Abstract
Airway smooth muscle (SM) develops from local mesenchymal cells located around the tips of growing epithelial buds. These cells gradually displace from distal to proximal position alongside the bronchial tree, elongate, and begin to synthesize SM-specific proteins. Mechanical tension (either generated by cell spreading/elongation or stretch), as well as epithelial paracrine factors, regulates the process of bronchial myogenesis. The specific roles of many of these paracrine factors during normal lung development are currently unknown. It is also unknown how and if mechanical and paracrine signals integrate into a common myogenic pathway. Furthermore, as with vascular SM and other types of visceral SM, we are just beginning to elucidate the intracellular signaling pathways and the genetic program that controls lung myogenesis. Here we present what we have learned so far about the embryogenesis of bronchial muscle.
Keywords: bronchial muscle, stretch, tension-induced myogenesis, cell shape, epithelial cell–derived morphogens
Airway smooth muscle (SM) develops from local mesenchymal cells that, at the initiation of the pseudoglandular period (gestation day 11 in mouse, 13 in rat, and week 5 in human), begin to elongate and express SM-specific proteins (1). Bronchial SM cells are first detected in the trachea/proximal main bronchi and follow the developing bronchial tree in a proximal to distal fashion (2–7). This pattern is the result of myogenesis proceeding in the opposite direction from mesenchymal cell precursors located at the tips of distal airways (8, 9). The appearance of bronchial SM precedes the appearance of vascular musculature by several days (3, 5, 6).
SM is found at sites that sustain mechanical tension in the form of hydrostatic pressure or shear stress, such as blood vessels and hollowed viscera, which during embryogenesis are filled with fluids. Furthermore, SM cells develop attached to basement membranes, which allow the former to spread and elongate, a process that generates additional cytoskeletal forces. Finally visceral SM, including that of the bronchial tree, originates around epithelia, which produce a variety of soluble factors with paracrine effect on the mesenchyme (10, 11). A growing body of evidence indicates that mechanical forces and epithelial-derived morphogenic factors are both involved in the development of airway SM.
EMBRYONIC MESENCHYMAL CELL SPREADING/ELONGATION AND AIRWAY SM MYOGENESIS
Evidence for the myogenic role of mechanical tension, in the form of cell spreading/elongation and stretching, comes mainly from our laboratory. Studies on the role of laminin (LN)-1, a main component of epithelial–mesenchymal basement membranes (12–14), demonstrate that during lung development LN-1 expression is induced in epithelial and mesenchymal cells upon contact with each other and further accumulates at the epithelial–mesenchymal interface as a polymer (15) essential for basement membrane formation (12–14). Either blocking LN-1 polymerization and thereby basement membrane formation, or attachment of mesenchymal cells to the LN-1 polymer with epitope-specific antibodies or antisense probes, prevents the normal elongation of mesenchymal cells surrounding the epithelium (15–17). When prevented from elongating, the embryonic mesenchymal cells do not differentiate into bronchial SM cells, regardless of epithelial cell presence (15–17). These observations led us to study the role of the cell shape per se on SM myogenesis. Undifferentiated embryonic mesenchymal cells from different organs were plated on microsurfaces of diameters that either maintained the original round cell shape or facilitated cell elongation (18). Irrespective of their fate in vivo, after 18 hours in culture all the elongated cells differentiated into SM myoblasts, as indicated by the expression of SM-specific proteins and development of membrane potentials of −60 mV and voltage-dependent Ca2+ currents, characteristic of SM cells. After 72 to 96 hours in culture, the cells attained the same level of SM-specific proteins as found in mature SM cells. SM differentiation is equally observed in proliferating and quiescent cells, suggesting that the process is not significantly affected by cell proliferation. In contrast, round cells remain undifferentiated as indicated by the expression of high levels of α-fetoprotein, an embryonic marker (18). Known modulators of SM differentiation like transforming growth factor (TGF)-β1 (19, 20), retinoic acid (21), platelet-derived growth factor (20, 22), sonic hedgehog (Shh) (23), and bone morphogenetic protein-4 (BMP-4) (9) neither stimulated round cells to differentiate nor prevented elongated cells from differentiating (18; Y. Zhou and L. Schuger, unpublished observations). Therefore, regardless of the organ of origin and normal fate of the cell in vivo, embryonic mesenchymal cells follow a myogenic pathway upon spreading/elongation. More recently it has been shown that human mesenchymal stem cells can be induced to differentiate either into SM or osteocytes by controlling the cell shape and tension with artificial matrices of different elasticity (24).
Undifferentiated mesenchymal cells do not express LN-2, the main LN produced by muscle (25), whereas when these cells are allowed to elongate, LN-2 expression and synthesis are induced (8). Similarly, LN-2 is synthesized by peribronchial mesenchymal cells when these elongate upon the onset of bronchial myogenesis (8). Blocking LN-2 with a specific antibody inhibits mesenchymal cell spreading/elongation and SM myogenic differentiation (8). Supporting these in vitro findings, decreases in the size and quantity of bronchial SM cells (8), as well as severe skeletal muscle abnormalities (26, 27), are seen in mice lacking LN-2 (26). In conclusion, these studies suggest that newly synthesized LN-1 and LN-2 accumulate around the developing bronchial tree, and by inducing spread/elongation of mesenchymal cell precursors, stimulate SM myogenesis (8).
EMBRYONIC MESENCHYMAL CELL STRETCHING AND BRONCHIAL SM DIFFERENTIATION
SM develops exclusively at sites that sustain mechanical tension such as hollow viscera and vasculature. In addition, cell spreading/elongation generate cytoskeletal tension forces. We therefore sought to determine whether stretch, by itself (in the absence of cell spreading) was sufficient to initiate SM myogenesis. Lung undifferentiated mesenchymal cells plated on silastic membranes were subjected to uniaxial continuous stretching. Five to ten percent stretching induced the expression of SM myosin, SM α-actin, desmin, and SM22 (28). Cells subjected to 0 or 1% stretch remain undifferentiated and synthesize high levels of α-fetoprotein (28). Undifferentiated mesenchymal cells from kidney and intestine do not respond to uniaxial stretch, suggesting a higher sensitivity of lung mesenchyme to mechanical tension. Embryonic lung explants from fetal mouse were further used to determine the effect of mechanical stretch upon SM myogenesis. The explants were cultured embedded in a thin collagen gel layer and their airway intraluminal pressure was increased, decreased, or eliminated. Increase in intrabronchial hydrostatic pressure enhances SM myogenesis as visualized by increase in SM α-actin surrounding the bronchial tree, whereas in absence of hydrostatic pressure, there is no bronchial muscle development (28) (Figure 1). To determine whether abnormal mechanical tension may cause abnormal SM development, in vivo studies were focused on two types of human hypoplastic lungs: those related to oligohydramnion and those related to diaphragmatic hernia, since in both there is decreased lung distension (29–35). Supporting the in vitro findings, these hypoplastic lungs show severe decrease to near absence of airway SM cells and proportional decrease in elastin, which is mainly produced by SM cells (28) (Figure 2). Significant bronchial SM paucity was recently documented in mutant embryos lacking skeletal muscle and therefore unable to generate fetal breathing-like movements, decreasing thereby the intraluminal hydrostatic pressure (36). It has been recently shown that the intracardiac fluid forces generated from blood flow are essential for cardiogenesis (37) and that stretch of vascular wall in adult mice significantly increases SM gene expression (38). Therefore it is plausible that vascular myogenesis is also modulated by mechanical forces.
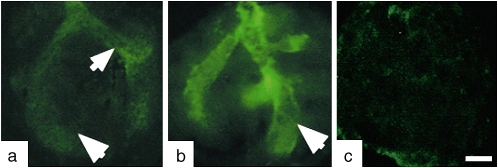
Figure 1.
Embryonic whole lung explants cultured in the presence of extra- and intra-luminal dextran (volume expander) and immunostained for smooth muscle (SM) α-actin. (a) SM α-actin in control explants cultured without dextran (unmodified pressure). Some noticeable bronchial SM development is present (arrows). (b) SM α-actin in lung explants cultured with 5% dextran inside airways (increased pressure) shows hyperplastic bronchial muscle (arrow). (c) No bronchial myogenesis in lung explants cultured with 1% dextran in the culture medium (eliminated pressure). Bar = 100 μm. Reprinted by permission from Reference 28.
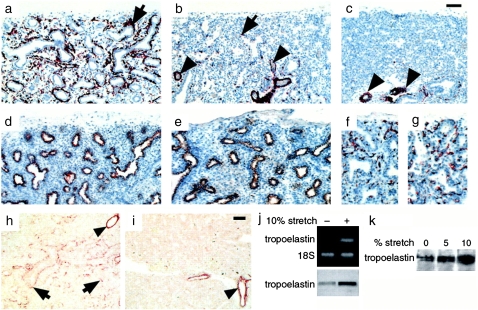
Figure 2.
Immunohistochemistry showing paucity of bronchial SM cells in human fetal hypoplastic lungs. Shown are histologic sections from (a) normal lung, (b) hypoplastic lung caused by oligohydramnion, and (c) hypoplastic lung caused by diaphragmatic hernia, all at 22 weeks of gestation, immunostained for SM α-actin. There is significant decrease in bronchial SM cells (arrows) in the hypoplastic lungs (b and c), particularly in those compressed by intrathoracic herniation of abdominal viscerae due to diaphragmatic hernia (c). The vascular musculature is unaffected (arrowheads). In the same hypoplastic lung shown in b, the epithelial cells, immunostained for cytokeratins (e), and the endothelial cells, immunostained for PECAM-1 (g), show no changes compared with controls (d and f). h and i demonstrate immunohistochemistry showing decrease in tropoelastin deposition in human hypoplastic lungs. (h) Histologic sections from normal lung at 20 weeks of gestation demonstrate tropoelastin deposition around bronchi and bronchioli (arrows). (i) Histologic sections from same age hypoplastic lung reveal essentially no tropoelastin deposition, with the exception of vascular SM that shows no changes in tropoelastin when compared with controls (arrowheads). Bar = 60 μm in a–e, h, and i, and 100 μm in f and g. RT-PCR and immunoblot show stretch-induced upregulation of tropoelastin expression in mouse lung embryonic mesenchymal cells undergoing myogenic differentiation. (k) Immunoblot shows stretch-induced upregulation of tropoelastin synthesis in human lung embryonic mesenchymal cells undergoing myogenic differentiation. Reprinted by permission from Reference 28.
MOLECULAR MECHANISMS INVOLVED IN TENSION-INDUCED BRONCHIAL SM MYOGENESIS
The molecular pathways that lead an undifferentiated lung mesenchymal cell to differentiate into a bronchial SM cell are largely unknown. Serum response factor (SRF) and its dominant-negative isoform SRFΔ5 play an important role in tension-induced myogenesis (28). SRF is one of the best-studied transcription factors regulating SM gene expression (39–41). SRF is a member of the MADS box family of transcription factors. SRF binds to the CArG box-like motif found in muscle-specific proteins and stimulates their transcription (39–41). SRFΔ5 serves as naturally occurring dominant-negative isoform that blocks SRF-dependent muscle gene expression (42, 43). Undifferentiated mesenchymal cells synthesize SRF and SRFΔ5. When these cells are subjected to mechanical tension, whether in the form of cell spreading/elongation or stretching, SRFΔ5 production is suppressed while SRF production is concomitantly increased. This change in isoform pattern induces or at least facilitates the onset of myogenesis (28). The role of SRF and SRFΔ5 is further confirmed by the fact that hypoplastic lungs associated with decreased intraluminal pressure still express both isoforms, whereas normal lungs of the same fetal age synthesized only full SRF (28). Indicating the importance of SRF for vascular SM development, recent studies demonstrated that conditional deletion of Srf in the vascular system results in a decrease in vascular SM cells proportional to the decrease in SRF-positive cells (44).
A search for genes differentially expressed during SM myogenesis indicated that Rho-a is one of the genes highly expressed in undifferentiated embryonic mesenchymal cells but expressed at low levels in their differentiated SM counterparts (45). RhoA is a small guanosine triphosphatase (GTPase) signaling protein that plays a critical role in the organization of the cytoskeleton (46). Additional studies confirmed that RhoA message, protein, and activity decrease during spread- and stretch-induced myogenesis (45; S. Jakkaraju and L. Schuger, unpublished observations) and in vivo, upon bronchial myogenesis (45). Functional studies in which constitutively active RhoA was expressed in undifferentiated embryonic mesenchymal cells show that high RhoA activity maintains the round, undifferentiated phenotype. However, if the cells begin to elongate and synthesize SM-specific proteins, the effect of RhoA is reversed from inhibitory to stimulatory (45). In round, undifferentiated mesenchymal cells both SRF and SRFΔ5 are enriched in the cytoplasm. Upon cell elongation, SRF translocates to the nucleus, whereas SRFΔ5 disappears. Transfection with dominant positive RhoA maintains the cells' round shape and prevents the SRF changes occurring upon cell elongation (45). Since LN-1 and mainly LN-2 were found to inhibit RhoA expression and activity (45), we proposed an integrative mechanism to explain how tension, in the form of cell spreading/elongation and stretch, may contribute to bronchial myogenesis (Figure 3) (47). More recently we identified a set of tension-responsive factors in lung embryonic mesenchymal cells, which we referred to as TIPs (tension-induced/inhibited proteins) (48). TIPs display signature motifs characteristic of nuclear receptor coregulators and chromatin remodeling enzymes. Functional studies revealed that TIP-1 and TIP-3 are involved in the cell's selection between the myogenic or the adipogenic pathway. TIP-1, induced by tension in the form of cell spreading and/or stretching, promotes myogenesis, while TIP-3, present in undifferentiated lung mesenchymal cells and inhibited by tension, stimulates adipogenesis. TIP-1 induces myogenesis by interacting with the SRF promoter and acetylating histones 3 and 4 at this promoter site, thereby activating its transcription. The process is dependent on TIP-1 nuclear receptor binding box (48). At the present it is unknown whether TIPs participate in the mechanism proposed in Figure 3.
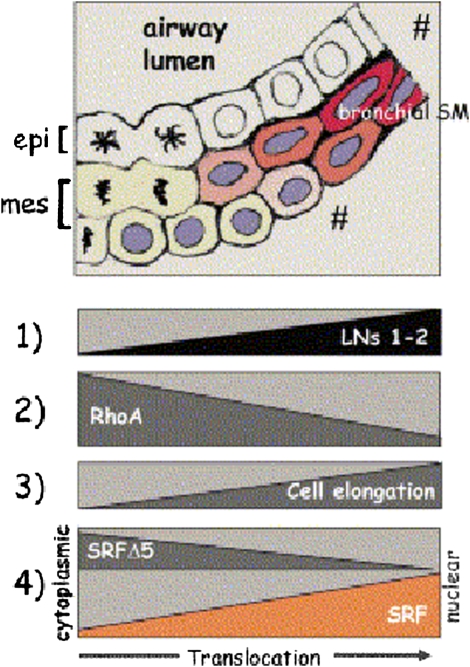
Figure 3.
New epithelial–mesenchymal contacts at the tips of growing airway buds induce laminin (LN)-1 expression and deposition at the epithelial–mesenchymal interface (1). LN-1 is a powerful inhibitor of RhoA activity (2). Because high RhoA activity maintains the undifferentiated mesenchymal cells' round shape, its decrease allows for initial cell elongation (3). Cell elongation then switches the synthesis of LN-2 (1), which is an even more powerful inhibitor of RhoA. A positive feedback loop is created between LN-2, RhoA inhibition, and cell elongation (1–3). In the round, undifferentiated mesenchymal cells serum response factor (SRF) and SRFΔ5 are present in the nucleus and cytoplasm (4). Upon cell elongation, the cytoplasmic SRF isoform translocates gradually to the nucleus, whereas SRFΔ5 disappears (4). The increment of SRF plus the disappearance of SRFΔ5 contribute to the initiation of bronchial myogenesis. Reprinted by permission from Reference 47.
EMBRYONIC EPITHELIAL CELL-DERIVED MORPHOGENS AND BRONCHIAL SM DIFFERENTIATION
Evidence coming mainly from transgenic animal studies demonstrates that epithelial-derived morphogens and their up- and down-stream effectors modulate bronchial myogenesis. Sonic hedgehog (Shh) is the vertebrate homolog of drosophila hedgehog (Hh) and is the best characterized epithelial-derived morphogen with a role in bronchial myogenesis. Shh is critical for the development of multiple organs (49, 50). Transduction of the Shh signal occurs by activation of its receptor Patched-1 (Ptc-1) (51). Binding of Shh to Ptc-1 relieves Ptc-1–mediated inhibition of a signaling protein referred to as Smoothened, which results in the transduction of the signal to the Gli transcription factors (Gli1, Gli2, and Gli3). These translocate into the nucleus and bind to Shh-responsive elements (52) (Figure 5). Shh activity is restricted by its interaction with Ptc-1 and with Hedgehog interacting protein-1 (HIP-1) (52). Starting at E10.5, Shh is expressed in the embryonic lung epithelium at the tips of the distal buds (53–55). Concomitantly, Ptc-1 (23, 56) and HIP-1 (57) are expressed at high levels in the mesenchyme surrounding the epithelial tips.
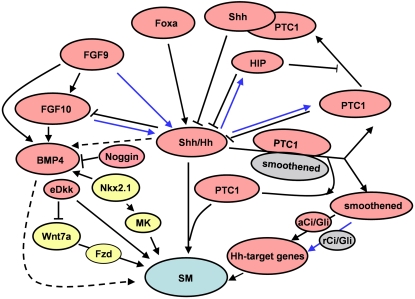
Figure 5.
A schematic representation of signaling pathways in lung smooth muscle myogenesis. Blue lines indicate a negative regulation on Shh-mediated smooth muscle myogenesis. Dotted lines represent relationships that are not yet clear. Yellow represents involvement in vascular smooth muscle myogenesis only. Gray represents repressed protein or complex. Shh, sonic hedgehog; Hh, hedgehog; PTC1, patched-1; HIP, hedgehog-interacting protein; aCi/Gli, Ci/Gli activator; rCi/Gli, Ci/Gli repressor; Foxa, Forkhead box-a; FGF, fibroblast growth factor; BMP4, bone morphogenetic protein-4; eDkk, epithelial Dickkopf-1; Fzd, Frizzled; MK, midkine, SM, smooth muscle myogenesis.
Mutant mice lacking Shh show esophageal atresia/stenosis, tracheo-esophageal fistula, and tracheal and lung abnormalities (58–60). In these animals a rudimentary lung is represented by a dilated sac lined by bronchial-type epithelium and surrounded by one or two layers of mesenchymal cells, some of them undergoing apoptosis (59, 60). Airway SM is absent in these highly abnormal lungs (59, 60). In mice with conditional deletion of Shh, bronchial cystic dilation, scant mesenchyme, and lack of bronchial and vascular SM are seen in the areas of the lung in which Shh is deleted (60) (Figure 4). The effect of Shh deletion seems to affect particularly bronchial and vascular SM, since Shh null mice develop other visceral muscle, albeit in slightly smaller amount than normal (59–61). Exposure of lung embryonic mesenchymal explants to Shh in culture results in induction of SM α-actin/SM myosin-positive cells (23). However, Shh overexpression in the developing airway epithelium does not alter bronchial myogenesis (9).
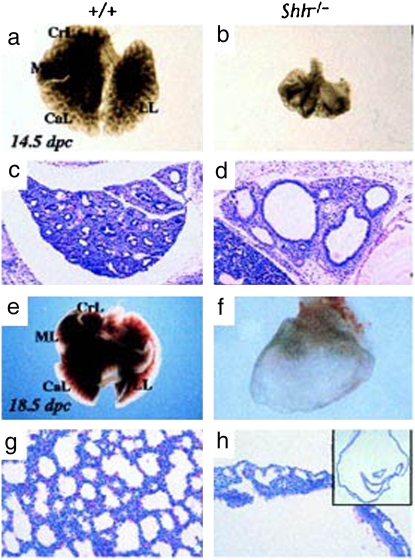
Figure 4.
Shh is essential in lung development. Whole mount view of (a, e) wild-type and (b, f) Shh−/− mutant lungs at (a, b) 14.5 and (e, f) 18.5 days after conception. Wild type shows characteristic lobulation (CrL, cranial lobe; ML, middle lobe; CaL, caudal lobe; LL, left lobe), which is not present in the mutant. Note that the mutant lung at 18.5 days after conception is reduced to a dilated sac (h, inset). (c, d, g, and h) Hematoxylin and eosin–stained sections of (c, g) wild type and (d, h) mutant lungs at (c, d) 14.5 and (g, h) 18.5 days after conception. Reprinted by permission from Reference 58.
Gli transcription factors act downstream of Shh. All three Gli genes are expressed in distinct but overlapping domains in lung mesenchyme, with expression being highest in the distal tips (62, 63). Mice in which the DNA-binding domain has been removed from Gli1 are viable and appear normal (64). However mutant mice lacking Gli2 function exhibit lung hypoplasia, which is more severe upon a 50% reduction in the gene dosage of Gli3 (65). In these animals the lung phenotype is very similar to that of Shh-null mice, and although airway SM has not been studied, it is probably affected.
Forkhead box (Fox)a1 and Foxa2 act upstream of Shh (66). Foxa (previously termed hepatocyte nuclear factor [HNF]-3) transcription factors are a family that includes Foxa1, Foxa2, and Foxa3 (previously termed HNF3a, HNF3b, and HNF3d, respectively). These transcription factors are expressed primarily in endodermally derived tissues, where they influence embryonic patterning, cell differentiation, and function (67). The embryonic lung epithelium expresses only Foxa1 and 2 in a diffuse manner (68), and their combined conditional deletion results in significant airway dilatation with production of large cysts in the areas in which both genes are deleted. These areas show decreased Shh expression and absence of airway SM development (66).
In the developing lung, fibroblast growth factor (FGF) 9 also acts upstream of Shh (69). At embryonic day (E)9.5 and E10.5 Fgf9 is expressed in both the mesothelial lining of the lung (future pleura) and the epithelium of the developing airways, but is restricted to the pleura by E12.5 and on (70). Lack of epithelial Fgf9 expression results in decreased Shh expression (69); however, SM development has not been studied in these lungs. Overexpression of Fgf9 in the airway epithelium increases Shh and Ptc-1 expression (69). Lungs overexpressing Fgf9 show bronchial dilation and increased mesenchymal cell proliferation, but notably lack bronchial SM (69) despite the increase in Shh and Ptc-1 expression. These findings are in agreement with a previous study that showed that Shh induction of SM α-actin in mesenchymal lung explants in culture is inhibited by FGF9-soaked beads (23). Therefore either absence of Shh or combined increase in Shh and FGF9 levels both lead to lack of bronchial SM. A similar dual response seems to occur with FGF9 and FGF10. Fgf9 overexpression up-regulates FGF10 along with the suppression of bronchial SM (69); however, FGF10 down-regulation decreases rather than increases bronchial myogenesis (9). Therefore either a decrease in FGF10 or a combined increase in FGF9 and FGF10 levels inhibit airway SM development (Figure 5).
BMP-4 is another epithelial-derived morphogen with a role in bronchial SM development, although it is currently unclear whether under physiologic conditions BMP-4 stimulates or inhibits myogenesis. BMP-4 is a member of the TGF-β superfamily of proteins and has been shown to play a role in diverse developmental processes (71). Similar to Shh, during lung development, BMP-4 transcripts are localized in high levels at the distal tips of the terminal epithelial buds (53), while BMP receptors are expressed throughout the lung epithelium as well as in the distal mesenchyme (53). Epithelial overexpression of BMP-4 seems to induce extensive mesenchymal SM differentiation (9) along with mesenchymal cell death (53). On the other hand, Fgf9-overexpressing lungs, which lack airway SM, have increased BMP-4 level (69). Similarly, BMP-4 is upregulated in Shh null mice, which lack bronchial muscle (59). Furthermore, a study in which the BMP antagonist noggin was overexpressed in the bronchial epithelium showed a significant increase, rather than a decrease, in SM α-actin–positive mesenchymal cells (72). Finally, noggin-null embryos show no abnormalities in bronchial SM (23), and no lung abnormalities are described in mice with BMP-4 haploinsufficiency (73). Therefore, the physiologic role of BMP-4 in the process of bronchial myogenesis is yet unclear (Figure 5).
Wnt signaling seems to play a role in bronchial morphogenesis, but as it happens with other morphogenic factors, it is currently unknown whether Wnt signaling stimulates or inhibits myogenesis during normal lung development. The vertebrate Wnt growth factor family contains at least 21 secreted cysteine-rich glycoproteins that regulate cell–cell interaction in many embryonic tissues (74). Wnts interact with at least 10 known Frizzled (Fzd) receptors and signals through a canonical and a noncanonical pathways. The canonical pathway involves Fzd receptor binding and nuclear translocation of hypophosphorylated β-catenin, which then activates lymphoid enhancer-binding factor/T cell factor (LEF/TCF)-mediated gene transcription. The noncanonical pathway involves either activation of c-Jun kinase or regulation of calcium flux (75, 76). Several Wnts are expressed in the lung and the biological function of Wnt5a and Wnt7a has been determined through the generation of mutant mice (77, 78). None of these mice seems to have abnormal bronchial SM, although Wnt7a-null mice present abnormalities in the differentiation and integrity of lung vascular SM (78). This finding points to a different regulation for vascular and bronchial myogenesis. The role of the Wnt signaling system on bronchial myogenesis is suggested by studies focusing on Dickkopf-1 (Dkk-1). Dkk-1 is a secreted Wnt antagonist that acts by inhibiting the Wnt/β-catenin signaling (79). Dkk-1 is produced in the embryonic lung by the distal epithelium (80). When Dkk-1 is overexpressed in the developing distal lung epithelium, lung bronchial myogenesis increases (78). However, and contrary to this observation, treatment of embryonic lung explants with Dkk-1 results in an almost complete absence of bronchial SM (80). Interestingly, Dkk1-null mice have no lung phenotype (81) and mice with conditional lung deletion of β-catenin have normal airway SM development (82).
Altogether these studies indicate that specific levels of morphogens originating from specific lung compartments and acting in specific combinations at specific times are required for normal bronchial myogenesis. Experimental alteration in any of these conditions results in either increased or decreased/absent bronchial SM.
LUNG VASCULAR SM DIFFERENTIATION
Early development of pulmonary vasculature include three processes: formation of central vessels (angiogenesis), peripheral vessels (vasculogenesis), and a lytic process to establish luminal connection between the two (83, 84). Pulmonary arteries develop close to the bronchi whereas veins form at sites away from bronchi. Arterial vascular SM originate partly from migration of adjacent airway SM cells and from surrounding undifferentiated mesenchymal cells (vasculogenesis), but venous vascular SM arises completely by vasculogenesis (85–87). In humans arteries acquired SM α-actin at 38 days, whereas veins did at 56 days (85). Besides mechanical forces/stretch, SRF, Shh, and Wnt7b (discussed in previous paragraphs), other factors affecting vascular smooth muscle myogenesis are Nkx2.1 (Thyroid transcription factor1, TTF1) and midkine (MK) (Figure 5). Suppression or targeted disruption of Nkx2.1, a homeodomain transcriptional regulator, resulted in defects in pulmonary vascular SM development, leading to the death of animals due to abnormal lung induced respiratory failure. In these animals bronchial airway SM is well formed with no changes in the expression of Shh mRNA (88, 89). It is noteworthy that Nkx2.1 is expressed in Shh(−/−) lungs (59). Midkine is a retinoic acid–responsive, heparin-binding growth factor regulated by Nkx2.1 (90, 91). MK transgenic mice showed increase in SM-specific genes (SM α-actin, calponin, and SM-22) in the vascular SM cells and vascular SM cell precursors, resulting in the increase in muscularization of small pulmonary arteries. The presence of normal bronchial SM in these animals indicate that MK has a selective effect on vascular SM (90, 91). MK expression was not detected in lungs of Nkx2.1 (TTF1)-null mice (90, 91).
CONCLUSIONS
The data currently available indicate that mesenchymal cell tension and epithelial-derived paracrine morphogenic factors control airway SM development. Whether these signals interrelate is a question that will require additional study. Several possibilities, however, may be proposed to link mechanical and biochemical myogenic signals. One possibility is that epithelial-derived morphogenic factors such as Shh may be required to render the embryonic mesenchymal cells responsive to mechanical forces. Another possibility is that morphogens act in part by promoting changes in mesenchymal cell shape. For example, Shh may induce cells to elongate when added to embryonic mesenchymal cell explants, and this in turn may induce SM α-actin and SM myosin expression, as seen in studies by Weaver and coworkers (23). This possibility may explain why embryonic mesenchymal cells do not respond to the myogenic effect of Shh when prevented from elongating (Y. Zhou and L. Schuger, unpublished observation). Alternatively, soluble factors and mechanical signals may both activate a common myogenic signaling pathway. Finally, there is a mechanical element that may be potentially contributing to the decrease or lack of bronchial SM observed in several transgenic lungs. In most of these lungs the airways are significantly dilated and therefore withstand increased hydrostatic pressures that may be high enough to inhibit rather than stimulate SM differentiation, as has been previously shown (28). In conclusion, during the last decade we learned most of what is known about bronchial myogenesis. Further research is needed to integrate mechanical and biochemical signaling pathways and to unveil intracellular myogenic mechanisms.
Acknowledgments
The authors thank Dr. David Buch for careful reading of this manuscript.
Supported by National Heart, Lung, and Blood Institute grants HL-48730, HL-77514, and HL-78752 (L.S.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sparrow MP, Lamb JP. Ontogeny of airway smooth muscle: structure, innervation, myogenesis and function in the fetal lung. Respir Physiolo Neurobiol 2003;137:361–372. [DOI] [PubMed] [Google Scholar]
- 2.Roman J, McDonald JA. Expression of fibronectin, the integrin alpha 5, and alpha-smooth muscle actin in heart and lung development. Am J Respir Cell Mol Biol 1992;6:472–480. [DOI] [PubMed] [Google Scholar]
- 3.McHugh KM. Molecular analysis of smooth muscle development in the mouse. Dev Dyn 1995;204:278–290. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Miano JM, Cserjesi P, Olson EN. Sm22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 1996;78:188–195. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell HW, Sparrow MP, Tagliaferri RP. Inhibitory and excitatory responses to field stimulation in fetal and adult pig airway. Pediatr Res 1990;28:69–74. [DOI] [PubMed] [Google Scholar]
- 6.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 1994;75:803–812. [DOI] [PubMed] [Google Scholar]
- 7.Woodcock-Mitchell J, White S, Stirewalt W, Periasamy M, Mitchell J, Low RB. Myosin isoform expression in developing and remodeling rat lung. Am J Respir Cell Mol Biol 1993;8:617–625. [DOI] [PubMed] [Google Scholar]
- 8.Relan NK, Yang Y, Beqaj S, Miner JH, Schuger L. Cell elongation induces laminin alpha2 chain expression in mouse embryonic mesenchymal cells: Role in visceral myogenesis. J Cell Biol 1999;147:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 2005;132:2157–2166. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624. [DOI] [PubMed] [Google Scholar]
- 11.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 2004;66:625–645. [DOI] [PubMed] [Google Scholar]
- 12.Schuger L, Varani J, Killen PD, Skubitz AP, Gilbride K. Laminin expression in the mouse lung increases with development and stimulates spontaneous organotypic rearrangement of mixed lung cells. Dev Dyn 1992;195:43–54. [DOI] [PubMed] [Google Scholar]
- 13.Thomas T, Dziadek M. Expression of collagen alpha 1(iv), laminin and nidogen genes in the embryonic mouse lung: implications for branching morphogenesis. Mech Dev 1994;45:193–201. [DOI] [PubMed] [Google Scholar]
- 14.Lallemand AV, Ruocco SM, Gaillard DA. Synthesis and expression of laminin during human foetal lung development. Anat Rec 1995;242:233–241. [DOI] [PubMed] [Google Scholar]
- 15.Schuger L, Skubitz AP, Zhang J, Sorokin L, He L. Laminin alpha1 chain synthesis in the mouse developing lung: requirement for epithelial-mesenchymal contact and possible role in bronchial smooth muscle development. J Cell Biol 1997;139:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Palmer KC, Relan N, Diglio C, Schuger L. Role of laminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development 1998;125:2621–2629. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, O'Shea S, Liu J, Schuger L. Bronchial smooth muscle hypoplasia in mouse embryonic lungs exposed to a laminin beta1 chain antisense oligonucleotide. Mech Dev 1999;89:15–23. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Relan NK, Przywara DA, Schuger L. Embryonic mesenchymal cells share the potential for smooth muscle differentiation: myogenesis is controlled by the cell's shape. Development 1999;126:3027–3033. [DOI] [PubMed] [Google Scholar]
- 19.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschi KK, Rohovsky SA, D'Amore PA. Pdgf, tgf-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10t1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 1998;141:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank RS, Swartz EA, Thompson MM, Olson EN, Owens GK. A retinoic acid-induced clonal cell line derived from multipotential p19 embryonal carcinoma cells expresses smooth muscle characteristics. Circ Res 1995;76:742–749. [DOI] [PubMed] [Google Scholar]
- 22.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-bb-induced suppression of smooth muscle cell differentiation. Circ Res 1992;71:1525–1532. [DOI] [PubMed] [Google Scholar]
- 23.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol 2003;258:169–184. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 25.Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol 1996;134:1483–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin m chain gene to dy locus. J Biol Chem 1994;269:13729–13732. [PubMed] [Google Scholar]
- 27.Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (lama2) gene. Nat Genet 1994;8:297–302. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest 2000;106:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W. Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 1984;19:658–665. [DOI] [PubMed] [Google Scholar]
- 30.Thurlbeck WM. Prematurity and the developing lung. Clin Perinatol 1992;19:497–519. [PubMed] [Google Scholar]
- 31.DiFiore JW, Fauza DO, Slavin R, Peters CA, Fackler JC, Wilson JM. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg 1994;29:248–256. (discussion 256–247). [DOI] [PubMed] [Google Scholar]
- 32.Harding R. Sustained alterations in postnatal respiratory function following sub-optimal intrauterine conditions. Reprod Fertil Dev 1995;7:431–441. [DOI] [PubMed] [Google Scholar]
- 33.Joe P, Wallen LD, Chapin CJ, Lee CH, Allen L, Han VK, Dobbs LG, Hawgood S, Kitterman JA. Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am J Physiol 1997;272:L95–L105. [DOI] [PubMed] [Google Scholar]
- 34.Nobuhara KK, Fauza DO, DiFiore JW, Hines MH, Fackler JC, Slavin R, Hirschl R, Wilson JM. Continuous intrapulmonary distension with perfluorocarbon accelerates neonatal (but not adult) lung growth. J Pediatr Surg 1998;33:292–298. [DOI] [PubMed] [Google Scholar]
- 35.Davenport M, Holmes K. Current management of congenital diaphragmatic hernia. Br J Hosp Med 1995;53:95–101. [PubMed] [Google Scholar]
- 36.Inanlou MR, Baguma-Nibasheka M, Keating MM, Kablar B. Neurotrophins, airway smooth muscle and the fetal breathing-like movements. Histol Histopathol 2006;21:931–940. [DOI] [PubMed] [Google Scholar]
- 37.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003;421:172–177. [DOI] [PubMed] [Google Scholar]
- 38.Albinsson S, Nordstrom I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem 2004;279:34849–34855. [DOI] [PubMed] [Google Scholar]
- 39.Belaguli NS, Schildmeyer LA, Schwartz RJ. Organization and myogenic restricted expression of the murine serum response factor gene: a role for autoregulation. J Biol Chem 1997;272:18222–18231. [DOI] [PubMed] [Google Scholar]
- 40.Browning CL, Culberson DE, Aragon IV, Fillmore RA, Croissant JD, Schwartz RJ, Zimmer WE. The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev Biol 1998;194:18–37. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Ip HS, Lu MM, Clendenin C, Parmacek MS. A serum response factor-dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol Cell Biol 1997;17:2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belaguli NS, Zhou W, Trinh TH, Majesky MW, Schwartz RJ. Dominant negative murine serum response factor: alternative splicing within the activation domain inhibits transactivation of serum response factor binding targets. Mol Cell Biol 1999;19:4582–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp PR, Metcalfe JC. Four isoforms of serum response factor that increase or inhibit smooth-muscle-specific promoter activity. Biochem J 2000;345:445–451. [PMC free article] [PubMed] [Google Scholar]
- 44.Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO Jr, Xiao Q, Weiler H, Ginty DD, Misra RP. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci USA 2004;101:17132–17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beqaj S, Jakkaraju S, Mattingly RR, Pan D, Schuger L. High rhoa activity maintains the undifferentiated mesenchymal cell phenotype, whereas rhoa down-regulation by laminin-2 induces smooth muscle myogenesis. J Cell Biol 2002;156:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall A. Rho gtpases and the actin cytoskeleton. Science 1998;279:509–514. [DOI] [PubMed] [Google Scholar]
- 47.Jakkaraju S, Zhe X, Schuger L. Role of stretch in activation of smooth muscle cell lineage. Trends Cardiovasc Med 2003;13:330–335. [DOI] [PubMed] [Google Scholar]
- 48.Jakkaraju S, Zhe X, Pan D, Choudhury R, Schuger L. Tips are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Dev Cell 2005;9:39–49. [DOI] [PubMed] [Google Scholar]
- 49.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev 2001;15:3059–3087. [DOI] [PubMed] [Google Scholar]
- 50.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 2003;53:1–114. [DOI] [PubMed] [Google Scholar]
- 51.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by ptc1. Cell 2001;105:599–612. [DOI] [PubMed] [Google Scholar]
- 52.McMahon AP. More surprises in the hedgehog signaling pathway. Cell 2000;100:185–188. [DOI] [PubMed] [Google Scholar]
- 53.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996;122:1693–1702. [DOI] [PubMed] [Google Scholar]
- 54.Bitgood MJ, McMahon AP. Hedgehog and bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995;172:126–138. [DOI] [PubMed] [Google Scholar]
- 55.Miller LA, Wert SE, Whitsett JA. Immunolocalization of sonic hedgehog (shh) in developing mouse lung. J Histochem Cytochem 2001;49:1593–1604. [DOI] [PubMed] [Google Scholar]
- 56.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of sonic hedgehog (shh) in mouse embryonic lung growth and morphogenesis. Development 1997;124:53–63. [DOI] [PubMed] [Google Scholar]
- 57.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian hedgehog signaling by the hedgehog-binding protein, hip1, modulates fgf signaling during branching morphogenesis of the lung. Genes Dev 2003;17:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet 1998;20:58–61. [DOI] [PubMed] [Google Scholar]
- 59.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998;8:1083–1086. [DOI] [PubMed] [Google Scholar]
- 60.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn 2004;231:57–71. [DOI] [PubMed] [Google Scholar]
- 61.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 2000;127:2763–2772. [DOI] [PubMed] [Google Scholar]
- 62.Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the gli gene family in embryonic mouse lung development. Dev Biol 1997;188:337–348. [DOI] [PubMed] [Google Scholar]
- 63.Platt KA, Michaud J, Joyner AL. Expression of the mouse gli and ptc genes is adjacent to embryonic sources of hedgehog signals suggesting a conservation of pathways between flies and mice. Mech Dev 1997;62:121–135. [DOI] [PubMed] [Google Scholar]
- 64.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse gli1 mutants are viable but have defects in shh signaling in combination with a gli2 mutation. Development 2000;127:1593–1605. [DOI] [PubMed] [Google Scholar]
- 65.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of gli2 and gli3 in the formation of lung, trachea and oesophagus. Nat Genet 1998;20:54–57. [DOI] [PubMed] [Google Scholar]
- 66.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of foxa1 and foxa2 during lung morphogenesis. J Biol Chem 2005;280:13809–13816. [DOI] [PubMed] [Google Scholar]
- 67.Hromas R, Costa R. The hepatocyte nuclear factor-3/forkhead transcription regulatory family in development, inflammation, and neoplasia. Crit Rev Oncol Hematol 1995;20:129–140. [DOI] [PubMed] [Google Scholar]
- 68.Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/hnf-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 1993;119:567–578. [DOI] [PubMed] [Google Scholar]
- 69.White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. Fgf9 and shh signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 2006;133:1507–1517. [DOI] [PubMed] [Google Scholar]
- 70.Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev Dyn 1999;216:72–88. [DOI] [PubMed] [Google Scholar]
- 71.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 1996;10:1580–1594. [DOI] [PubMed] [Google Scholar]
- 72.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999;126:4005–4015. [DOI] [PubMed] [Google Scholar]
- 73.Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in bmp4 heterozygous null mice and modification by mutations in gli3 and alx4. Dev Biol 1997;188:235–247. [DOI] [PubMed] [Google Scholar]
- 74.Wodarz A, Nusse R. Mechanisms of wnt signaling in development. Annu Rev Cell Dev Biol 1998;14:59–88. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with fzd1, fzd10, and lrp5. Mol Cell Biol 2005;25:5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim N, Vu TH. Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res C Embryo Today 2006;78:80–89. [DOI] [PubMed] [Google Scholar]
- 77.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol 2002;248:68–81. [DOI] [PubMed] [Google Scholar]
- 78.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, et al. Wnt/beta-catenin signaling acts upstream of n-myc, bmp4, and fgf signaling to regulate proximal-distal patterning in the lung. Dev Biol 2005;283:226–239. [DOI] [PubMed] [Google Scholar]
- 79.Kawano Y, Kypta R. Secreted antagonists of the wnt signalling pathway. J Cell Sci 2003;116:2627–2634. [DOI] [PubMed] [Google Scholar]
- 80.De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (dkk1) reveals that fibronectin is a major target of wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol 2005;277:316–331. [DOI] [PubMed] [Google Scholar]
- 81.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001;1:423–434. [DOI] [PubMed] [Google Scholar]
- 82.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238. [DOI] [PubMed] [Google Scholar]
- 83.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 1997;16:568–581. [DOI] [PubMed] [Google Scholar]
- 84.deMello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol 2000;3:439–449. [DOI] [PubMed] [Google Scholar]
- 85.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol 2005;288:L141–L149. [DOI] [PubMed] [Google Scholar]
- 86.Hall SM, Hislop AA, Haworth SG. Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol 2002;26:333–340. [DOI] [PubMed] [Google Scholar]
- 87.Fernandes DJ, McConville JF, Stewart AG, Kalinichenko V, Solway J. Can we differentiate between airway and vascular smooth muscle? Clin Exp Pharmacol Physiol 2004;31:805–810. [DOI] [PubMed] [Google Scholar]
- 88.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in nkx2.1(−/−) mouse embryos. Dev Biol 1999;209:60–71. [DOI] [PubMed] [Google Scholar]
- 89.Yuan B, Li C, Kimura S, Engelhardt RT, Smith BR, Minoo P. Inhibition of distal lung morphogenesis in nkx2.1(−/−) embryos. Dev Dyn 2000;217:180–190. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 2004;279:37124–37132. [DOI] [PubMed] [Google Scholar]
- 91.Reynolds PR, Mucenski ML, Whitsett JA. Thyroid transcription factor (ttf) -1 regulates the expression of midkine (mk) during lung morphogenesis. Dev Dyn 2003;227:227–237. [DOI] [PubMed] [Google Scholar]