. J Pharmacol Exp Ther 1991;256:141–148. [PubMed] [Google Scholar]
- 3.Sims SM, Jiao Y, Zheng ZG. Intracellular calcium stores in isolated tracheal smooth muscle cells. Am J Physiol 1996;271:L300–L309. [DOI] [PubMed] [Google Scholar]
- 4.Baron CB, Cunningham M, Strauss JF III, Coburn RF. Pharmacomechanical coupling in smooth muscle may involve phosphatidylinositol metabolism. Proc Natl Acad Sci USA 1984;81:6899–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray RK, Bennett CF, Fluharty SJ, Kotlikoff MI. Mechanism of phorbol ester inhibition of histamine-induced IP3 formation in cultured airway smooth muscle. Am J Physiol 1989;257:L209–L216. [DOI] [PubMed] [Google Scholar]
- 6.Murray RK, Fleischmann BK, Kotlikoff MI. Receptor-activated Ca influx in human airway smooth muscle: use of Ca imaging and perforated patch-clamp techniques. Am J Physiol 1993;264:C485–C490. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Farley JM. Depletion and refilling of acetylcholine- and caffeine-sensitive Ca++ stores in tracheal myocytes. J Pharmacol Exp Ther 1996;277:789–795. [PubMed] [Google Scholar]
- 8.Nuttle LC, Farley JM. Frequency modulation of acetylcholine-induced oscillations in Ca++ and Ca(++)-activated Cl- current by cAMP in tracheal smooth muscle. J Pharmacol Exp Ther 1996;277:753–760. [PubMed] [Google Scholar]
- 9.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol 1997;272:C966–C975. [DOI] [PubMed] [Google Scholar]
- 10.Somlyo AP, Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J 1989;3:2266–2276. [DOI] [PubMed] [Google Scholar]
- 11.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 1994;372:231–236. [DOI] [PubMed] [Google Scholar]
- 12.Prakash YS, Pabelick CM, Kannan MS, Sieck GC. Spatial and temporal aspects of ACh-induced. Cell Calcium 2000;27:153–162. [DOI] [PubMed] [Google Scholar]
- 13.White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J 2003;17:482–484. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Lederer MR, Xiao RP, Gomez AM, Zhou YY, Ziman B, Spurgeon H, Lakatta EG, Lederer WJ. Excitation-contraction coupling in heart: new insights from Ca2+ sparks. Cell Calcium 1996;20:129–140. [DOI] [PubMed] [Google Scholar]
- 15.Parker I, Zang WJ, Wier WG. Ca2+ sparks involving multiple Ca2+ release sites along Z-lines in rat heart cells. J Physiol 1996;497:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabelick CM, Prakash YS, Kannan MS, Sieck GC. Spatial and temporal aspects of calcium sparks in porcine tracheal smooth muscle cells. Am J Physiol 1999;277:L1018–L1025. [DOI] [PubMed] [Google Scholar]
- 17.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 1995;270:633–637. [DOI] [PubMed] [Google Scholar]
- 18.Klein MG, Cheng H, Santana LF, Jiang YH, Lederer WJ, Schneider MF. Two mechanisms of quantized calcium release in skeletal muscle. Nature 1996;379:455–458. [DOI] [PubMed] [Google Scholar]
- 19.Lacampagne A, Lederer WJ, Schneider MF, Klein MG. Repriming and activation alter the frequency of stereotyped discrete Ca2+ release events in frog skeletal muscle. J Physiol 1996;497:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science 1995;269:1723–1726. [DOI] [PubMed] [Google Scholar]
- 21.Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca(2+) sparks in smooth muscle. J Gen Physiol 2004;123:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol 1997;272:L659–L664. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol 2004;286:C538–C546. [DOI] [PubMed] [Google Scholar]
- 24.Hyvelin JM, Martin C, Roux E, Marthan R, Savineau JP. Human isolated bronchial smooth muscle contains functional ryanodine/caffeine-sensitive Ca-release channels. Am J Respir Crit Care Med 2000;162:687–694. [DOI] [PubMed] [Google Scholar]
- 25.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of. Am J Physiol 1998;274:C1653–C1660. [DOI] [PubMed] [Google Scholar]
- 26.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L909–L917. [DOI] [PubMed] [Google Scholar]
- 27.Worley JF III, Kotlikoff MI. Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol 1990;259:L468–L480. [DOI] [PubMed] [Google Scholar]
- 28.Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986;7:1–12. [DOI] [PubMed] [Google Scholar]
- 29.Takemura H, Putney JW Jr. Capacitative calcium entry in parotid acinar cells. Biochem J 1989;258:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci 2001;2:387–396. [DOI] [PubMed] [Google Scholar]
- 31.Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci 2006;27:25–32. [DOI] [PubMed] [Google Scholar]
- 32.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol 2006;35:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem 1996;271:20385–20391. [DOI] [PubMed] [Google Scholar]
- 34.Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol 2002;282:H1304–H1310. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem 1997;272:3133–3136. [DOI] [PubMed] [Google Scholar]
- 36.Li PL, Tang WX, Valdivia HH, Zou AP, Campbell WB. cADP-ribose activates reconstituted ryanodine receptors from coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 2001;280:H208–H215. [DOI] [PubMed] [Google Scholar]
- 37.Lee HC, Aarhus R, Graeff R, Gurnack ME, Walseth TF. Cyclic ADP ribose activation of the ryanodine receptor is mediated by calmodulin. Nature 1994;370:307–309. [DOI] [PubMed] [Google Scholar]
- 38.Lee HC, Aarhus R, Graeff RM. Sensitization of calcium-induced calcium release by cyclic ADP-ribose and calmodulin. J Biol Chem 1995;270:9060–9066. [DOI] [PubMed] [Google Scholar]
- 39.Takasawa S, Ishida A, Nata K, Nakagawa K, Noguchi N, Tohgo A, Kato I, Yonekura H, Fujisawa H, Okamoto H. Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization. J Biol Chem 1995;270:30257–30259. [DOI] [PubMed] [Google Scholar]
- 40.Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem 1987;262:9561–9568. [PubMed] [Google Scholar]
- 41.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993;262:1056–1059. [DOI] [PubMed] [Google Scholar]
- 42.Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul 1991;2:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guse AH. Second messenger function and the structure-activity relationship of cyclic adenosine diphosphoribose (cADPR). FEBS J 2005;272:4590–4597. [DOI] [PubMed] [Google Scholar]
- 44.Rusinko N, Lee HC. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem 1989;264:11725–11731. [PubMed] [Google Scholar]
- 45.White TA, Johnson S, Walseth TF, Lee HC, Graeff RM, Munshi CB, Prakash YS, Sieck GC, Kannan MS. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta 2000;1498:64–71. [DOI] [PubMed] [Google Scholar]
- 46.Walseth TF, Aarhus R, Zeleznikar RJ Jr, Lee HC. Determination of endogenous levels of cyclic ADP-ribose in rat tissues. Biochim Biophys Acta 1991;1094:113–120. [DOI] [PubMed] [Google Scholar]
- 47.Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in CD38-deficient mice. Am J Respir Cell Mol Biol 2005;32:149–156. [DOI] [PubMed] [Google Scholar]
- 48.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 2003;17:452–454. [DOI] [PubMed] [Google Scholar]
- 49.Schuber F, Lund FE. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr Mol Med 2004;4:249–261. [DOI] [PubMed] [Google Scholar]
- 50.Ortolan E, Vacca P, Capobianco A, Armando E, Crivellin F, Horenstein A, Malavasi F. CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct 2002;20:309–322. [DOI] [PubMed] [Google Scholar]
- 51.Prasad GS, McRee DE, Stura EA, Levitt DG, Lee HC, Stout CD. Crystal structure of Aplysia ADP ribosyl cyclase, a homologue of the bifunctional ectozyme CD38. Nat Struct Biol 1996;3:957–964. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto-Katayama S, Ariyoshi M, Ishihara K, Hirano T, Jingami H, Morikawa K. Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities. J Mol Biol 2002;316:711–723. [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q. Crystal structure of human CD38 extracellular domain. Structure 2005;13:1331–1339. [DOI] [PubMed] [Google Scholar]
- 54.States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci 1992;17:495. [DOI] [PubMed] [Google Scholar]
- 55.Lee HC. Enzymatic functions and structures of CD38 and homologs. Chem Immunol 2000;75:39–59. [DOI] [PubMed] [Google Scholar]
- 56.Cakir-Kiefer C, Muller-Steffner H, Schuber F. Unifying mechanism for Aplysia ADP-ribosyl cyclase and CD38/NAD(+) glycohydrolases. Biochem J 2000;349:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem 1995;270:30327–30333. [DOI] [PubMed] [Google Scholar]
- 58.Bak J, Billington RA, Timar G, Dutton AC, Genazzani AA. NAADP receptors are present and functional in the heart. Curr Biol 2001;11:987–990. [DOI] [PubMed] [Google Scholar]
- 59.Mandi M, Toth B, Timar G, Bak J. Ca2+ release triggered by NAADP in hepatocyte microsomes. Biochem J 2006;395:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yusufi AN, Cheng J, Thompson MA, Chini EN, Grande JP. Nicotinic acid-adenine dinucleotide phosphate (NAADP) elicits specific microsomal Ca2+ release from mammalian cells. Biochem J 2001;353:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yusufi AN, Cheng J, Thompson MA, Burnett JC, Grande JP. Differential mechanisms of Ca(2+) release from vascular smooth muscle cell microsomes. Exp Biol Med (Maywood) 2002;227:36–44. [DOI] [PubMed] [Google Scholar]
- 62.Boittin FX, Galione A, Evans AM. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ Res 2002;91:1168–1175. [DOI] [PubMed] [Google Scholar]
- 63.Munshi C, Aarhus R, Graeff R, Walseth TF, Levitt D, Lee HC. Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J Biol Chem 2000;275:21566–21571. [DOI] [PubMed] [Google Scholar]
- 64.Sauve AA, Munshi C, Lee HC, Schramm VL. The reaction mechanism for CD38. A single intermediate is responsible for cyclization, hydrolysis, and base-exchange chemistries. Biochemistry 1998;37:13239–13249. [DOI] [PubMed] [Google Scholar]
- 65.Liu Q, Kriksunov IA, Graeff R, Lee HC, Hao Q. Structural basis for formation and hydrolysis of calcium messenger cyclic ADP-ribose by human CD38. J Biol Chem 2006;281:32861–32869. [DOI] [PubMed] [Google Scholar]
- 66.Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem 2006;281:28951–28957. [DOI] [PubMed] [Google Scholar]
- 67.De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci 2004;1028:176–191. [DOI] [PubMed] [Google Scholar]
- 68.Umar S, Malavasi F, Mehta K. Post-translational modification of CD38 protein into a high molecular weight form alters its catalytic properties. J Biol Chem 1996;271:15922–15927. [DOI] [PubMed] [Google Scholar]
- 69.Ceni C, Muller-Steffner H, Lund F, Pochon N, Schweitzer A, De Waard M, Schuber F, Villaz M, Moutin MJ. Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38-deficient mice. J Biol Chem 2003;278:40670–40678. [DOI] [PubMed] [Google Scholar]
- 70.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2005;288:L773–L788. [DOI] [PubMed] [Google Scholar]
- 71.Barone F, Genazzani AA, Conti A, Churchill GC, Palombi F, Ziparo E, Sorrentino V, Galione A, Filippini A. A pivotal role for cADPR-mediated Ca2+ signaling: regulation of endothelin-induced contraction in peritubular smooth muscle cells. FASEB J 2002;16:697–705. [DOI] [PubMed] [Google Scholar]
- 72.Fritz N, Macrez N, Mironneau J, Jeyakumar LH, Fleischer S, Morel JL. Ryanodine receptor subtype 2 encodes Ca2+ oscillations activated by acetylcholine via the M2 muscarinic receptor/cADP-ribose signalling pathway in duodenum myocytes. J Cell Sci 2005;118:2261–2270. [DOI] [PubMed] [Google Scholar]
- 73.Kip SN, Smelter M, Iyanoye A, Chini EN, Prakash YS, Pabelick CM, Sieck GC. Agonist-induced cyclic ADP ribose production in airway smooth muscle. Arch Biochem Biophys 2006;452:102–107. [DOI] [PubMed] [Google Scholar]
- 74.Hirshman CA, Lande B, Croxton TL. Role of M2 muscarinic receptors in airway smooth muscle contraction. Life Sci 1999;64:443–448. [DOI] [PubMed] [Google Scholar]
- 75.Kotlikoff MI, Dhulipala P, Wang YX. M2 signaling in smooth muscle cells. Life Sci 1999;64:437–442. [DOI] [PubMed] [Google Scholar]
- 76.Roux E, Guibert C, Savineau JP, Marthan R. [Ca2+]i oscillations induced by muscarinic stimulation in airway smooth muscle cells: receptor subtypes and correlation with the mechanical activity. Br J Pharmacol 1997;120:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge ZD, Zhang DX, Chen YF, Yi FX, Zou AP, Campbell WB, Li PL. Cyclic ADP-ribose contributes to contraction and Ca2+ release by M1 muscarinic receptor activation in coronary arterial smooth muscle. J Vasc Res 2003;40:28–36. [DOI] [PubMed] [Google Scholar]
- 78.Giulumian AD, Meszaros LG, Fuchs LC. Endothelin-1-induced contraction of mesenteric small arteries is mediated by ryanodine receptor Ca2+ channels and cyclic ADP-ribose. J Cardiovasc Pharmacol 2000;36:758–763. [DOI] [PubMed] [Google Scholar]
- 79.Riffo-Vasquez Y, Pitchford S, Spina D. Cytokines in airway inflammation. Int J Biochem Cell Biol 2000;32:833–853. [DOI] [PubMed] [Google Scholar]
- 80.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest 2003;111:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Curr Allergy Asthma Rep 2004;4:123–131. [DOI] [PubMed] [Google Scholar]
- 82.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 83.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 2004;31:36–42. [DOI] [PubMed] [Google Scholar]
- 84.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 2006;291:L1286–L1293. [DOI] [PubMed] [Google Scholar]
- 85.Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology 2004;145:881–889. [DOI] [PubMed] [Google Scholar]
- 86.Erzurum SC. Inhibition of tumor necrosis factor alpha for refractory asthma. N Engl J Med 2006;354:754–758. [DOI] [PubMed] [Google Scholar]
- 87.Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol 2001;79:132–140. [DOI] [PubMed] [Google Scholar]
- 88.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 2005;60:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med 2006;354:697–708. [DOI] [PubMed] [Google Scholar]
- 90.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-α in asthma. Am J Respir Crit Care Med 2006;174:753–762. [DOI] [PubMed] [Google Scholar]
- 91.Kang BN, Deshpande DA, Tirumurugaan KG, Panettieri RA, Walseth TF, Kannan MS. Adenoviral mediated anti-sense CD38 attenuates TNF-alpha-induced changes in calcium homeostasis of human airway smooth muscle cells. Can J Physiol Pharmacol 2005;83:799–804. [DOI] [PubMed] [Google Scholar]
- 92.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of NF-kappaB and sensitivity to glucocorticoids. FASEB J 2006;20:1000–1002. [DOI] [PubMed] [Google Scholar]
- 93.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-{alpha} induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-{kappa}B and AP-1. Am J Physiol Lung Cell Mol Physiol (In press) [DOI] [PubMed]