Abstract
Superimposition of force fluctuations on contracted tracheal smooth muscle (TSM) has been used to simulate normal breathing. Breathing has been shown to reverse lung resistance of individuals without asthma and animals given methacholine to contract their airways; computed tomography scans also demonstrated bronchial dilation after a deep inhalation in normal volunteers. This reversal of airway resistance and bronchial constriction are absent (or much diminished) in individuals with asthma. Many studies have demonstrated that superimposition of force oscillations on contracted airway smooth muscle results in substantial smooth muscle lengthening. Subsequent studies have shown that this force fluctuation–induced relengthening (FFIR) is a physiologically regulated phenomenon. We hypothesized that actin filament length in the smooth muscle of the airways regulates FFIR of contracted tissues. We based this hypothesis on the observations that bovine TSM strips contracted using acetylcholine (ACh) demonstrated amplitude-dependent FFIR that was sensitive to mitogen-activated protein kinase (p38 MAPK) inhibition- an upstream regulator of actin filament assembly. We demonstrated latrunculin B (sequesters actin monomers thus preventing their assimilation into filaments resulting in shorter filaments) greatly increases FFIR and jasplakinolide (an actin filament stabilizer) prevents the effects of latrunculin B incubation on strips of contracted canine TSM. We suspect that p38 MAPK inhibition and latrunculin B predispose to shorter actin filaments. These studies suggest that actin filament length may be a key determinant of airway smooth muscle relengthening and perhaps breathing-induced reversal of agonist-induced airway constriction.
Keywords: actin filament length, latrunculin B, jasplakinolide
Many studies have demonstrated that superimposition of force oscillations on contracted airway smooth muscle results in substantial smooth muscle lengthening in vitro (1–4), a phenomenon we refer to as force fluctuation–induced relengthening (FFIR). It has been proposed that imposition of these force oscillations simulate the effect of breathing on airway responsiveness and caliber. Shen and coworkers demonstrated in ventilated rabbits that breathing reduced methacholine-elicited airway resistance in a tidal volume–dependent manner (5). In normal individuals who demonstrated increased airway resistance to inhaled methacholine, the degree of bronchoconstriction could be reversed again by normal, tidal breathing (6, 7) or by taking a single deep inspiration (8–10). This airway dilation with deep inspiration also was demonstrated directly by looking at computed tomography (CT) scans of normal volunteers (8). Lung tethering forces on the conducting airways probably contributed to this dilation by loading the smooth muscle in the bronchiolar walls (8–10). However, this reversal of bronchoconstriction with breathing or deep inspiration is absent or minimal in individuals with asthma (9, 10); their airways remain constricted to methacholine with breathing and, although CT scans demonstrate increased bronchial diameters with deep inspiration, the airways from individuals with asthma reconstrict almost immediately (8). Thus, it becomes apparent that determining the mechanisms relating these differences between normal individuals and individuals with asthma could be of great importance in understanding not only the disease but also in suggesting novel therapies to reverse bronchospasm. The results reported in this article have been published previously in two articles (In vitro tracheal smooth muscle studies. J Appl Physiol 2005;98:489–497 and In vivo mouse ventilatory studies. J Appl Physiol 2006;101:249–255).
DIFFERENCES IN BREATHING EFFECT BETWEEN NORMAL SUBJECTS AND SUBJECTS WITH ASTHMA (HYPERRESPONSIVENESS)
Using an animal model, we previously assessed the anti-obstructive effects of different breathing patterns in ventilated C57Bl/6J and A/J mice (11). The A/J mouse strain is more hyperresponsive (modeling innate airway hyperresponsiveness, if not asthma) compared with the C57Bl/6J strain (12–15). Anesthetized mice underwent tracheostomy, and were ventilated with a computer-controlled small-animal ventilator (flexiVent; SCIREQ, Montreal, Canada). Positive end-expiratory pressure of 2 cm H2O was applied throughout. An external jugular vein was isolated for intravenous infusion of methacholine. To gauge the influence of tidal volume/frequency combination on airway caliber at baseline or during methacholine-induced bronchoconstriction, we varied tidal volume (10, 5, or 20 ml/kg) while adjusting frequency (150, 300, or 75 breaths/min, respectively) to keep minute ventilation constant. Each ventilation pattern was continued for approximately 3 minutes at baseline and in the presence of methacholine (0.1 mg/ml intravenously) infusion or boluses (11). Respiratory system resistance was recorded every 20 seconds, during 1 second of 6 Hz oscillation at 0.08 ml, using the flexiVent “Snapshot-6Hz” protocol. We found in both strains of mice that increasing tidal volume resulted in less resistance at baseline and less methacholine-induced resistance. Similarly, identical intravenous boluses resulted in less methacholine-induced resistance in mice breathing at higher tidal volumes, but A/J mice were more sensitive to the lower tidal volume breathing pattern; that is, they demonstrated an accentuated bronchoconstrictor response compare with C57Bl/6 mice (11). Thus, breathing deeper in itself reduced airway resistance in these mice.
Studies in humans have also demonstrated differences in the ability of breathing to inhibit agonist-induced bronchoconstriction between normal individuals and those with asthma. Brown and colleagues (8) used high-resolution computed tomography to examine the ability of a deep inspiration (DI) to distend the airways of subjects with asthma compared with normal individuals at baseline and after increasing airway tone with methacholine. They observed that both at baseline and after administering methacholine, a DI distended the airways of healthy control subjects and those of subjects with asthma to a similar extent. However, after administration of methacholine, when the DI was released, the healthy subjects maintained some bronchodilation compared with their pre-DI state, whereas subjects with asthma demonstrated further bronchoconstriction compared with their pre-DI state. That is, it seems that the airways (airway smooth muscle) of healthy individuals reacted differently to the stretch from a DI than did those of individuals with asthma. Therefore, we designed in vitro experiments (1, 4) to assess whether oscillating (to simulate breathing) contracted and shortened airway smooth muscle (to simulate narrowed bronchi) could be relengthened (airway opening) and to determine whether the relengthening could be physiologically regulated.
MECHANISTIC EXPLANATIONS FROM IN VITRO STUDIES
We reasoned from the observations noted above and the following in vitro studies that there was something different about the airway smooth muscle from individuals with asthma. Work from Newman Stephens laboratory has demonstrated increased expression of myosin light chain kinase (MLCK) in airway smooth muscle from individuals with asthma and a ragweed-sensitized dog model of airway hyperresponsiveness (16, 17). Airway smooth muscle from these individuals with asthma and from sensitized dogs concomitantly demonstrated increased shortening velocity and capacity (17, 18). Furthermore, seventh-generation human bronchi passively sensitized using human sera with high titers to house dust mite antigen showed, in addition to increased shortening velocity and capacity (19), robust myogenic responsiveness to quick stretch compared with sham-sensitized control airways (20). These in vitro findings paralleled the increased airway sensitivity and responsiveness observed in individuals with asthma.
However, those studies do not explain the mechanisms behind these differences. We need to sort out how breathing reverses and/or protects against bronchoconstriction in normal individuals before we can determine why this does not work as efficiently in individuals with asthma. Fredberg and coworkers hypothesized that breathing causes a disequilibrium of the activated myosin crossbridges in contracted smooth muscle and that this disequilibrium produced the bronchodilation observed in normal individuals (2, 21, 22). To examine this possibility, they took strips of bovine tracheal smooth muscle (TSM), hooked them up to a lever-arm assembly under physiologic conditions, and after an equilibration period to determine a reference length (Lref) and maximal isometric response (Fmax) to acetylcholine (ACh), they then contracted the TSM strips against a load equivalent to 32% Fmax, allowing the muscles to shorten isotonically. After allowing the tissues to reach a new equilibrium at shortened length, sinusoidal force oscillations of increasing amplitude (± 2–16% at 0.2 Hz, to simulate tidal breathing) were then superimposed on the TSM. They found that the tissues relengthened even in the presence of continued maximal stimulation to ACh in an oscillation, amplitude-dependent manner (2). Although their results did indicate a role for myosin binding disequilibrium in muscle relengthening with force oscillations, they could not explain why, when oscillations were stopped, the tissues did not re-shorten to their isotonic maxima. These data suggested that some other mechanism must be operative beyond myosin binding disequilibrium (2, 22). Lakser, working in Fredberg's laboratory, extended the original studies by demonstrating that the force fluctuation–induced relengthening is a physiologically regulated phenomenon (4). They found that incubating the TSM strips in SB203580, a p38 MAP kinase inhibitor, resulted in an even greater degree of relengthening with the force oscillations. They speculated that p38 MAPK inhibition resulted in reduced phosphorylation of heat shock protein (HSP)27, a downstream protein that has been found to cap actin filaments when inactivated, uncapping when phosphorylated (23, 24). They speculated that activated HSP27 allowed for increased actin filament polymerization, longer actin filaments in the airway smooth muscle cytoskeleton resulting in a stiffer muscle (23), and, concomitantly, less oscillation-induced relengthening (2–4, 21, 22). When HSP27 activation was inhibited by SB203580, the actin cytoskeleton was less stiff (23), resulting in greater oscillation-induced relengthening (2–4, 21, 22).
ACTIN FILAMENT LENGTH AS A REGULATOR OF FFIR
We took Fredberg and Lakser's speculation one step further. We considered that the actin filaments of the cytoskeleton were not the only targets of p38 MAPK and HSP27, and hypothesized that actin filament length of the contractile apparatus also was a potential site for modulation of smooth muscle relengthening by force oscillations. We were intrigued by studies from Lincoln Ford's and Chun Seow's laboratories demonstrating the adaptability of tracheal smooth muscle (TSM) strips to actively redefine their length–tension relationship over a threefold change in tissue length (25–27). They demonstrated that when the length of the TSM strips was increased or decreased over a range of 0.5 to 1.5 Lref, initially force generation (using electrical field stimulation) was depressed, but after a series of contractions the muscles would re-adapt and generate the same force relative to cross-sectional area independent of change in length (25). The overlap of the actin and myosin filaments would not be as optimal in the stretched or shortened state initially, but with time and contractions the contractile units adapted. They and we define a contractile unit as a myosin filament, and the actin filaments with which it interacts in terms of crossbridges. Wang and coworkers also demonstrated that tissues passively stretched or shortened over this same length range would alter their parallel/series contractile units overnight such that the new length–tension relationships did not appear significantly different from the control curve obtained at Lref (27). They concluded that this adaptation to length change suggested a series to parallel re-arrangement of contractile units in smooth muscle when the tissue is shortened and back to a series arrangement upon re-stretching the tissues; that is, the greater the number of contractile units in parallel, the greater the force development. Their data also demonstrated the dynamics of such a system: the contractile units adapted to the new lengths within five or six contractions (30 min).
We speculated that TSM demonstrated greater re-lengthening in the presence of SB203580 (p38 MAPK inhibitor) because the phosphorylation of HSP27 was prevented (thus, uncapping and actin filament lengthening was diminished). Based on the dynamics of actin filaments, this prevention of assembly predisposed to shorter filaments favoring rearrangement of the contractile units into a series arrangement with the force oscillations. This low force arrangement allowed for a greater degree of oscillation-induced relengthening in the presence of the inhibitor.
We reasoned that if individuals with asthma had longer actin filaments in their airway smooth muscle, the contractile units would not need to adapt as readily to stretch elicited by tidal breathing or deep inspiration (26–28). Without the need for rearrangement of contractile units, the airway smooth muscle of an individual with asthma could re-contract immediately after the stretch imposed on the bronchi by the lung-tethering forces. Longer actin filaments in people with asthma are not that hard to hypothesize, since the environment in asthmatic, inflamed airways is rife with cytokines and mediators that promote hypertrophy and hyperplasia in most of the tissues in the bronchiolar wall (28, 29). Furthermore, it is well established that stress, stretch, and cytokines can directly activate p38 MAPK, an upstream regulator of HSP27 and actin filament length (23, 24, 31). However, actin filament length in smooth muscle is an elusive and moving target (32); unlike skeletal muscle, actin filament length in smooth muscle has yet to be definitively measured. Herrera and colleagues have demonstrated that associated actin (32) and myosin (25–27) filaments in TSM increase in length with contraction, and dynamically shorten and lengthen with airway smooth muscle cell migration (23, 24). This dynamic nature of actin filament assembly and evanescence cannot be accounted for by the actin filament–stabilizing effects of such proteins as tropomyosin (29–31) or caldesmon (33, 34). Therefore, we used pharmacologic tools to test the hypothesis that altering actin dynamics and therefore actin filament length would indeed modify FFIR of contracted airway smooth muscle.
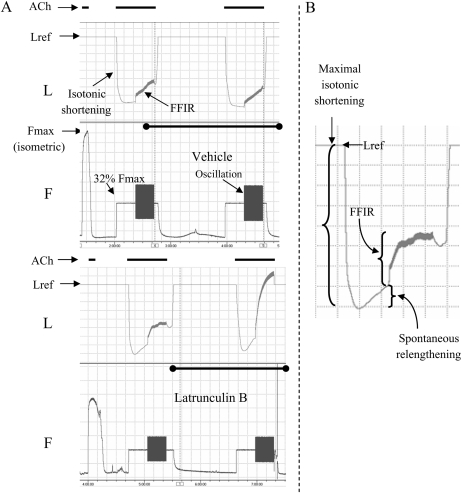
We tested our hypothesis using experimental protocols akin to that of Lakser and coworkers (4); however, we modified the experimental procedure so that we could assess FFIR before and after treatment with either latrunculin B (an agent that binds to monomeric actin, preventing incorporation into filaments) or jasplakinolide (an agent that stabilizes actin filaments), or both (1). After equilibration, the reference length of the TSM strip (Lref—that length at which isometric contractile responses to 43 mM KCl-substituted Krebs-Henseleit were maximal and repeatable) and maximal isometric response (Fmax) to 100 μM ACh were determined. ACh was washed out and the tissues left to relax and re-equilibrate at baseline resting tension for approximately 20 minutes. Then the muscle was again maximally contracted to ACh, this time against a 32% Fmax load, allowing the tissue to shorten isotonically (Figure 1). After 20 minutes, when the contracted tissue had stabilized at a new shortened length, ± 16% Fmax sine wave force oscillations were superimposed for a further 20 minutes. Oscillations were at 0.2 Hz simulating a tidal breathing pattern of 12 breaths per minute. At the end of this 20 minutes, ACh was washed out and the TSM strip was allowed to relax. We measured FFIR as the difference between muscle length just before and just after the 20-minute superimposition of force oscillations (Figure 1). Over the next hour, tissues were incubated with 30 nM latrunculin B (sequesters monomeric actin), 500 nM jasplakinolide (stabilizes filamentous actin), or vehicle (DMSO control). In preliminary studies, we found that these concentrations just did not affect isometric force development, nor did they affect isotonic shortening in either the absence or presence of imposed force oscillations (Figure 1). A second isotonic protocol was then performed and FFIR was again measured (Figure 1). The difference in FFIR before and after treatment (ΔFFIR) was calculated by subtracting the pre-treatment from the post-treatment value and expressed as a percentage of Lref (1).
Figure 1.
Representative tracings of length (L) and force (F) for vehicle control and latrunculin B–treated canine tracheal smooth muscle (TSM) strips. (A) These tracings are compressed to visualize and compare before and after treatment of TSM strips with either vehicle (control or latrunculin B). After determining the maximum isometric response to 100 μM ACh (Fmax), muscles were isotonically contracted from Lref against a load = 32% Fmax again using 100 μM ACh. Superimposed force oscillations (± 16% Fmax) caused tissue relengthening that was significantly augmented in the presence of 30 nM latrunculin B (lower panels) compared with vehicle controls (upper panels). (B) This trace is a blowup of the pre–latrunculin B protocol length myogram at left. The following length parameters were noted and comparisons made between treatments, before and after interventions: maximal isotonic shortening, spontaneous relengthening, and force oscillation–induced relengthening. (Reprinted by permission from Reference 1.)
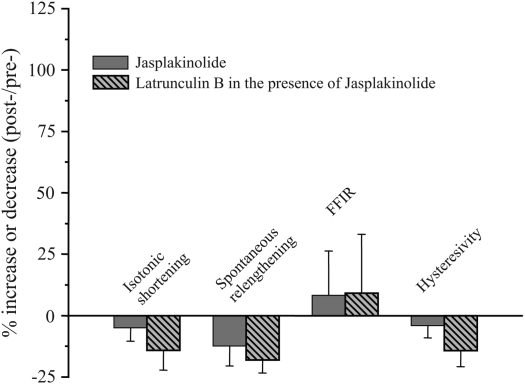
For experiments in which TSM strips were exposed to both latrunculin B and jasplakinolide, three isotonic protocols were elicited. After the initial control contraction, tissues were incubated with jasplakinolide, re-contracted and relaxed (to verify that jasplakinolide had no effect on FFIR), then incubated with latrunculin B. A third isotonic protocol was then performed. Changes in FFIR after the third protocol in the presence of latrunculin B were compared with pre-latrunculin B values.
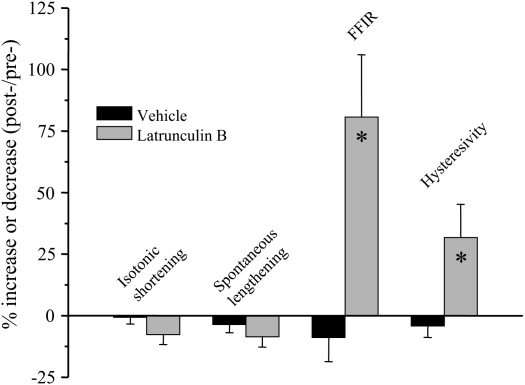
We found that latrunculin B significantly and profoundly augmented FFIR of isotonically shortened, ACh-contracted TSM strips—this despite having no significant effects on isotonic shortening or spontaneous relengthening (Figures 1 and 2). We also found that the phosphorylation level of the regulatory myosin light chain 20 (rMLC20) was not different between control and latrunculin B–treated tissues that had been flash-frozen near the end of the 20-minute oscillation protocol (1). These data indicated that the increased FFIR in the presence of latrunculin B could not be accounted for by a reduction of crossbridge activation in these TSM preparations. Furthermore, jasplakinolide treatment had no effect on muscle shortening, spontaneous relengthening, or FFIR (Figure 2). However, in tissues pretreated with the actin filament stabilizer and then incubated with latrunculin B, we found that jasplakinolide completely prevented the profound increase in FFIR previously observed in TSM strips treated only with latrunculin B (Figure 3). At this time there is no available technique to directly measure actin filament length, but in an attempt to get an indirect indication we measured the ratio of filamentous (F-) actin to globular (G-) actin. We analyzed fluorescence of TSM stained with Texas red phalloidin (F-actin) and Oregon green DNase (G-actin). We found that the ratio of F:G actin was less for latrunculin B–treated compared with control tissues. This suggests that less actin was in the filamentous state in the latrunculin B–treated TSM strips, which would be consistent with shorter actin filaments. These data implicate actin filament length as a major regulator of FFIR.
Figure 2.
Effect of latrunculin B and vehicle on shortening and relengthening in canine TSM. Latrunculin B (30 nM) significantly augmented tissue force fluctuation–induced relengthening (FFIR) and hysteresivity in response to superimposed 32% Fmax force oscillations compared with vehicle treated control tissues. Latrunculin B had no effect on isotonic shortening or spontaneous relengthening. These data suggest that shorter actin filaments cause greater FFIR in isotonically contracted TSM strips. Data were compared using unpaired t tests. *Statistically significant difference from vehicle-treated control tissues. (Reprinted by permission from Reference 1.)
Figure 3.
Effect of latrunculin B on shortening and relengthening in canine TSM after treatment with jasplakinolide. In the presence of 500 nM jasplakinolide, 30 nM latrunculin B did not affect FFIR or hysteresivity. Compared with jasplakinolide alone, latrunculin B treatment (in the continued presence of jasplakinolide) had similar minimal effects on isotonic shortening, spontaneous relengthening, force oscillation–induced relengthening, and hysteresivity when compared with initial control (pre-) isotonic contractions. These data indicate that stabilization of actin filaments with jasplakinolide prevented the effects of latrunculin B and further suggest a relation between actin filament length and FFIR in TSM strips. Data were compared using paired t tests. (Reprinted by permission from Reference 1.)
CONCLUSIONS
Numerous human and animal studies have demonstrated that breathing antagonizes agonist-induced airway constriction. This effect seems to be very powerful in normal individuals but much less effective in hyperresponsive individuals or those with asthma. The mechanisms regulating this effect are poorly understood, and thus the reason that individuals with asthma respond differently is unclear. Here we have reviewed a number of possible theories for this difference. We have presented published data that strongly implicate FFIR as a key component of this breathing effect (1–4). Furthermore, we have suggested, based on our previous work, that actin filament length is a major determinant of FFIR (1). Actin filament length is regulated by many pathways, including those that may be activated by inflammatory mediators known to be present in asthmatic airways. It has been demonstrated that inhibition of p38 MAPK activity augments FFIR (4); thus this smooth muscle relengthening is physiologically regulated and potentially subject to pharmacologic intervention. We suspect that the augmentation of FFIR with p38 MAPK inhibition may be due in part to inhibition of HSP27 phosphorylation, preventing its uncapping from actin and thus favoring shorter actin filaments. Shorter actin filaments allow for a greater degree of relengthening with force fluctuations (1). Of note, p38 MAPK is activated by a number of inflammatory mediators and cytokines (23, 24). It is therefore possible that the inflammatory environment of the asthmatic airway provides for a background supporting longer actin filaments resulting in impaired relengthening with breathing.
There are likely a number of additional regulators of FFIR other than actin filament length. Newman Stephens has suggested that asthmatic airway smooth muscle demonstrates more MLCK content and activity than that of normal airways (16, 17). Certainly, in addition to the contractile apparatus, the actin cytoskeleton may play a role in regulating FFIR, as suggested by Lakser and Fredberg (4, 33). Also, actomyosin crossbridge dynamics (i.e., cycling rate) likely plays a role. Fredbergs's laboratory and our work (Figures 2 and 3) have demonstrated increased hysteresivity with larger force fluctuations (1–3). Hysteresivity is a reflection of actomyosin crossbridge cycling. Larger force fluctuations disrupt actomyosin interactions resulting in faster crossbridge cycling. Fredberg has suggested, however, that an increased intrinsic rate of crossbridge cycling would make it more difficult for the force fluctuations to disrupt the reaction. This suggestion would be consistent with studies demonstrating increased intrinsic crossbridge cycling rates (shortening velocity) in allergen-sensitized muscle (16–19). Thus asthmatic airway smooth muscle may have a greater actomyosin crossbridge cycling rate that may be intrinsic to the muscle and/or enhanced by the surrounding inflammatory environment. Once again this would result in impaired effectiveness of FFIR and thus reduced ability of breathing to antagonize agonist-induced bronchoconstriction.
Further understanding of the mechanisms regulating FFIR and the potent effect of breathing on airway constriction would provide clues as to differences between the airways of healthy individuals and those with asthma. This in turn may suggest novel therapeutic strategies that may benefit patients suffering from asthma and other hyperresponsive conditions.
Supported by AADRC P01 AI56352, HL079398, and K08HL086604.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Stelmack GL, Halayko AJ, Solway J, Mitchell RW. Latrunculin B increases force fluctuation-induced relengthening of ACh-contracted, isotonically shortened canine tracheal smooth muscle. J Appl Physiol 2005;98:489–497. [DOI] [PubMed] [Google Scholar]
- 2.Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 1999;159:959–967. [DOI] [PubMed] [Google Scholar]
- 3.Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 1996;81:2703–2712. [DOI] [PubMed] [Google Scholar]
- 4.Lakser OJ, Lindeman RP, Fredberg JJ. Inhibition of the p38 MAP kinase pathway destabilizes smooth muscle length during physiological loading. Am J Physiol Lung Cell Mol Physiol 2002;282:L1117–L1121. [DOI] [PubMed] [Google Scholar]
- 5.Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol 1997;83:1202–1208. [DOI] [PubMed] [Google Scholar]
- 6.Freedman S, Lane R, Gillett MK, Guz A. Abolition of methacholine induced bronchoconstriction by the hyperventilation of exercise or volition. Thorax 1988;43:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stirling DR, Cotton DJ, Graham BL, Hodgson WC, Cockcroft DW, Dosman JA. Characteristics of airway tone during exercise in patients with asthma. J Appl Physiol 1983;54:934–942. [DOI] [PubMed] [Google Scholar]
- 8.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 2001;163:994–1001. [DOI] [PubMed] [Google Scholar]
- 9.Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol 1981;50:1079–1086. [DOI] [PubMed] [Google Scholar]
- 10.Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol 2001;91:506–515. (discussion 504–505). [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Liu G, Shardonofsky F, Dowell M, Lakser O, Mitchell RW, Fredberg JJ, Pinto LH, Solway J. Tidal breathing pattern differentially antagonizes bronchoconstriction in C57BL/6J vs. A/J mice. J Appl Physiol 2006;101:249–255. [DOI] [PubMed] [Google Scholar]
- 12.Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JH, Eidelman DH. Bronchial responsiveness among inbred mouse strains: role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med 2000;161:839–848. [DOI] [PubMed] [Google Scholar]
- 13.Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol 2001;91:1962–1970. [DOI] [PubMed] [Google Scholar]
- 14.Shinagawa K, Kojuima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med 2003;168:959–967. [DOI] [PubMed] [Google Scholar]
- 15.Tankersly CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. J Appl Physiol 1999;86:1764–1769. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Rao K, Halayko AJ, Kepron W, Stephens NL. Bronchial smooth muscle mechanics of a canine model of allergic airway hyperresponsiveness. J Appl Physiol 1992;72:39–45. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol 2002;283:L1181–L1189. [DOI] [PubMed] [Google Scholar]
- 18.Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol 1979;46:681–687. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell RW, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol 1994;267:L218–L222. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell RW, Magnussen H, Rabe KF, Leff AR. Passive sensitization of human airways induces myogenic contractile responses in vitro. J Appl Physiol 1997;83:1276–1281. [DOI] [PubMed] [Google Scholar]
- 21.Latourelle J, Fabry B, Fredberg JJ. Dynamic equilibration of airway smooth muscle contraction during physiological loading. J Appl Physiol 2002;92:771–779. [DOI] [PubMed] [Google Scholar]
- 22.Mijailovich SM, Butler JP, Fredberg JJ. Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys J 2000;79:2667–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 1999;274:24211–24219. [DOI] [PubMed] [Google Scholar]
- 24.Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol Lung Cell Mol Physiol 1997;273:L930–L940. [DOI] [PubMed] [Google Scholar]
- 25.Pratusevich VR, Seow CY, Ford LE. Plasticity in canine airway smooth muscle. J Gen Physiol 1995;105:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seow CY, Pratusevich VR, Ford LE. Series-to-parallel transition in the filament lattice of airway smooth muscle. J Appl Physiol 2000;89:869–876. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Pare PD, Seow CY. Effect of chronic passive length change on airway smooth muscle length-tension relationship. J Appl Physiol 2001;90:734–740. [DOI] [PubMed] [Google Scholar]
- 28.Dulin NO, Fernandes DJ, Dowell M, Bellam S, McConville J, Lakser O, Mitchell R, Camoretti-Mercado B, Kogut P, Solway J. What evidence implicates airway smooth muscle in the cause of BHR? Clin Rev Allergy Immunol 2003;24:73–84. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes DJ, Mitchell RW, Lakser O, Dowell M, Stewart AG, Solway J. Do inflammatory mediators influence the contribution of airway smooth muscle contraction to airway hyperresponsiveness in asthma? J Appl Physiol 2003;95:844–853. [DOI] [PubMed] [Google Scholar]
- 30.Solway J, Bellam S, Dowell M, Camoretti-Mercado B, Dulin N, Fernandes D, Halayko A, Kocieniewski P, Kogut P, Lakser O, et al. Actin dynamics: a potential integrator of smooth muscle (Dys-)function and contractile apparatus gene expression in asthma. Parker B. Francis lecture. Chest 2003;123:392S–398S. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 1995;15:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L1161–L1168. [DOI] [PubMed] [Google Scholar]
- 33.Bursac P, Fabry B, Trepat X, Lenormand G, Butler JP, Wang N, Fredberg JJ, An SS. Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun 2007;355:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'angelo G, Adam LP. Inhibition of ERK attenuates force development by lowering myosin light chain phosphorylation. Am J Physiol Heart Circ Physiol 2001;282:H602–H610. [DOI] [PubMed] [Google Scholar]